Abstract
Phosphorus (P) is one of the main nutrients for all plants, including grasses. However, sources of P fertilizer are not renewable, are not evenly distributed and overfertilization can lead to serious environmental degradation. Smart combinations of grasses may be able to more efficiently take up P from soils through complementarity. In a two-year field mesocosm experiment, we compared the performance of Lolium perenne L. and Festuca arundinacea Schreb. in monocultures and in combination, as well as a mixture of both species with a tetraploid variety of Lolium perenne L and Phleum pratense L. Plants were grown in an unfertilized low P soil and in P fertilized soil for two growing seasons. We measured biomass production, root traits, nutrient uptake, microbial biomass and enzymatic activities. In the unfertilized plots the combination of Lolium perenne and Festuca arundinacea generated the highest cumulative yields (25,951 ± 4059 kg ha−1), relative total yield (> 1) and P nutrition index (0.79). We related this to the complementarity found in root traits and lower intraspecific competition of Festuca arundinacea and Lolium perenne diploid. Festuca arundinacea produced higher root biomass than Lolium perenne diploid at deeper soil layers (98 vs. 44 g m−2; p < 0.05). On the other hand, Lolium perenne diploid had significantly finer roots than Festuca arundinacea both at topsoil and bottom layers (0.19 vs. 0.22 mm and 0.19 vs. 0.23 mm at top and bottom layers respectively). The 4 species combination did not result in higher yields. Our results show that, in low P soils, combinations of grass species with contrasting root traits could lead to significantly higher yields than monocultures.
Similar content being viewed by others
Introduction
Phosphorus (P) is one of the main nutrients for plants and therefore essential for grassland production (Aydin and Uzun 2005). However, P is a non-renewable resource that is concentrated in very few countries (Penuelas et al. 2023). P has also been included in the list of critical raw materials by the European Union because of its uneven distribution across the globe (Bertrand et al. 2016). At the same time, overfertilization with P in the past has turned almost 60% of European grassland soils unresponsive to P fertilization (Recena et al. 2022) and has caused eutrophication and degradation of natural ecosystems (Yuan et al. 2018). It is therefore urgent to reduce the environmental impacts of P fertilization by reducing the overall demand for P fertilizers in agriculture without compromising agricultural production (Garske et al. 2020).
Seeding mixtures of plants can be an option to reduce P dependency while increasing plant yields and nutrient uptake in agricultural systems (Postma and Lynch 2012; Xue et al. 2016). In situations of P limitation, complementary root traits and delayed resource access by different plants could result in higher yields in mixtures than in monocultures (Bakker et al. 2018; Oram et al. 2018). Moreover, some grass species can facilitate the uptake of P of the other species they are grown in combination. This can be achieved through a change in soil chemical properties, such as pH, due to the release of root exudates, or by the promotion of soil microbial activity that could ultimately lead to a higher P availability (Khan et al. 2009; Xue et al. 2016; Giles et al. 2017). Research on such mechanisms has focused mostly in natural and permanent grasslands, yet complementary use of P in intensively managed grasslands remains poorly investigated.
In Europe, perennial or English ryegrass (Lolium perenne L.) is one of the main cultivated grass species (Rogers et al. 2019; Becker et al. 2020). This grass species is well adapted to a temperate climate and it provides high yields and feeding values (Becker et al. 2020). In recent years, the combination of L. perenne with tall fescue (Festuca arundinacea Schreb.) has gained interest because of F. arundinacea’s higher tolerance to drought (Cougnon et al. 2014), providing a better access to water under drought events. This quite common combination of L. perenne and F. arundinacea, however, has received little attention as a potential way to increase P access to grasslands. This combination could be of special interest in areas of the world where the inputs of P fertilizer are expected to decline such as north-western Europe. There are a few challenges related to F. arundinacea that could inhibit its widespread cultivation in temperate regions. One of its major drawbacks is its low palatability compared to L. perenne (Cougnon et al. 2014, 2018). Moreover, the high silicon content of the leaves of F. arundinacea results in low digestibility (Hartley et al. 2015). To mitigate these disadvantages, one strategy is to combine F. arundinacea in grass mixtures with species with a higher palatability.
Here we quantified the P uptake and yield benefits of introducing F. arundinacea in combination with L. perenne or in association with other palatable grass species (timothy (Phleum pratense L.) and a tetraploid variety of L. perenne). We compared performance with and without P fertilization, and investigated the relationships between yields and root traits, soil microbial activity or soil properties. We tested the general hypothesis that F. arundinacea, with its deeper rooting system, would facilitate access by the grassland mixtures to P in the soil and improve yields in low P conditions, and that this could be related to a complementarity effect in root traits as well as an increased P solubilization by the microbial community.
Materials and methods
Experimental setup
We conducted a two-year (2019–2020) field mesocosm experiment on the campus of Wageningen University, The Netherlands (51.989° N, 5.657′ E). The climate at the field location is temperate maritime (Cfb according to Köppen-Geiger classification). Further detailed climatic information during the two seasons can be found in the supplementary material (Supplementary material, Fig. S1). The mesocosms consisted of wooden boxes (0.75 m wide × 0.75 m tall × 0.40 m deep) that were installed in the field (Supplementary material, Fig. S2). The boxes were then divided in half, so each mesocosm consisted of two plots with a surface of 0.28 m2 each. The mesocosms had a weed control fabric on the bottom side that allowed water to leach out but prevented roots from exploring the soil outside the wood box, both downwards and sideways. Two years before the start of the experiment, the boxes were filled with a pre-homogenised low P sandy soil from Achterberg, The Netherlands (51.593° N, 5.352° E). This soil is classified as a plaggic podzol (WRB) and was collected from the top 0–0.25 m from an extensively managed grassland that had not been fertilized with P for more than 25 years. This soil had a low CaCl2 extractable P (0.5 mg P kg−1) fraction, acidic pH (5.59) and a cation exchange capacity of 7 cmol kg−1. More information about this soil is available in Supplementary material (Table S1).
