Abstract
Phosphorus (P) reactions in calcareous soils limit the concentration of P in the soil solution for plant uptake. Calcareous soils with high calcium carbonate content (CaCO3) and high pH have low P fertiliser efficiency, leading to crop deficiency and limited crop productivity. The aim of this work was to test if soil acidifying amendments could reduce soil pH and improve the solubility of fertiliser P to improve crop P nutrition and biomass. Three calcareous soils with varying CaCO3 content (14–29% w/w) were used to test acidifying amendments both with and without mono-ammonium phosphate (MAP). Soil was amended with oxalic acid, sulfuric acid, glucose, ammonium sulfate and elemental sulfur (S0). Initial titrations demonstrated the ability of oxalic and sulfuric acids to reduce pH and improve P solubility in all three soils. Incubation of the acidifying amendments in the soil with the lowest carbonate content over 14 days (52 days for S0) showed increased P solubility and diffusion from MAP granules in soil amended with glucose, sulfuric acid and oxalic acid. There was, however, no improvement in P fertiliser uptake in wheat crops grown in these highly calcareous soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcareous soils are found on approximately one third of the Earth’s land surface, primarily in arid and semi-arid regions and they raise numerous agronomic challenges that limit crop productivity, in particular the plant availability of essential nutrients such as phosphorus (P) (Alloway 2012; Taalab et al. 2019). Calcareous soils are defined as soils containing calcium carbonate (CaCO3) in one or more of their horizons varying from 3 to 95% w/w (Ruellan 1973). High CaCO3 concentrations reduce crop productivity through low nutrient availability and cycling, high soil pH (generally between pH 7.0–8.5) and low microbial activity (Davey et al. 2021; Taalab et al. 2019; Wahba et al. 2019). Calcareous soils are common in cropping regions including the Eyre Peninsula and Yorke Peninsula in South Australia (Adcock et al. 2007; Bertrand et al. 2003). With a growing global population and an increasing gap between agricultural production and food demand, implementing strategies such as improved nutrient use efficiency to reduce the limitations of calcareous soils on crop production is becoming increasingly necessary (Bertrand et al. 2003; Wahba et al. 2019).
Calcium carbonate in calcareous soils can severely limit nutrient availability in the soil leading to reduced yields and the development of nutrient deficiencies such as P deficiency (Rafiullah et al. 2020; Shrivastava et al. 2018; Wahba et al. 2019). Phosphorus is a vital structural component of nucleic acids, phospholipids, adenosine triphosphate and adenosine diphosphate, and plays important roles in numerous cellular reactions (Emsley 1980; Shrivastava et al. 2018). Phosphorus deficiency symptoms include stunted plants, poor root growth and light green or purplish leaf colouration, but often there are reduced yields with no visual symptoms (Mengel and Kirkby 2001; Taalab et al. 2019).
The limited supply of natural soil P reserves has led to a dependence on P fertilisers such as mono-ammonium phosphate (MAP) to maintain the crop’s P nutritional demand (McLaughlin et al. 2011; Weeks and Hettiarachchi 2019). Phosphorus fertilisers, however, generally have very low efficiency, with crop fertiliser P uptake ranging between 3 and 33% in the year of application (McBeath et al. 2012). Low P solubility, diffusion and crop uptake is mainly due to fertiliser-soil reactions removing plant available P from the soil solution pool and forming poorly soluble P pools (Rafiullah et al. 2020; Taalab et al. 2019). Sorption and precipitation reactions are strongly favoured in calcareous soils due to high concentrations of CaCO3 increasing the soil’s P buffer capacity, sorption strength, and precipitation reactions forming stable calcium (Ca) phosphates (Bertrand et al. 2003; McLaughlin et al. 2011; Ryan et al. 1985; Shen et al. 2011; Shrivastava et al. 2018). Consequently, the concentration of P in the soil solution is generally very low (< 1% of total soil P) with peak availability at a pH of 5.0–7.0 (Hopkins and Ellsworth 2005; Ros et al. 2020).
Despite years of fertiliser application, low P availability in southern Australian calcareous soils limits crop yields (Frischke et al. 2004; Wahba et al. 2019). Enhancing P solubilisation and limiting soil-fertiliser reactions could improve the availability of fertiliser and accumulated soil P (Bertrand et al. 2003; Degryse and McLaughlin 2013; McLaughlin et al. 2011). Improving P fertiliser efficiency is very important as global P resources are non-renewable, meaning they will become increasingly expensive to mine and produce, and may have even lower efficiency due to lower P content and increasing levels of impurities (Schneider et al. 2019; Richardson 2001). Many strategies have been tested and implemented to increase P solubility. Adjusting soil pH around the fertiliser band is one strategy which has shown considerable potential in alkaline and calcareous soils (Khorsandi 1994b; Wahba et al. 2019; Shrivastava et al. 2018); however, an effective solution has not been found for the calcareous soils of southern Australia.