L. perenne diploid (Lp2) and F. arundinacea (Fa) were planted in monocultures and in a 50/50 combination (Lp2Fa). We used the seeding rate recommended by the seed producer (Barenbrug BV, Nijmegen, The Netherlands) which was 4 and 6 g seeds m−2 for Lp2 and Fa respectively. For the Lp2Fa combination we used 50% of the recommended rate for each grass species, resulting in 2 and 3 g m−2 respectively. In addition, to improve the palatability of the Lp2Fa grass combination, we studied a 4-species combination (4sp) with timothy (P. pratense) (Pp) and a tetraploid variety of Lolium perenne L. (Lp4). Both Pp and Lp4 are considered highly palatable (Balocchi and López 2009; Ogle et al. 2010). The seeding rate for each species in the 4sp treatment was 25% of the recommended seeding rate for each species, resulting in 1, 1.5, 0.4 and 1.5 g m−2 for Lp2, Lp4, Pp and Fa respectively. We also grew Pp and Lp4 in monocultures to calculate the relative yield total for the 4sp treatment. The experiment resulted in a randomized block design with 6 plant mixtures replicated 4 times, leading to 24 boxes each split in two plots (with and without P fertilization on the northern and southern sides respectively, not randomized) for a total of 48 plots distributed over 4 blocks. Nine harvests were performed over two growing seasons (S1 and S2) for the above-ground plant biomass, and roots and soils were sampled at the last harvest in the end of the experiment. Specific dates of the harvests can be found in the Supplementary material (Supplementary material, Table S2).
All plots were fertilized with nitrogen (N) and potassium (K). N fertilizer was added in the form of calcium ammonium nitrate (CAN), which was added before sowing the grasses as well as after each grass cut, leading to a total application 350 kg N ha−1 for S1 and 311 kg N ha−1 for S2. K fertilizer was added in the form of potassium sulphate, once at the beginning of each growing season, at a rate of 30 kg K ha−1 for S1 and 36 kg K ha−1 for S2. In addition, P was applied in the form of triple super phosphate (Ca(H2PO4)2·H2O) on the northern half of each box (P+), at a rate of 22 kg P ha−1 for S1 and 50 kg of P ha−1 for S2. We increased the P application in S2 to ensure alleviation of any P limitation in the P+ plots. The southern side of each box did not receive any P fertilizer (P−). Rates were based on the Dutch fertilizer guidelines for grasslands (Schoonvelde et al. 2017). Detailed information on the amount of fertilization treatments and sowing can be found in the Supplementary material (Table S2). All fertilizers used were broadcasted uniformly by hand over the soil surface in solid granular form as provided by the commercial suppliers.
Plant analyses
Aboveground biomass samples were taken at each harvest (Supplementary material, Table S2) by cutting grass shoots 0.05 m above soil surface. A metal frame (0.2 × 0.2 m) was used as a guide to cut the grass at the same location at each harvest. The grass outside the metal frame was also clipped at each harvest and discarded. After harvesting, samples were sorted by grass species and dried for 48 h at 70 °C.
We calculated the relative yields (RY) of the grass species grown in the mixtures and the relative yield total (RYT) of the full mixture [1] (de Wit 1960).
where “a” represents the grass species. RY is calculated similarly but only for one of the species.
Root samples were taken at two different depths (0–0.15 and 0.15–0.40 m) at the end of the experiment using a soil core with a diameter of 30 mm. Three cores were taken from each plot and pooled. Roots were rinsed with water and separated from soil using a 1 mm mesh sieve. For analyses of root traits, a subsample of fresh roots was scanned after being stained with a neutral red solution (0.5 g L−1). A resolution of 600 dpi on a 0.15 × 0.25 m tray was used for scanning. Root length (m) and diameter (mm) were calculated using the software WinRHIZO Pro (Regent Instruments, Quebec, Canada) (Ros et al. 2018). Specific root length (SRL) was calculated by dividing root length by root biomass (m g−1). The remaining roots were dried at 70 °C for 48 h.
N and P content in shoots were measured colorimetrically in a segmented flow analyser (SFA, Skalar, SAN++, Breda, The Netherlands) after digestion with H2SO4/Se (Houba et al. 1995). In short, shoot samples were placed in digestion tubes with a mixture of salicylic acid, sulphuric acid and selenium to prevent loss of N. The sample was incubated for 2 h at room temperature followed by heating at 100 °C for 2 h. After the tubes were allowed to cool down, hydrogen peroxide was added and heated at 330 °C. This step was repeated twice. Finally, the samples were allowed to stand overnight before homogenization and measurement. Measurements were done separately for each of the species in the grass mixtures treatments (Lp2Fa and 4sp). The N and P content at the treatment level was calculated as the weighted average of the N and P content present in a given treatment according to the relative biomass contribution of each species for that treatment.
We calculated the phosphorus nutrition index (PNI) [2] (Duru and Ducrocq 1996) and the nitrogen nutrition index (NNI) [3] (Lemaire and Gastal 1997) in the aboveground biomass as follows:
Values of PNI or NNI > 1 indicate no nutrient limitation and < 0.8 show plant growth limitation. These indexes were calculated on pastures at field level and are designed to assess the direct and indirect effects of P and N fertilization on grass growth and nutritional status.
Soil analyses
Soil samples were taken at the end of the experiment from a depth of 0–0.1 m. Representative samples were taken using a Grass Plot Sampler (Eijkelkamp, Giesbeek, The Netherlands) with a diameter of 23 mm. The soil cores were sampled randomly over the soil surface of the plot and pooled after sampling c.a. 500 g of fresh soil in each plot. Soil samples were stored at − 80 °C for biological analyses or dried at 40 °C for 48 h for soil chemical analyses.
Soluble P, dissolved organic nitrogen (DON), ammonium (NH4), nitrate (NO3), dissolved organic carbon (DOC) and pH were determined on dried samples. For the determination of pH, soil was shaken with a 0.01 M CaCl2 solution for 2 h and measured with a combined glass electrode. The solution was then also used for the colorimetric determination of the beforementioned C, N and P parameters using a SFA (Skalar, SAN++, Breda, The Netherlands) (Houba et al. 2000).
Microbial C, N and P, and enzymatic activities were measured on fresh or frozen (-80 °C) samples. Due to technical constraints, we were not able to measure biological variables in the Pp and Lp4 monoculture treatments. Microbial C and N were measured with the chloroform fumigation/extraction method in fresh samples (Jenkinson and Powlson 1976). Briefly, 30 g of soil was fumigated under vacuum conditions for 24 h. Subsequently C and N were extracted with 100 mL of a 0.05 M K2SO4 solution for 1 h. The measurements of C and N were performed in a TOC analyser (Shimadzu SSM-5000A/TOC-VCSH Carbon, Shimadzu, Kyoto, Japan). In parallel, unfumigated samples were also extracted as the controls. Microbial C and N were then calculated by subtracting the values of C and N in the control unfumigated samples from the values in the fumigated samples. Microbial P was also estimated using the chloroform fumigation/extraction method (Brookes et al. 1982). In short, 5 g of fresh soil were fumigated under vacuum conditions for 24 h. P was then extracted with 100 mL of a 0.5 M sodium bicarbonate solution at pH 8.5 for 30 min. Similarly, in parallel, P was extracted from unfumigated samples as controls. P content was measured colorimetrically by the molybdate blue method (Murphy and Riley 1962). Microbial P was calculated by subtracting the values of P in the control samples from the values in the fumigated samples. Microbial C, N and P are expressed in mg of C, N or P kg−1. No correction factors were applied for microbial C, N or P.
Potential soil enzymatic activities were measured on frozen soil colorimetrically using a 96-well microplate technique (ISO 20130:2018) (Cheviron et al. 2021). Enzymes linked to the C, N and P cycle were analysed. The enzymes that were measured were β-glucosidase (BGLU; EC: 3.2.1.21), N-acetyl-glucosaminidase (NAG; EC: 3.2.1.30), arylamidase (ARYLN; EC: 3.5.1.5), acid phosphatase (PAC; EC: 3.1.3.1) and alkaline phosphatase (PAK; EC: 3.1.3.2). In short, 4 g of fresh soil were mixed with H2O for BGLU and NAG during 10 min. For ARYLN, PAC and PAK, a solution of Trizma buffer (50 mM) at pH 7.5, 5.5 and 11 respectively was used. Soil solutions were incubated with 4-nitrophenyl β-D-glucopyranoside 0.05 M for BGLU, 4-N-acetyl-β-D-glucosaminide 0.01 M for NAG, L-leucine β-naphthylamide hydrochloride 0.008 M for ARYLN and 4-nitrophenylphosphate disodium salt hexahydrate 0.05 M for PAC and PAK. After incubation, the reaction was stopped and the coloration revealed by the addition of 0.5 M CaCl2 and 0.1 M Tris at pH 12 for BGLU, NAG, PAC and PAK activities. For ARYLN, ethanol and p-dimethylaminocinnamaldehyde were added (DMCA). The absorbance was measured on a Varioskan Flash-Thermo microplate reader (Thermo Fisher Scientific, Waltham, USA) at 405 nm for BGLU, NAG, PAC and PAK and at 540 for ARYLN. Enzymatic activities were expressed in nmol PNP (paranitrophenol) min−1 g−1 of dry soil for BGLU, NAG, PAC and PAK activities and in β-naphthylamine min−1 g−1 of dry soil for ARYLN activities.
Statistical analyses
Statistical analyses and figures were done using the software RStudio v1.4.1717 (R Core Team 2022). Figures were made using the R package “ggplot2” (Wickham 2016).
The effect of plant species and time on yields, nutrient content in the leaf, PNI and NNI were analysed by conducting a linear mixed effect model, with the plot’s ID nested within blocks as a random factor using the R package “lme4” separately on P− and P+ plots (Bates et al. 2015). When the effect of the plant species was significant (p < 0.05) we conducted Tukey’s post-hoc test using the R package “emmeans” (Lenth 2023). When the interaction of the model is significant, it is not possible to interpret the effect of the plant species, as it depends on the time of observation. In this situation, we performed Tukey’s post-hoc test on single main effects after estimating marginal means using the R package “emmeans”. This allows to test the effect of factor accounting for the interaction effect.
The effects of plant species on root morphology, soil chemical variables and soil biological variables were analysed using one-way analyses of variance (ANOVA) separately on P− and P+ plots including block as a random effect using the package “lme4” separately on P+ and P− plots. When the ANOVA showed statistical differences, p < 0.05, we conducted Tukey’s HSD post-hoc test to reveal pairwise differences between plant treatments using the R package “emmeans”. We also performed one way ANOVAs at a plant species level within the mixtures to understand which of the grass species within the mixture was driving P uptake or increased yields at a given harvest. One-way ANOVA models residuals were examined for normality, homogeneity and heteroscedasticity assumptions.
Over- or underyielding of the grass species grown in mixture were tested by performing a one tailed t-test on the RY of each species. When the RY of Lp2 or Fa grown in the Lp2Fa mixture were significantly (p < 0.05) higher or lower than 0.5 overyielding or underperformance respectively is assumed. For the grasses grown in the 4sp mixture, over- or underyielding was assumed when Fa or Pp’s RY was significantly higher or lower than 0.25. The distinction between Lp2 and Lp4 in the 4sp mixture is not possible, for this reason, overyielding or underperformance was assumed when Lp’s RY was significantly higher or lower than 0.5.
Raw data is available at ZENODO online repository (Velasco-Sánchez et al. 2023).
Results
Aboveground biomass and yields
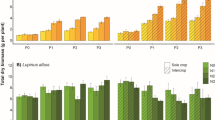
The results of the mixed linear model showed a significant effect of grass species on cumulative yields (p = 0.03) and a significant interaction of grass species and harvest date (p < 0.01) in the plots that received no P fertilization (P−). After examination of single main effects we observed that Lp2Fa mixture showed significantly higher cumulative yields than any other treatment, including Lp2 (L. perenne) and Fa (F. arundinacea) in monoculture (p < 0.05) at months 14, 16 and 18 after sowing (Fig. 1). On average, Lp2Fa produced 25,950 ± 8117 kg ha−1 (mean ± standard error, n = 4) of dry biomass over two growing seasons, a 69% increase compared to the mean of all other treatments combined (15,333 ± 924, n = 20). No statistical differences in cumulative yields were found between plant treatments in the P+ plots (Fig. 1). The cumulative yields were also, on average, the highest in the Lp2Fa mixture in the P- plots at the end of season 1 (10,720 ± 1242 kg ha−1) and season 2 (15,231 ± 2882 kg ha−1) yet the differences were not significant.
Cumulative biomass production of P unfertilized (P−) and fertilized plots (P+) for the different grass species mixtures. Lp2 = Lolium perenne L. diploid, Fa = Festuca arundinacea Schreb., Pp = Phleum pratense L., Lp4 = Lolium perenne L. tetraploid, Lp2Fa = Lp2 and Fa combination and 4sp = Lp2, Lp4, Fa and Pp combination. Bars indicate standard errors of the mean, n = 4. Asterisks indicate Tukey’s significant differences of single main effects. Months indicate months after sowing
The RYT was, generally, higher than 1 in all the harvests of the P− plots for the Lp2Fa treatment but not the 4sp treatment (Fig. 2). In the P+ plots, neither the Lp2Fa nor the 4sp mixture resulted in consistent RYT values above 1. The highly variable RYT values in the P+ and P− plots in June of season 1 (first harvest) are associated with the low yields at the first cut of the experiment.