Past research on acidifying calcareous soils to improve P availability has shown the most success with amendments which affect soil pH and P solubility via different mechanisms. Sulfuric acid and oxalic acid rapidly reduce soil pH through the release of H+ upon equilibrium with the soil solution, reducing soil pH and leading to the conversion of P into more soluble forms (Jones and Darrah 1994; Khorsandi 1994a; Ryan and Stroehlein 1979). Oxalic acid can also solubilse P through forming insoluble Ca complexes, reducing the formation of insoluble Ca phosphates (Mihoub et al. 2017; Wang et al. 2015). Once oxidised, S0 reduces pH through the production of H+ and reacts with P fertilisers, Ca phosphates and CaCO3, improving the concentration of soluble P (Deluca et al. 1989; Mitchell et al. 1952). Ammonium sulfate can reduce soil pH and potentially contribute to increased P solubility in calcareous soils through complexation reactions of Ca with sulfate, and nitrification of ammonium (Cabral et al. 2020). Glucose improves microbial activity and turnover, improving the mineralisation of organic P and increasing soil solution P concentrations (Oehl et al. 2001). Soil pH can also reduce in response to various microbial processes such as nitrification, S0 oxidation, the dissolution of carbon dioxide (CO2) produced through respiration and the release of organic acids (Brautigan et al. 2014; Richardson 2001).
The aim of this study was to quantify the effect of acidification induced by soil amendments on the solubility of fertiliser P in calcareous soils. Five soil amendments, namely sulfuric acid, oxalic acid, S0, ammonium sulfate and glucose were tested in three calcareous soils in soil incubation and plant growth response experiments. A successful amendment could reduce fertiliser inputs and improve crop productivity.
Materials and methods
Soil analysis
Three soils were collected from broadacre cropping regions in South Australia, namely Venus Bay (33° 15′ 03′′ S 134° 45′ 46" E) and Poochera (32° 44′ 34′′ S 134° 54′ 07′′ E) on the Upper Eyre Peninsula and Port Broughton (33° 34′ 55′′ S 138° 00′ 07′′ E) on the Yorke Peninsula. These soils represent typical highly calcareous soils within southern Australia (> 15% CaCO3 in the surface layer). Air-dried soil samples of the top 10 cm were sieved and analysed (Table 1).
Soil acidifying amendments
Sulfuric acid (97.5–98.5%) (MW = 98.08 g/mol, D = 1.84 g/mL) was sourced from RCI Labscan with a pH (@ 25 °C) of 0.9 @49 g/L. Oxalic acid dihydrate (99.5–102.5%) (MW = 126.07) was sourced from Chem-supply with at pH (@ 25 °C) of 1.0 @100 g/L. The S0 (99.998%) (MW = 32.07) used in this study had a particle size of 149 µm. Elemental S, ammonium sulfate (≥ 99.0%) (MW = 132.14 g/mol) and D-( +)-glucose (≥ 99.5%) (MW = 180.16 g/mol) used were all sourced from Sigma-Aldrich. The ammonium sulfate had a pH (@ 25 °C) of 5.0–6.0 @136 g/L and there was no pH data supplied with the S0 and glucose.
Experiment 1: Soil pH buffering capacity with and without phosphorus fertiliser
Titrations were performed to quantify the moles of hydrogen ions (H+) required to reduce soil pH to a target level (optimal for P solubility) in the three calcareous soils, which was defined here as the soil pH buffering capacity (pHBC). Sulfuric acid (H2SO4) was titrated into a 200 mL 1:5 soil water suspension, with three replications of each soil. When the pH stabilised at 8.5, 8.0, 7.5, 7.0, 6.5 and 6.0, the volume of acid added to the suspension was recorded and a 6 mL sub-sample of the suspension was taken for analysis. A stable pH was defined as remaining within 0.05 of the target pH for at least 5 min. The sub-sample was filtered through a 0.45 µm filter (Sartorius), diluted and analysed using a Perkin-Elmer Avio 200 inductively coupled plasma optical emission spectroscopy (ICP-OES) for water-extractable P and Ca concentrations.
Titrations following the same method but comparing sulfuric acid (H2SO4)(1 M) with oxalic acid (C2H2O4)(0.5 M) at titrants were carried out with the addition of MAP (57.5 g/L) to the suspensions of all three calcareous soils. This represents the P concentration within a 1 cm radius of soil around a MAP fertiliser granule. This tested how soil pHBC was affected by the addition of P fertiliser. Due to a rapid pH reduction with the addition of MAP, the suspension was left for 2 h to equilibrate, and then titrated to target pH values of 6.5, 6.0 and 5.5.
Experiment 2: Phosphorus diffusion visualisation
This experiment involved adding each acidifying amendment at two rates (low and high) to the Poochera soil for an incubation period of 14 days with MAP fertiliser granules, and a high amendment rate without MAP fertiliser granules. The experimental methodology has been described previously (Lombi et al. 2004a). Through incubating soil treated with or without a MAP granule and an amendment, the longer-term effects of the amendments on fertiliser availability and changes in soil pH were examined. Along with sulfuric acid and oxalic acid, additional soil amendments were explored: ammonium sulfate, glucose and S0 which could not be tested in Experiment 1 as they require aerobic conditions to acidify the soil. As S0 requires oxidation prior to acidifying the soil, it was incubated for 56 days rather than 14 days. Based on its particle size of 149 µm, it was estimated that 70% of the added S0 would oxidise with an incubation time of 56 days (Fertiliser Technology Research Centre 2020b).