Relative yield total (RYT) of the LpFa and 4sp combinations in the P fertilized (P+) and P unfertilized (P−) plots at each harvest. S1 = First season, S2 = Second season, Lp2Fa = Lolium perenne L. diploid and Festuca arundinacea Schreb. combination and 4sp = Lolium perenne L. diploid, Lolium perenne L. tetraploid, Festuca arundinacea Schreb. and Phleum pratense L. combination. Bars indicate standard error, n = 4. Values of RYT > 1 indicate higher yields than monoculture
In the P- plots, Lp2 dominated the harvests of season 1 and Fa dominated the ones of season 2 (Table 1). Lp2 produced significantly (p < 0.05) higher relative yields (RY > 0.5, overyielding) in the Lp2Fa mixture than in monoculture throughout the grass harvests of the first season (July, August, September and October). Conversely, Fa overyielded significantly throughout the second season (June, August and October). In the 4sp mixture, Lp overyielded in the harvest of May, June and August of season 2. Fa overyielded in the first cut of season 1, yet it significantly underyielded (RY < 0.25) in the harvest of August, September and October in season 1 and in May in season 2. Pp (P. pratense) also significantly underyielded in the 4sp mixture in the last cut of season 2.
In the P+ plots, the trends were more erratic. Overyielding was found for Lp2 in the Lp2Fa mixture only in the cut of August in season 1 and underyielded in the cut of October in season 2 (Table 1). Fa grown in the Lp2Fa mixture overyielded in the harvests of July of season 1 and in the harvests of May and October of season 2. Grass species generally underyielded in the 4sp mixture grown in the P+ plots, Fa underyielded throughout the first season (July, August, September and October) and also in the cut of June in season 2. Similarly, Pp underyielded in the cut of October in season 2. Only Lp (not possible to differentiate Lp2 from Lp4 in the 4sp mixture) overyielded (RY > 0.5) in the cut of October in season 2.
Results of the mixed linear model showed a significant effect of grass species on P nutrition (N P−1 and PNI) and a significant interaction between grass species and season (S1 vs. S2) (p < 0.01) in the P− plots (Fig. 3). No differences were observed between grass species at the end of S1 in P− plots, the average N P−1 value was 13.49 ± 0.36, n = 4 and the PNI values at the end of S1 were close to the 0.8 threshold for P limitation (Fig. 3, panels A and B). At the end of S2, in the P− plots, the N P−1 ratio increased significantly (p < 0.05) in the Lp2, Lp4 and 4sp mixtures compared to S1. Likewise, PNI decreased significantly at the end of S2 compared to S1 for Lp2 and the 4sp mixture in the P- plots. The N P−1 ratios were significantly higher in the Lp2, Lp4 and 4sp treatments, followed by Pp, Lp2Fa and Fa in the P- plots (Fig. 3, panel A) and the PNI values indicated near adequate P levels (0.8) in the Lp2Fa and Fa treatments and strong P limitation in the Lp2, Lp4 and 4sp combinations (Fig. 3, panel B) at the end of S2. In P+ plots, no statistical differences were found among grass species on N P−1 or PNI and these values did not change across seasons (Fig. 3, panels C and D). The PNI values in the P+ plots were close to 0.8 in both S1 and S2, suggesting adequate P nutrition (Fig. 3, panel D).
Averaged nutrient uptake in P unfertilized (P−) (panels A and B) and P fertilized (P+) (panels C and D) plots at last harvest of season 1 (S1) and season 2 (S2). PNI = Phosphorus nutrition index. Lp2 = Lolium perenne L. diploid, Fa = Festuca arundinacea Schreb., Pp = Phleum pratense L., Lp4 = Lolium perenne L. tetraploid, Lp2Fa = combination of Lp2 and Fa and 4sp = combination of Lp2, Lp4, Fa and Pp. Letters indicate significant differences among grass species after single main effect evaluation (Tukey test, p < 0.05). Brackets and asterisks indicate significant differences between S1 and S2 (p < 0.05). Horizontal line at 0.8 in panels B and D shows threshold for adequate PNI. Bars show standard errors, n = 4
When examining the differences in P nutrition at a species level within the mixtures we observed no significant differences in N P−1 ratios of PNI by the end of S1 and S2 in the P− and P+ plots (Supplementary material, Table S3). In the P+ plots, the PNI values were close to the 0.8 threshold, indicating no strong P limitation (Supplementary material, Table S3).
We did not find strong N limitation in either P− and P+ plots as shown by the high nitrogen nutrition index (NNI) values, which were on average higher than 0.8 (Supplementary material, Table S4). We observed that the 4sp was the mixture with the lowest NNI values both in the P+ and P− plots at S1 and S2 (Supplementary material, Table S4). Detailed information on N and P nutrition as well as NNI and PNI indexes can be found in Supplementary material, Table S4.
Root morphology
Root biomass showed no statistical differences between plant treatments in the P− and P+ plots in the top soil layer (0–0.15 m) (Fig. 4, panels A and D). SRL was found significantly lowest for Fa in both P− and P+ plots (141 ± 8 and 148 ± 15 m g−1 for P− and P+ respectively). Root diameter was found the highest for Fa in both P− and P + with an average diameter of 0.23 ± 0.01 and 0.22 ± 0.01 mm respectively (Fig. 4, panels C and F).
Root traits in P unfertilized (P−) (panels A, B and C) and P fertilized (P+) (panels D, E and F) plots at last harvest in the top soil (0–0.15 m). Lp2 = Lolium perenne L. diploid, Fa = Festuca arundinacea Schreb., Pp = Phleum pratense L., Lp4 = Lolium perenne L. tetraploid, Lp2Fa = combination of Lp2 and Fa and 4sp = combination of Lp2, Lp4, Fa and Pp, SRL = Specific root length. Letters are displayed when ANOVA’s p < 0.05 and indicate statistically significant differences between plants based on Tukey’s HSD test. Boxes describe interquartile range, whiskers represent first and fourth quartiles, the horizontal line shows the median and dots show observations outside of the first and fourth quartiles
In the deeper soil layers (0.15–0.40 m) we observed significantly (p < 0.05) higher root biomass and diameter for Fa (Fig. 5, panels A, C, D and F). The average root biomass of Fa at the deeper soil layers was 98.14 ± 18.61 g m−2 for the P- plots and 93.9 ± 24.69 for the P+ plots g m−2, n = 4 (Fig. 5, panels A and D). Root diameter was highest in the Fa monoculture and lowest in the Pp monoculture in both P− and P+ plots (Fig. 5, panels C and F). SRL was also significantly lower in the Fa compared to the rest of plant treatments in the P+ plots. In the P- plots, no statistically significant differences between species were found, yet Fa also showed, on average, the lowest SRL (Fig. 5, panels B and E).
Root traits in P unfertilized (P−) (panels A, B and C) and P fertilized (P+) (panels D, E and F) plots at last harvest in the deeper layers of the soil (0.15–0.40 m). Lp2 = Lolium perenne L. diploid, Fa = Festuca arundinacea Schreb., Pp = Phleum pratense L., Lp4 = Lolium perenne L. tetraploid, Lp2Fa = combination of Lp2 and Fa and 4sp = combination of Lp2, Lp4, Fa and Pp. SRL = Specific root length. Letters are displayed when ANOVA’s p < 0.05 and indicate statistically significant differences between plants based on Tukey’s HSD test. Boxes describe interquartile range, whiskers represent first and fourth quartiles, the horizontal line shows the median and dots show observations outside of the first and fourth quartiles
Root traits were also significantly correlated with P nutrition values (Table 2). In P− plots, we observed that PNI and P uptake were positively correlated with top root diameter, deep root biomass and deep root diameter. PNI was also negatively correlated with top and deep SRL. P concentration was not correlated with any of the root traits in the P− plots. In the P+ plots, P concentration and PNI were positively correlated with top and deep SRL. Moreover, P concentration was negatively correlated with top and deep root diameter and total root biomass.
Soil chemical and biological differences
No statistical differences were found between the plant treatments for most of the soil chemical variables analysed (readily available P, DOC, DON, NH4, NO3 and pH) (Supplementary material, Table S5). Fertilization with P roughly doubled the levels of readily available P (CaCl2 extractable P); on average soluble P was 0.02 ± 0.01 mg kg−1 of dry soil for the P- plots and 0.05 ± 0.01 mg kg−1 for the P + plots. Plant available P (Olsen P) was also increased in the fertilized plots, 9.76 ± 0.42 versus 20.63 ± 0.75 mg P kg−1. The pH of the soil was unaffected by the plant treatments, the pH of the P− plots was 5.23 ± 0.03 and 5.26 ± 0.04 in the P+ plots, n = 24. On average, the 4sp treatment had the highest DOC values (6.43 ± 0.16 mg C kg−1, n = 24) in the P- plots, yet the differences were found non-significant (p = 0.096).
Similarly to soil chemical variables, the effect of the different plant treatments was non-significant in most of the soil biological variables measured (microbial C, microbial N, microbial P, N-acetyl-glucosaminidase (NAG), arylamidase (ARYLN), acid phosphatase (PAC) and alkaline phosphatase (PAK)) with the exception of β-glucosidase (BGLU) (Table 3). BGLU potential activity in the P- plots was the highest for the 4sp treatment (18.92 ± 1.86 PNP min−1 g−1 of dry soil, n = 4). The 4sp treatment also showed the highest average of microbial C, N and P biomass and highest NAG, ARYLN, PAC and PAK, yet these differences were found non-significant.
Discussion
Root traits and reduced intraspecific competition improve P nutrition
The aim of this experiment was to evaluate the introduction of F. arundinacea (Fa) in grass mixtures with L. perenne (Lp) alone or with other grass species (tetraploid variety of Lp and P. pratense (Pp)) to improve P acquisition and therefore reduce dependency on P fertilisers. We hypothesized that Fa, because of its deeper root system, could potentially explore and make use of more stocks of P compared to other grass species. Our results confirm our initial hypothesis showing a significantly higher cumulative biomass production in the Lp2Fa treatment compared to the Lp2 or Fa monocultures (Fig. 1) and a RYT consistently greater than 1 in the plots with no P fertilisation (P−), indicating overyielding (Fig. 2 and Table 1).
The increased cumulative yields and RYT of the Lp2Fa combination can be explained by a complementary use of resources over time and space and lower intraspecific competition. During the first season Lp2 grown in the Lp2Fa mixture significantly overyielded compared to the monoculture, whereas, in the second season, Fa was the species that overyielded (Table 1). During the first season, Lp2, a faster-growing grass (Finn et al. 2013), possibly benefited from more available P, as shown by the ideal PNI values (Fig. 3, panels B and D). Lp2 also could have benefited from a lower intraspecific competition, as the seeding rate was reduced in the Lp2Fa compared to the monoculture (2 vs. 4 g m−2). Conversely, at the end of the second season, Fa, a slower-growing grass (Gastal et al. 2010), caught up and started to benefit from its distinct root traits and associated increased access to more nutrients (Cougnon et al. 2014) (Table 2). Likewise, Fa could also have benefitted from a lower intraspecific competition, as the seeding rate in the mixture was also reduced compared to the monoculture (3 vs. 6 g m−2).
We argue that the Lp2Fa mixture benefited from the niche complementarity of Lp2 and Fa, which ultimately also facilitated P nutrition. By the end of the experiment, irrespective of the P fertilization regime, Lp2 in monoculture had consistently and significantly finer roots than Fa (Figs. 4, panels C and F and 5, panels C and F) and Fa had significantly more root biomass at deeper soil layers (Fig. 5, panels A and D). Finer roots because of an associated higher specific root length (Eissenstat 1992; Tshewang et al. 2022) and deeper root biomass because of increased access to nutrient pools (Ros et al. 2018) have been suggested as relevant for P uptake in grass mixtures. Our results are in line with previous research in which Fa was shown to have higher root biomass at deeper soil layers (Cougnon et al. 2014, 2017; Ros et al. 2018) and relatively thick roots (Cougnon et al. 2017). The higher root biomass in the deeper soil layers potentially allowed Fa to keep up P uptake under limiting conditions in both monoculture and when grown in the Lp2Fa mixture (Fig. 3 and Table 2). This was not the case for Lp2 in monoculture, which was strongly P limited as shown by the PNI and N P−1 values at the end of season 2 (Fig. 3, panels A and B). In this case, the higher intraspecific competition in the monoculture could have resulted in a decrease in P acquisition, as also shown in global meta-analyses (Adler et al. 2018; Zhu et al. 2023).
We also hypothesized that an increased complementarity between roots would result in a higher P uptake and yields under P limitation in the mixture with 4 species (Bi et al. 2019). However, when we introduced Fa in the more palatable 4sp mixture, we did not observe any increased yield or RYT compared to the monocultures (Figs. 1 and 2). In fact, we observed underyielding for some of the species in the mixture at many of the harvests, particularly for Fa (Table 1). Other studies have also demonstrated that increasing the number of species in a grass mixture does not necessarily result in increased yields (Mangan et al. 2011; Roscher et al. 2011) and that high interspecific competition could result in lower P uptake in grasslands (Guiz et al. 2018).