Soil was wetted to 80% field capacity and Petri dishes (diameter of 5.5 cm) were packed with soil to field bulk density. The soil amendments were applied to the centre of the dish to acidify an approximate 1 cm radius sphere around the fertiliser granule. For the application of concentrated sulfuric acid, the acid was pipetted into a small well in the centre of the soil-filled Petri dish. After the release of CO2 stopped (reaction of acid with CaCO3), the well was covered with soil. The remaining amendments were applied as powders mixed through the centre section of soil (1.8 cm diameter ring). The high and low amendment application rates were based on the acidification rates recorded in Experiment 1. Sulfuric acid was applied at 51 µL/dish (low) and 167 µL/dish (high), oxalic acid was applied at 0.481 g/dish (low) and 0.783 g/dish (high), ammonium sulfate was applied at 0.599 mg/dish (low) and 0.997 mg/dish (high), glucose was applied at 0.551 g/dish (low) and 1.30 g/dish (high), and S0 was applied at 0.043 g/dish (low) and 0.098 g/dish (high).
After the amendments were applied, the Petri dishes were covered, sealed in a tray with cling film and left to equilibrate overnight in an incubator at 25 °C. To maintain the soil water content, moist paper towel was placed inside the sealed tray. The next day, a well was made in the soil at the centre of the Petri dishes, and a MAP granule (40 ± 2 mg) was placed into the well and covered with soil. The Petri dishes were closed, and the tray was sealed and returned to the incubator. After 4 h, 24 h, and 3, 7 and 14 days, P diffusion was measured using iron-oxide impregnated filter papers and visualised using a malachite green solution (Degryse and McLaughlin 2013). The S0 amendment P diffusion was measured after 7, 14, 28, 42 and 56 days. After being left to dry, the filter papers were scanned and analysed using imaging software (GNU Image Manipulation Program—2.0) to determine the diameter of P diffusion. Throughout the incubation, the surface of each Petri dish was observed for microbial growth.
At the end of the incubation period, soil in concentric rings (1.8 cm and 2.5 cm diameters) was removed for individual analysis. The soils analysed were < 0.9 cm, 0.9–1.25 cm and > 1.25 cm away from the centre of the Petri dish (placement of MAP granule). The inner ring represented an approximate 1 cm radius of soil which is the soil most influenced by MAP in highly calcareous soil (Lombi et al. 2004b). Prior to soil analysis, the soil sub-samples were ground using a mortar and pestle and weighed. Soil was mixed in a 1:5 soil:water suspension, shaken for one hour, allowed to settle for 20 min, and pH was measured. The sample was filtered using a 0.45 µm filter and analysed through ICP-OES for water extractable P and Ca concentrations.
Experiment 3: Wheat growth response to acidifying amendments
A plant growth response experiment was established to measure the growth of wheat plants (Triticum aestivum cv. Scepter) in response to the fertiliser × amendment treatments in two of the experimental soils grown in pots in a growth chamber for 4 weeks. Soils from Poochera and Venus Bay were used in this experiment as they had the lowest and highest carbonate contents of the three soils used. Acidifying amendments of oxalic acid, sulfuric acid, ammonium sulfate and glucose were compared against a MAP treatment and a nil fertiliser or amendment treatment control. One amendment application rate was chosen based on the best results in Experiment 2. Amendment application rates in Venus Bay soil were 7.99 g/pot oxalic acid, 0.94 mL/pot sulfuric acid, 10.08 g/pot glucose and 0.0629 g/pot ammonium sulfate. In Poochera soil, the application rates were 5.12 g/pot oxalic acid, 0.41 mL/pot sulfuric acid, 3.59 g/pot glucose and 0.0728 g/pot ammonium sulfate (~ 26 kg N/ha). As S0 requires extended time to oxidise in the soil, it was not included in this experiment.
Topsoil (0-10 cm, 500 g) was fertilised with a basal nutrient solution, liquid urea and wetted to 80% field capacity. The basal solution per pot consisted of 1.25 mg manganese sulfate (MnSO4.H2O), 3.15 mg zinc sulfate (ZnSO4.7H2O), 1.55 mg copper sulfate (CuSO4.5H2O), 0.03 mg boric acid (H3BO3), 0.025 mg molybdenum oxide (MoO3) and 25 mg potassium sulfate (K2SO4). A urea solution was applied at 32 mg/kg soil (equivalent to ~ 50 kg N/ha) to all soil treatments apart from ammonium sulfate to balance N applied and ensure N was not limiting. The amendments were mixed through a soil sub-sample 4 cm below the soil surface in a 1 cm radius fertiliser band across the pot diameter. Oxalic acid and glucose were mixed with this soil sub-sample and sulfuric acid was applied by dripping it along a band that ran the length of the pot. Ammonium sulfate was mixed into the top 1 cm layer of soil representing the most common application on-farm which is topdressing and incorporation by rainfall. After packing the remaining soil into the pots, 4 pre-germinated Scepter wheat seeds were sown 1 cm deep into each pot (thinned to 2 plants per pot after 1 week), along with 3 MAP granules (36 ± 1 mg each) at a rate equivalent to 40 kg P/ha in all pots apart from the nil treatment control. To reduce soil evaporation, 15 g of low-density polyethylene beads (Qenos, Altona, VIC) were applied to the soil surface of each pot. Pots were grown for 4 weeks in a controlled environment growth chamber with average day/night temperatures of 23 °C/18 °C at 80% relative humidity and with a daytime light period of 18 h. Pots were watered daily with deionised water to maintain a water content of 80% field capacity and randomly arranged on a weekly basis.
At the end of the 4 weeks, wheat plants at Zadoks growth stage 22 (Zadoks et al. 1974) were removed from the pots and roots were removed. Plant shoots were dried at 60 °C for 72 h and weighed. After being ground, sub-samples (0.25 g) of the dry shoot biomass samples were acid digested following the closed-tube digest method outlined by Wheal et al. (2011) and analysed for shoot P concentrations using ICP-OES analysis. From this plant P uptake was calculated as:
Statistical analysis
Statistical analysis utilised analysis of variance (ANOVA) and regression in the Genstat® V.19 statistical package. For ANOVA both the normality of distribution and constant error variance assumptions were tested for each analysis and differences between treatments were determined by least significant difference (LSD) at the 5% significance level using Fisher’s unprotected LSD.
Regression analysis of amendment vs moles of H+ added vs soil was conducted in Experiment 1 to test whether the buffer curves for sulfuric and oxalic acid differed between amendments and soils (separate slopes analysis) using the Genstat® V.19 statistical package. GraphPad Prism® was used for curve fitting and plotting the buffer curves with their regression lines. To analyse the effect of treatment on soil solution P and Ca, the ANOVA design was amendment × fertiliser (plus or minus) × soil × pH, with a block structure of replication.
For the soil measurements in Petri dishes (Experiment 2), the design was the amendment treatment (combination of acidifying amendment and application rate) × position, with a block structure of position/replication to represent the nested design of the Petri dish. This was analysed separately for without and with MAP treatments. In Experiment 2, the filter papers were scanned, and the area of the high-P zone was calculated using imaging software (GNU Image Manipulation Program, v. 2.6.11, Free Software Foundation, Boston, MA) which converts the scanned images to black-white binary images using a threshold colour value and quantifies the area of the high P-zone (Degryse and McLaughlin 2013).
For the wheat growth experiment (Experiment 3), the design was acidifying amendment with a block structure of replicates, analysed by ANOVA in Genstat. This was analysed independently in each soil due to the high levels of variation in data collected from the Poochera soil.
Results
Experiment 1: Soil pH buffering capacity with and without phosphorus fertiliser
Sulfuric acid addition initially produced a rapid pH reduction and then a slower rate of pH decline from pH 6.5 following a power trend (P < 0.001) (Fig. 1). Through regression analysis, a separate slopes analysis showed no change (P = 0.993) between the soils indicating that the pH buffering capacity of the three soils should be interpreted as a single curve (Fig. 1).
The change in soil pH (1:5 soil:water suspension) in three different calcareous soils (Venus Bay, Poochera and Port Broughton) which were titrated with sulfuric acid to measure the soil pH buffering capacity. The pH buffer curve of the three soils is pH = 9.1235x−0.061, where x represents the µmol H+ from sulfuric acid per g of soil added (r2 = 98.8%, P < 0.001)
Fertiliser MAP was added to the soil suspension to measure the effect of P fertiliser on the soil pHBC in response to sulfuric (Fig. 2a) and oxalic acids (Fig. 2b). With P, a regression analysis found that the soil pHBC was significantly different between the three soils (P < 0.001, r2 values of 95.5–96.4%) and when the data was not grouped into soils the correlation was very low (r2sulfuric+P = 32.4%, r2oxalic+P = 39.8%) (Fig. 2).
The change in soil pH (1:5 soil:water suspension) in three calcareous soils: Venus Bay(VB), Poochera (Po) and Port Broughton (PB), titrated with sulfuric acid (a) and oxalic acid (b) to calculate the soil pH buffering capacity with P fertiliser (MAP) addition. The soil pHBC (slope) for each soil were calculated using a linear regression analysis (Psulfuric+P < 0.001, Poxalic+P < 0.001, r2sulfuric+P = 95.5%, r2oxalic+P = 96.4%). The linear equations where x = µmol H.+/g soil for sulfuric acid treatments are: \(pH\left(VB\right)= -0.000751x+6.871, \, pH\left(Po\right)=-0.00110x+6.419, \, pH\left(PB\right)-0.00167x+6.199\), and for oxalic acid are: \(pH\left(VB\right)=-0.000223x+6.914, \, pH(Po)=-0.000272x+6.469, \, pH(PB)=-0.000337x+6.162\)
To reduce soil pH in the presence of P, much greater amounts of H+ were needed and the H+ requirement increased linearly (P < 0.001), with significant effects due to the amendment and soil type on the pHBC (P < 0.05) (Fig. 2). At a given pH, the H+ requirement of oxalic acid was greater than sulfuric with MAP application (P < 0.001). The initial addition of MAP caused a dramatic reduction in soil pH to 6.94, 6.36 and 6.02 in Venus Bay, Poochera and Port Broughton soils, respectively. Due to the acidifying effect of MAP, the amount of sulfuric acid required to reduce soil pH to 6.0 was significantly less in the Poochera and Port Broughton soils (Fig. 2). The acidification from MAP was calculated as 550 µmol H+/g soil, suggesting it has a similar acidifying strength to sulfuric acid (Fig. 1). Venus Bay, however, required significantly more H+ to reduce pH in the presence of P compared to the other two soils. In both sulfuric and oxalic acid titrations, Venus Bay had the highest pHBC followed by Poochera and then Port Broughton (P < 0.001, Fig. 2).