The adaptation of Fa in different types of soils should be considered. In our mesocosms, we used a homogeneous sandy soil with a low P content. It is unknown how different grasses would react in soils with a finer texture or different P availability distribution, as these variables are important for root traits and plant growth (Becker et al. 2020). Moreover, we found contrasting correlations for the P+ and P− plots, suggesting different root pathways of P acquisition depending on soil P status (Table 2). Likewise, previous studies have proposed that different root traits, such as root diameter and length, are influenced by soil P availability (Ros et al. 2018; Kumar et al. 2019), aligning with the observed differences in correlations presented in Table 2. Lastly, concerns of Fa palatability should be taken into consideration when examining the higher yields of the Lp2Fa mixture under P limitation. Nevertheless, newer varieties of Fa are considerably improved in palatability (Becker et al. 2020; Kindiger 2021). Moreover, post-harvest techniques, such as silage, are known to increase the nutritional value of harvested grass (Peratoner et al. 2011). In this sense, Lp2Fa could be an interesting grass mixture in situations of P limitation.
Contribution of soil microorganisms towards P nutrition
Soil microorganisms are able to solubilize poorly soluble forms of P and eventually increase the plant available pool of P (Khan et al. 2009; Richardson and Simpson 2011). As such, we hypothesized that soil microorganisms could improve the uptake of P by grasses in situations of P limitation. However, we did not observe any relationship between microbial activities (enzyme activities) or microbial biomass with P uptake in both P+ and P− plots. Our results indicate that none of the species studied contributed to significantly change the microbial community of the soil in a way that resulted in higher yield or P mobilization.
We did observe that, in the P− plots, the 4sp treatment resulted in significantly higher BGLU activities. There was also a trend for increased microbial C, N and P biomass and increased ARYLN, NAG, PAC and PAK activities compared to Lp2, albeit non-significant. This might be related to an increased interspecific competition between grass species in the 4sp treatment (Bybee-Finley et al. 2022). For example, the Pp species was well established in the 4sp mix early in the experiment, with an initial RY of 0.49, indicating neither over nor under-yielding. At the end of the second season, however, at the time of soil sampling, Pp had almost entirely been outcompeted, and had therefore likely provided additional biomass available for microbial degradation. So, higher turnover of organic material because of a higher competition among species could have caused the increased microbial activities and biomass (Schofield et al. 2019; Sun et al. 2021). Moreover, a higher species diversity could have resulted in a higher exudation of organic compounds that might have stimulated microbial activities (Steinauer et al. 2016).
Measuring soil microbial activities and biomass throughout the growing season might have yielded different results as the activities of soil microorganisms could be time-dependent (Gao et al. 2021). Other P related enzymes, such as phytase, could have also dominated the P release from poorly soluble P forms (Rizwanuddin et al. 2023) and their effects might have been different depending on the most abundant poorly soluble P species in the soil (Velasco‐Sánchez et al. 2024). Activities could also have been markedly different at deeper soil layers, where the root biomass was dominated by Fa (Fairbanks et al. 2020). Similarly, measuring arbuscular mycorrhiza fungi (AMF) colonization could have also explained the differences between grass species, yet preliminary measurements during the first season showed no statistical differences.
Conclusion
We tested grass mixture combinations containing F. arundinacea as a way to reduce the inputs of P fertilizer in intensively managed grasslands. We hypothesized that F. arundinacea, because of its deeper root system would be able to access more P than other grass species. We also postulated that in a situation of P limitation soil microorganisms would be of importance to achieve high yields. Our results showed that the combination of L. perenne diploid and F. arundinacea resulted in significantly higher yields than the monocultures possibly because of the contrasting root systems of L. perenne diploid and F. arundinacea and the ability of F. arundinacea to withstand P limitation. On the other hand, we did not observe any contribution of the soil microbial community towards higher yields or P mobilization.
More research is needed on the combination of L. perenne diploid and F. arundinacea to allow its widespread use as a P limitation tolerant grass mixture. In particular, further experiments should aim to better understand the palatability issues of Fa, potentially including grazing animals in their experimental design. Moreover, experiments conducted on different soils that account for vertical heterogeneity of soil properties. Lastly, further experiments should also focus on exploring the role of soil microorganisms on P cycling at the deeper soil layers where F. arundinacea was predominant. We conclude that a combination of L. perenne and F. arundinacea species in intensively managed grassland could lead to the alleviation of P limitation and higher yields in situations of low P availability.
References
Adler PB, Smull D, Beard KH, Choi RT, Furniss T, Kulmatiski A, Meiners JM, Tredennick AT, Veblen KE (2018) Competition and coexistence in plant communities: intraspecific competition is stronger than interspecific competition. Ecol Lett 21:1319–1329. https://doi.org/10.1111/ELE.13098
Aydin I, Uzun F (2005) Nitrogen and phosphorus fertilization of rangelands affects yield, forage quality and the botanical composition. Eur J Agron 23:8–14. https://doi.org/10.1016/J.EJA.2004.08.001
Bakker LM, Mommer L, Van Ruijven J (2018) Can root trait diversity explain complementarity effects in a grassland biodiversity experiment? J Plant Ecol 11:73–84. https://doi.org/10.1093/JPE/RTW111
Balocchi OA, López IF (2009) Herbage production, nutritive value and grazing preference of diploid and tetraploid perennial ryegrass cultivars (Lolium perenne L.). Chil J Agric Res 69:331–339