The concentrations of water-extractable P and Ca concentrations were measured at each target soil pH throughout each titration (Table 2). In Venus Bay soil in the absence of MAP, sulfuric acid initially decreased the concentration of P from a pH of 8.5 to 7.5 (P < 0.001), but there was a subsequent increase in P concentration between pH 7.0 and pH 6.0 (P < 0.001). While the other soils followed the same trend of P concentration changes, it was only the comparison between initial pH and pH 6.0 in Poochera and 6.5 in Port Broughton that was different. As expected, titrations carried out in the presence of MAP had significantly higher concentrations of solution P compared to without MAP, although as pH reduced the concentration of P also reduced (P < 0.001) (Table 2). This was evident in all soils, however, Venus Bay soil had significantly lower concentrations of P than Poochera and Port Broughton soils. Titrations with sulfuric acid additions led to higher concentrations of P in the soil solution compared to oxalic acid in all soils (P < 0.001).
Additions of sulfuric acid to the unfertilised soil suspension increased the concentration of Ca (P < 0.001) with significant differences from the initial pH at soil pH values less than 7.5, however there was no significant difference between soils (P > 0.05) (Table 2). Addition of MAP reduced the concentration of Ca in the soil (P < 0.001) with significant difference between soils titrated with sulfuric acid. Venus Bay soil generally had the highest Ca concentrations (Table 2). Titrations of soil solutions containing MAP showed effects of acidifying agents (P < 0.001). Lower concentrations of Ca were found in soil titrated with oxalic acid (Table 2) with Port Broughton soil the least responsive and generally having the highest Ca concentrations in this situation.
Experiment 2: Phosphorus diffusion visualisation
The diameter of the high-P zone of MAP diffused from the MAP granules was affected by the interaction of the incubation time and treatment applied (P < 0.001) (Supplementary Information, Fig. 1). Among all amendments throughout the incubation period, oxalic acid and glucose had the greatest effects on P diffusion with oxalic acid causing significantly more P diffusion after 24 h and glucose after 14 days compared with MAP alone (Fig. 3). Higher diffusion was mostly evident in the high amendment application rate in the presence of MAP, although in some cases the low amendment application rate led to greater diffusion of MAP (data not shown). The application rates × timing that showed the biggest effect on P diffusion for each amendment are compared in Fig. 3. There was rapid diffusion of P away from the granule in the first 24 h, however, for the remainder of the incubation there was no significant change (Fig. 3). Oxalic acid and sulfuric acid significantly enhanced the diffusion of P from the MAP granule; this was only significant until day 7 (Fig. 3). Ammonium sulfate and S0 had no effect on P diffusion compared with MAP alone. Glucose showed no effect on P diffusion compared with MAP alone until day 14 when the diameter of the high-P zone was greater than all other treatments. Amendments applied at the high rate without MAP had limited effects on P dissolution and diffusion, with the only visual evidence of a high-P zone found after 4 h in the oxalic acid amendment and 14 days in the glucose amendment (data not shown).
Diameter of the high-P zone around the MAP granule over time for selected amendment application rates with MAP using the P visualisation method of Degryse and McLaughlin (2013) in Poochera soil. Across incubation times and treatments, the least significant difference between treatments is 0.20 cm (at P = 0.05)
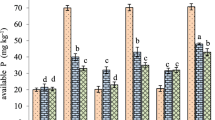
Analysis of water-extractable P both with and without the application of MAP indicated that the application of acidifying amendments had a significant effect on the amount of P in the Petri dishes over time and space (i.e. section of Petri dish) (P < 0.05) (Fig. 4). In the absence of P, glucose was the only amendment which improved the amount of water-extractable P compared to the nil treatment control (Fig. 4a). This was consistent with the findings in the P diffusion analysis at the end of the incubation (Fig. 3). Among fertilised treatments, the amount of P was higher in the inner section with little indication of P diffusion into the middle and outer rings. An exception to this was glucose with MAP (both rates) which had significantly higher amounts of P in the soil section most distant from the point of application (Fig. 4b). The total amount of water-extractable P in the dish was also significantly higher in glucose treatments compared with MAP alone. The only other amendment which improved P solubility compared to MAP alone was sulfuric acid (both rates) (Fig. 4b).
The concentration of water-extractable phosphorus (µg P/dish) in Poochera soil after 14 days incubation as a function of distance from the application point of treatments—inner (< 0.9 cm), middle (0.9–1.25 cm) and outer (> 1.25) sections of the Petri dish. Petri dishes were amended with acidifying treatments, a without and b with the application of MAP. Significant differences between treatments are represented by lettering (LSD at P = 0.05)
The addition of amendments produced significant differences in the water-extractable Ca concentration (P < 0.05) (Table 3). In the absence of P, soil amended with sulfuric acid or glucose had a higher water-extractable Ca concentration than the nil treatment control. This was highest in the inner ring for sulfuric acid applications and the outside ring for glucose applications. In MAP-fertilised soil, sulfuric acid was the only amendment which significantly affected Ca with higher concentrations throughout the whole dish.
Soil pH had the largest changes in the inner ring with pH change in the rest of the dish often not significantly different to that induced by MAP alone (Table 3). Sulfuric acid had the greatest effect on soil pH reduction both with and without MAP. In soil with MAP, pH change in response to sulfuric acid was significant throughout the whole dish at both application rates. Ammonium sulfate also reduced soil pH in the presence of MAP, although this was only found in soil close to the area of application. Without MAP, glucose significantly reduced pH at the point of application compared to the nil treatment control, however, the application of glucose at the low rate with MAP caused an increase in soil pH above 9 in soil close to the point of application. Both with and without MAP, oxalic acid significantly reduced soil pH from the control in the soil most distant from the point of application. Additionally, when MAP was applied to the dish, oxalic acid amended soil led to high pH compared to the MAP control.
Experiment 3: Wheat growth response to acidifying amendments
Wheat plants growing in both soils were highly responsive to MAP application, demonstrating higher plant biomass and P uptake (P < 0.001, Table 4). However, the concentration of P was higher in the control plants than those fertilized with MAP (P < 0.001), indicating a growth dilution effect.
Applying acidifying amendments to Poochera soil in pots had no significant effect over and above that of MAP alone on plant biomass, concentration of P, or plant uptake of P (Table 4). In the Venus Bay soil, the addition of glucose with MAP depressed yields and reduced P uptake compared to MAP alone, with no significant effects of the remaining amendment (Table 4).
Discussion
Application of soil acidifying amendments did not improve the plant utilisation of P fertilisers in southern Australian calcareous soils. As expected, all treatments with MAP improved plant growth compared to the control (Table 4). This indicated that the wheat plants were responsive to P fertilisation in the soils studied and would benefit from further gains in availability. Symptoms of P deficiency were observed in the control plants through reduced plant height and tillering (data not shown). Despite better plant growth and higher P uptake in MAP-fertilised pots, plant P concentrations were still low. The critical deficiency level of P in wheat shoots at this stage of growth is 0.28% (Reuter and Robinson 1997), with most plants being at, or lower, than this critical concentration. This indicates that P availability likely continued to limit plant growth, even in MAP fertilised soils. Additionally, the amendment application rates used were possibly not sufficient to significantly increase the availability of soil P.
This study did not identify a soil amendment which improved plant biomass or P uptake compared to soils fertilised with MAP alone. Some amendments did have large effects on soil pH, the solubility of P and Ca and P diffusion that are discussed in detail below.
Oxalic acid
Through soil experiments, oxalic acid demonstrated some potential as an acidifying amendment, however, there was no enhancement of plant growth or P uptake. To reach a soil pH in soil suspension of 6.5 where P solubility should be enhanced (Hopkins and Ellsworth 2005), greater amounts of oxalic acid were required compared with sulfuric acid. Oxalic acid has pKa values of 1.25 and 3.81 meaning H+ ions are readily lost as the pKa is less than the soil pH. Sulfuric acid has lower pKa values of < 1 and 1.99 meaning it is a stronger acid and achieves a greater effect on pH (Averill 2012; Hall 2002). Despite reducing soil pH to target levels in stirred soil suspension experiments (Fig. 2), when oxalic acid was applied to P-fertilised soil and incubated, the soil pH compared with MAP alone significantly increased at the point of application and significantly decreased in soil most distant from the application point (Table 3). Fertiliser may have initially acidified the soil close to the point of application and then returned to original pH; this has been found in a previous incubation of 12 days duration (Macias-Benitez et al. 2020). A possible explanation for the increased soil pH is the exhaustion of oxalic acid as a microbial food source (Jones and Darrah 1994; Jones et al. 2003). This may cause microbial acidifying reactions to reduce and alternative soil reactions that increase soil pH to occur. These include reactions between carbonate and water forming bicarbonate (CO32− + H2O → HCO3– + OH−), and volatile fatty acids produced by soil microorganisms and soil carbonates (R-COO− + H+ → R–H + CO2) (Brautigan et al. 2014). Microbial growth (most likely fungal) was observed in Petri dishes treated with oxalic acid (not shown), suggesting a potential limitation to the efficacy of oxalic acid as a soil amendment.