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Becker T, Isselstein J, Jürschik R, Benke M, Kayser M (2020) Performance of modern varieties of Festuca arundinacea and Phleum pratense as an alternative to Lolium perenne in intensively managed sown grasslands. Agronomy 10:540. https://doi.org/10.3390/AGRONOMY10040540
Bertrand G, Cassard D, Arvanitidis N, Stanley G (2016) Map of critical raw material deposits in Europe. Energy Procedia 97:44–50. https://doi.org/10.1016/J.EGYPRO.2016.10.016
Bi Y, Zhou P, Li S, Wei Y, Xiong X, Shi Y, Liu N, Zhang Y (2019) Interspecific interactions contribute to higher forage yield and are affected by phosphorus application in a fully-mixed perennial legume and grass intercropping system. Field Crops Res 244:107636. https://doi.org/10.1016/J.FCR.2019.107636
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329. https://doi.org/10.1016/0038-0717(82)90001-3
Bybee-Finley KA, Cordeau S, Yvoz S, Mirsky SB, Ryan MR (2022) Finding the right mix: a framework for selecting seeding rates for cover crop mixtures. Ecol Appl 32:e02484. https://doi.org/10.1002/EAP.2484
Cheviron N, Grondin V, Marrauld C, Poiroux F, Bertrand I, Abadie J, Pandard P, Riah-Anglet W, Dubois C, Malý S, Marques CR, Asenjo IV, Alonso A, Díaz DM, Mougin C (2021) Inter-laboratory validation of an ISO test method for measuring enzyme activities in soil samples using colorimetric substrates. Environ Sci Pollut Res. https://doi.org/10.1007/S11356-021-17173-3
Cougnon M, Baert J, Van Waes C, Reheul D (2014) Performance and quality of tall fescue (Festuca arundinacea Schreb.) and perennial ryegrass (Lolium perenne L.) and mixtures of both species grown with or without white clover (Trifolium repens L.) under cutting management. Grass Forage Sci 69:666–677. https://doi.org/10.1111/GFS.12102
Cougnon M, De Swaef T, Lootens P, Baert J, De Frenne P, Shahidi R, Roldán-Ruiz I, Reheul D (2017) In situ quantification of forage grass root biomass, distribution and diameter classes under two N fertilisation rates. Plant Soil 411:409–422. https://doi.org/10.1007/S11104-016-3034-7
Cougnon M, Shahidi R, Schoelynck J, Van Der Beeten I, Van Waes C, De Frenne P, Van Labeke MC, Reheul D (2018) Factors affecting grazing preference by sheep in a breeding population of tall fescue (Festuca arundinacea Schreb.). Grass Forage Sci 73:330–339. https://doi.org/10.1111/GFS.12340
de Wit CT (1960) On competition. Verslagen Van Landboukundige Onderzoekingen, vol 66, pp 1–82
Duru M, Ducrocq H (1996) A nitrogen and phosphorus herbage nutrient index as a tool for assessing the effect of N and P supply on the dry matter yield of permanent pastures. Nutr Cycl Agroecosyst 47:59–69. https://doi.org/10.1007/BF01985719
Eissenstat DM (1992) Costs and benefits of constructing roots of small diameter. J Plant Nutr 15:763–782. https://doi.org/10.1080/01904169209364361
Fairbanks D, Shepard C, Murphy M, Rasmussen C, Chorover J, Rich V, Gallery R (2020) Depth and topographic controls on microbial activity in a recently burned sub-alpine catchment. Soil Biol Biochem 148:107844. https://doi.org/10.1016/J.SOILBIO.2020.107844
Finn JA, Kirwan L, Connolly J, Sebastià MT, Helgadottir A, Baadshaug OH, Bélanger G, Black A, Brophy C, Collins RP, Čop J, Dalmannsdóttir S, Delgado I, Elgersma A, Fothergill M, Frankow-Lindberg BE, Ghesquiere A, Golinska B, Golinski P, Grieu P, Gustavsson AM, Höglind M, Huguenin-Elie O, Jørgensen M, Kadziuliene Z, Kurki P, Llurba R, Lunnan T, Porqueddu C, Suter M, Thumm U, Lüscher A (2013) Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: a 3-year continental-scale field experiment. J Appl Ecol 50:365–375. https://doi.org/10.1111/1365-2664.12041
Gao W, Reed SC, Munson SM, Rui Y, Fan W, Zheng Z, Li L, Che R, Xue K, Du J, Cui X, Wang Y, Hao Y (2021) Responses of soil extracellular enzyme activities and bacterial community composition to seasonal stages of drought in a semiarid grassland. Geoderma 401:115327. https://doi.org/10.1016/J.GEODERMA.2021.115327
Garske B, Stubenrauch J, Ekardt F (2020) Sustainable phosphorus management in European agricultural and environmental law. Rev Eur Comp Int Environ Law 29:107–117. https://doi.org/10.1111/REEL.12318
Gastal F, Dawson LA, Thornton B (2010) Responses of plant traits of four grasses from contrasting habitats to defoliation and N supply. Nutr Cycl Agroecosyst 88:245–258. https://doi.org/10.1007/S10705-010-9352-X
Giles CD, Brown LK, Adu MO, Mezeli MM, Sandral GA, Simpson RJ, Wendler R, Shand CA, Menezes-Blackburn D, Darch T, Stutter MI, Lumsdon DG, Zhang H, Blackwell MSA, Wearing C, Cooper P, Haygarth PM, George TS (2017) Response-based selection of barley cultivars and legume species for complementarity: root morphology and exudation in relation to nutrient source. Plant Sci 255:12–28. https://doi.org/10.1016/J.PLANTSCI.2016.11.002
Guiz J, Ebeling A, Eisenhauer N, Hacker N, Hertzog L, Oelmann Y, Roscher C, Wagg C, Hillebrand H (2018) Interspecific competition alters leaf stoichiometry in 20 grassland species. Oikos 127:903–914. https://doi.org/10.1111/OIK.04907
Hartley SE, Fitt RN, McLarnon EL, Wade RN (2015) Defending the leaf surface: intra- and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front Plant Sci 6:116553. https://doi.org/10.3389/FPLS.2015.00035
Houba VJG, Temminghoff EJM, Gaikhorst GA, van Vark W (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal 31:1299–1396. https://doi.org/10.1080/00103620009370514
Houba VJG, Van der Lee JJ, Novozamsky I (1995) Soil analysis procedures; other procedures (soil and plant analysis, part 5B). Dept Soil Sci Plant Nutr, Wageningen Agricultural University 217
Jenkinson DS, Powlson DS (1976) The effects of biocidal treatments on metabolism in soil—V: a method for measuring soil biomass. Soil Biol Biochem 8:209–213. https://doi.org/10.1016/0038-0717(76)90005-5
Khan MS, Zaidi A, Wani PA (2009) Role of phosphate solubilizing microorganisms in sustainable agriculture—A review. Sustainable agriculture. Springer, pp 551–570
Kindiger B (2021) A preliminary evaluation on the performance of tall fescue F1 hybrids. J Hortic 8:1
Kumar A, Shahbaz M, Koirala M, Blagodatskaya E, Seidel SJ, Kuzyakov Y, Pausch J (2019) Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. J Plant Nutr Soil Sci 182:945–952. https://doi.org/10.1002/jpln.201900322
Lemaire G, Gastal F (1997) N uptake and distribution in plant canopies. Diagnosis of the nitrogen status in crops. Springer, Berlin, pp 3–43
Lenth RV (2023) emmeans: estimated marginal means, aka least-squares means