Soil reactions with oxalic acid affect the behaviour of P and its availability in soil solution (Mihoub et al. 2017). Through conversion to oxalate, oxalic acid releases H+ which reacts with CaCO3 in the soil. Although this beneficially reduces the soil pH, the release of Ca2+ can precipitate with fertiliser P forming Ca phosphates and reducing the concentration of soluble P and Ca even though pH may be reduced. Oxalic acid can also form strong insoluble Ca oxalate complexes in the soil, which are produced in preference to Ca phosphates due to their low solubility (Jones et al. 2003; Mihoub et al. 2017). Evidence of this pathway was not observed as the availability of water-soluble P and Ca did not significantly change over the 14-day incubation compared to MAP application alone (Fig. 4, Table 3). Additionally, in the soil suspension both P and Ca concentrations reduced suggesting the formation of Ca phosphates (Table 2). Using the solution data from Table 2, and assuming charge balance in the solutions was maintained mainly by concentrations of ammonium (NH4+), chemical equilibria were modelled using Visual Minteq V3.1 (model output not shown) (Gustafsson 2021). Solutions in the presence of MAP were all supersaturated with several Ca-phosphate species, even the most likely Ca-phosphate solid phase, dicalcium phosphate dihydrate (CaHPO4.2H2O). High concentrations of oxalate (6 mM) were predicted not to interfere with this precipitation of Ca-phosphate—indeed, most of the added oxalate at this concentration was predicted to precipitate as the Ca-oxalate solid phase. Phosphorus diffusion, however, was enhanced through the application of oxalic acid in the first 7 days of incubation (compared with MAP alone) (Fig. 3). Initially, the release of H+ from oxalic acid would have increased the solubility of P by reducing precipitation and absorption reactions in the soil (Mihoub et al. 2017). However, during the incubation, the reduction in soil solution P may have occurred through precipitation reactions (Fig. 4b). One explanation for the short-term effect of oxalic acid could be that it was degraded by soil microorganisms, limiting its effect on soil availability and use as a soil acidifier (Jones and Darrah 1994; Jones et al. 2003).This could also provide some explanation to why the P solubility with reduced pH was not well translated to improved P uptake in plant growth experiments and could suggest that a high rate may be required for longer term effects.
Sulfuric acid
Sulfuric acid showed potential as an acidifying amendment with significant reductions in soil pH both in the titration and incubation experiments (Figs. 1 and 2, Table 3), but like oxalic acid had no effect on plant growth (Table 4). Longer lasting acidification and reduced H+ requirements through the application of sulfuric acid compared to oxalic acid in soil suspension could be attributed to the very low pKa values and rapid deprotonation of sulfuric acid in the soil solution (Averill 2012; Hall 2002). Sulfuric acid has the greatest effect on soil pH compared to other amendments in the absence and presence of MAP in the 14-day soil incubation, reaching a minimum pH of 7.43 in soil close to the area of application and increased concentrations of water-soluble P in the acidified soil (Table 3). Sulfuric acid showed improved P diffusion for the first 7 days of the incubation (Fig. 3) and had significantly higher amounts of P close to the point of application than MAP alone at the end of the soil incubation (Fig. 4b). High P solubility through the application of sulfuric acid has been found in previous research (Khorsandi 1994a) and was linked to the reduced soil pH where dissolution and desorption reactions between P and the soil occur (Hopkins and Ellsworth 2005; Pierzynski et al. 2005). The high reactivity of sulfuric acid with the incubated soil was further supported by elevated concentrations of Ca found at all application rates (Table 3) (Mace et al. 2010). This suggested that sulfuric acid is effective in solubilising Ca phosphates and hence making soil residual P more available, as found by Ryan and Stroehlein (1979). However, adding sulfuric acid with MAP is a different scenario where solution P concentrations are very high—mobilising Ca from carbonates in this scenario creates the possibility for more precipitation of P as Ca-phosphates, even at pH values as low as pH 5.5. Nevertheless, sulfuric acid increased concentrations of water-extractable P in the soil around the MAP granules (Fig. 4b).
Glucose
Among amendments, glucose had the largest effect on the parameters observed in all studies, but like the other amendments did not have a positive effect on plant P uptake. The acidifying effect of glucose is driven by elevated microbial activity which utilises glucose as a carbon feedstock (Bünemann et al. 2008). Microorganisms mediate several processes such as nitrification, organic acid production and CO2 production through respiration which hold potential to reduce pH (Msimbira and Smith 2020; Richardson 2001). The expected reduction in soil pH was not found when MAP with glucose was applied to the soil, and in fact the pH in soil incubated with glucose and MAP significantly increased (Table 3). An increase in soil pH could have been explained by the same change to soil reactions observed in oxalic acid where the microbial food source becomes exhausted (Brautigan et al. 2014). Alkalinity caused by glucose amendment could have been one reason for the reduced plant growth observed in glucose-treated soils as high concentrations of HCO3− are known to limit root respiration (Table 4) (Msimbira and Smith 2020). Reduced plant growth and the negative effects of band-applied glucose were associated with reduced root growth in the amendment application area (Supplementary Information, Fig. 2), reduced plant biomass, reduced tillering compared to all other treatments (data not shown; one rather than 2 tillers) and evidence of chlorosis. A second possibility is that glucose also prompted the growth of pathogens such as Rhizoctonia (Davey 2013). Rhizoctonia fungus causes disease in cereal roots, reducing growth, crop yields and nutrient uptake (Gupta 2016). There was some evidence of spear-tipped roots which is a common symptom of Rhizoctonia root rot (Supplementary Information, Fig. 2) (Gupta 2016).