Mangan ME, Sheaffer C, Wyse DL, Ehlke NJ, Reich PB (2011) Native perennial grassland species for bioenergy: establishment and biomass productivity. Agron J 103:509–519. https://doi.org/10.2134/AGRONJ2010.0360
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Ogle D, St John L, Jensen K (2010) Species selection and grazing management guidelines. Pasture Grazing Manag Northwest PNW 614:7–20
Oram NJ, Ravenek JM, Barry KE, Weigelt A, Chen H, Gessler A, Gockele A, de Kroon H, van der Paauw JW, Scherer-Lorenzen M, Smit-Tiekstra A, van Ruijven J, Mommer L (2018) Below-ground complementarity effects in a grassland biodiversity experiment are related to deep-rooting species. J Ecol 106:265–277. https://doi.org/10.1111/1365-2745.12877
Penuelas J, Coello F, Sardans J (2023) A better use of fertilizers is needed for global food security and environmental sustainability. Agric Food Secur 12:1–9. https://doi.org/10.1186/S40066-023-00409-5
Peratoner G, Florian C, Klotz C, Figl U, Gottardi S (2011) Effect of forage conservation on the leaf texture of tall fescue. In: Proceedings of the 16th symposium of the European Grassland Federation, Gumpenstein, Austria, 29–31, 2011. Wallig, pp 223–225
Postma JA, Lynch JP (2012) Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Ann Bot 110:521–534. https://doi.org/10.1093/AOB/MCS082
R Core Team (2022) R: a language and environment for statistical computing
Recena R, García-López AM, Quintero JM, Skyttä A, Ylivainio K, Santner J, Buenemann E, Delgado A (2022) Assessing the phosphorus demand in European agricultural soils based on the Olsen method. J Clean Prod 379:134749. https://doi.org/10.1016/J.JCLEPRO.2022.134749
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996
Rizwanuddin S, Kumar V, Singh P, Naik B, Mishra S, Chauhan M, Saris PEJ, Verma A, Kumar V (2023) Insight into phytase-producing microorganisms for phytate solubilization and soil sustainability. Front Microbiol 14:1127249. https://doi.org/10.3389/FMICB.2023.1127249
Rogers ME, Lawson AR, Kelly KB, Rogers ME, Lawson AR, Kelly KB (2019) Summer production and survival of perennial ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea) genotypes in northern Victoria under differing irrigation management. Crop Pasture Sci 70:1163–1174. https://doi.org/10.1071/CP18542
Ros MBH, De Deyn GB, Koopmans GF, Oenema O, van Groenigen JW (2018) What root traits determine grass resistance to phosphorus deficiency in production grassland? J Plant Nutr Soil Sci 181:323–335. https://doi.org/10.1002/JPLN.201700093
Roscher C, Scherer-Lorenzen M, Schumacher J, Temperton VM, Buchmann N, Schulze ED (2011) Plant resource-use characteristics as predictors for species contribution to community biomass in experimental grasslands. Perspect Plant Ecol Evol Syst 13:1–13. https://doi.org/10.1016/J.PPEES.2010.11.001
Schofield EJ, Brooker RW, Rowntree JK, Price EAC, Brearley FQ, Paterson E (2019) Plant-plant competition influences temporal dynamism of soil microbial enzyme activity. Soil Biol Biochem 139:107615. https://doi.org/10.1016/J.SOILBIO.2019.107615
Schoonvelde H, van Middelkoop JC, Philipsen AP, van Dongen C, Bussink DW, Bos AJ, Velthof GL, de Haan JJ, Schröder JJ, Reijneveld JA, van Eekeren N (2017) Bemestingsadvies Commissie Bemesting Grasland en Voedergewassen: Versie 2017. Commissie Bemesting Grasland en Voedergewassen
Steinauer K, Chatzinotas A, Eisenhauer N (2016) Root exudate cocktails: the link between plant diversity and soil microorganisms? Ecol Evol 6:7387–7396. https://doi.org/10.1002/ECE3.2454
Sun Y, Zang H, Splettstößer T, Kumar A, Xu X, Kuzyakov Y, Pausch J (2021) Plant intraspecific competition and growth stage alter carbon and nitrogen mineralization in the rhizosphere. Plant Cell Environ 44:1231–1242. https://doi.org/10.1111/PCE.13945
Tshewang S, Rengel Z, Siddique KHM, Solaiman ZM (2022) Microbial consortium inoculant increases pasture grasses yield in low-phosphorus soil by influencing root morphology, rhizosphere carboxylate exudation and mycorrhizal colonisation. J Sci Food Agric 102:540–549. https://doi.org/10.1002/JSFA.11382
Velasco-Sánchez Á, Bennegadi-Laurent N, Trinsoutrot-Gattin I, van Groenigen JW, Moinet GYK (2024) Soil microorganisms increase Olsen phosphorus from poorly soluble organic phosphate: a soil incubation study. Soil Use Manag. https://doi.org/10.1111/sum.12960
Velasco-Sanchez Á, Ferron LM, Bennegadi-Laurent N, Trinsoutrot-Gattin I, van Groenigen JW, Moinet GY (2023) Dataset for Combination of ryegrass (Lolium perenne L.) and tall fescue (Festuca aurundinacea Schreb.) improve yields in a low P soil. https://doi.org/10.5281/ZENODO.8124393
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Xue Y, Xia H, Christie P, Zhang Z, Li L, Tang C (2016) Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: a critical review. Ann Bot 117:363–377. https://doi.org/10.1093/AOB/MCV182
Yuan Z, Jiang S, Sheng H, Liu X, Hua H, Liu X, Zhang Y (2018) Human perturbation of the global phosphorus cycle: changes and consequences. Environ Sci Technol 52:2438–2450. https://doi.org/10.1021/ACS.EST.7B03910
Zhu SG, Tao HY, Li WB, Zhou R, Gui YW, Zhu L, Zhang XL, Wang W, Wang BZ, Mei FJ, Zhu H, Xiong YC (2023) Phosphorus availability mediates plant–plant interaction and field productivity in maize-grass pea intercropping system: field experiment and its global validation. Agric Syst 205:103584. https://doi.org/10.1016/J.AGSY.2022.103584
Acknowledgements
This work was funded by the Horizon 2020 projects Circular Agronomics (Grant Number 773649) and REFLOW (Grant Number 814258). We would like to acknowledge the contribution of the following people: Henk Martens, Peter Garamszegi, Ron de Goede, Lisa Castel, Tamas Salanki, Marleen Ursem, Hannah Vos and Nina Ruoho for their help in the analysis of the samples, Barenbrug BV for providing the seeds and Gerlinde de Deyn for her advice in the selection of grass species. Lastly, we would like to thank the two anonymous reviewers who contributed to improve our manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by A.V.S., L.M.E.F. and D.T.C.M. Data analysis, data visualization, first draft and revised draft of the manuscript was done by A.V.S. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Velasco-Sánchez, Á., Ferron, L.M.E., Mani, D.T.C. et al. Combination of Lolium perenne L. and Festuca arundinacea Schreb. improve yields under low phosphorus availability. Nutr Cycl Agroecosyst 128, 199–215 (2024). https://doi.org/10.1007/s10705-024-10346-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-024-10346-9