Reduced root growth restricts the ability for roots to interact with the MAP granule and with little P diffusion throughout the rest of the pot, P uptake was low compared to MAP in the Venus Bay soil. This could be explained by immobilisation of P by soil microbial populations which became more active with the addition of glucose as a food source (Kouno et al. 2002). Soil amended with glucose showed the greatest diffusion of P (Fig. 3) and amount of water-extractable P (Fig. 4) in the soil both with and without MAP, following a pH gradient of a high pH in the centre section to neutral pH in the outer section. Diffusion of P to the outer section of the dish was only significantly higher at the end of the incubation. High growth of microbial biomass (likely fungal) was visually observed on the soil surface of all soils amended with glucose which coincided with these observed changes in P towards the end of the incubation. Phosphorus diffusion could possibly have been improved through desorption of P in the centre of the dish by bicarbonate and carbonate produced through microbial respiration from CO2.
Other acidifying agents
Soil amended with ammonium sulfate and S0 showed minimal or no effect on the concentration, diffusion or plant uptake of P, soil pH, Ca concentrations or plant biomass compared to the fertilisation of soils with MAP alone. Based on the application rates and methods used, these amendments showed little potential for use as soil amendments to enhance P-use efficiency. Ammonium sulfate was limited in its application rate to an agronomically realistic level, meaning the H+ supplied to the soil was significantly less than the other amendments. Elemental sulfur also had limited effects, possibly due either to a slow oxidation rate (Zhao et al. 2017) or use of an application rate that was insufficient to overcome the pHBC of the soils.
MAP
The soil acidification effect of MAP was large with soil pH reducing below 7.0 in the soil suspension titration (Fig. 2). Phosphate anion has pKa values of 2.16, 7.20 and 12.3 (Averill 2012), although is mostly present as dihydrogen phosphate (H2PO4−) and monohydrogen phosphate (HPO42−) in the pH range that commonly occurs in soils (Shrivastava et al. 2018). Mono-ammonium phosphate is applied in the form of H2PO4−, therefore at an initial soil pH of > 7.2, H2PO4− converts to HPO42− releasing a proton and rapidly acidifies the soil (Fertiliser Technology Research Centre 2020a). With time the ammonium ion is also nitrified, and this can further acidify the soil, but the effect may not be as localised as ammonium diffuses away from the granule more rapidly through soil than P. Through titration, it appeared that MAP had a similar acidifying effect to sulfuric acid, however when incubated in the soil, the longer-term pH effect of MAP alone was not as significant as sulfuric acid.
Conclusion
The benefits for P solubility through the application of oxalic acid, sulfuric acid and glucose, did not translate to plant growth or P nutrition benefits. None of the amendments studied showed improvements compared with MAP alone.
References
Adcock D, McNeill AM, McDonald GK, Armstrong RD (2007) Subsoil constraints to crop production on neutral and alkaline soils in southern Australia: a review of current knowledge and management strategies. Aust J Exp Agric 47(11):1245–1261. https://doi.org/10.1071/EA06250
Alloway BJ (2012) Bioavailability of elements in the soil. Essentials Med Geol. https://doi.org/10.1007/978-94-007-4375-5_15
Arias DM, Teasdale PR, Doolette CL, Lombi E, Farquhar S, Huang J (2021) Development and evaluation of a new colorimetric DGT technique for the 2D visualisation of labile phosphate in soils. Chemosphere 269:128704. https://doi.org/10.1016/j.chemosphere.2020.128704
Averill BA (2012) Principals of General Chemistry vol. 1, Appendix C: Dissociation constants and pKa values for acids at 25°C. https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s31-appendix-c-dissociation-consta.html. Accessed 27 October 2021
Bertrand I, Holloway RE, Armstrong RD, McLaughlin MJ (2003) Chemical characteristics of phosphorus in alkaline soils from southern Australia. Aust J Soil Res 41(1):61–76. https://doi.org/10.1071/SR02021
Brautigan DJ, Rengasamy P, Chittleborough DJ (2014) Amelioration of alkaline phytotoxicity by lowering soil pH. Crop Pasture Sci 64(12):1278–1287. https://doi.org/10.1071/CP13435
Bünemann EK, Smernik RJ, Marschner P, McNeill AM (2008) Microbial synthesis of organic and condensed forms of phosphorus in acid and calcareous soils. Soil Biol Biochem 40(4):932–942. https://doi.org/10.1016/j.soilbio.2007.11.012
Cabral CEA, Cabral CHA, Santos ARM, Carvalho KS, Bonfim-Silva EM, Mattos JS, Alves LB, Bays AP (2020) Ammonium sulfate enhances the effectiveness of reactive natural phosphate for fertilizing tropical grasses. Tropical Grasslands-Forrajes Tropicales 8(2):86–92. https://doi.org/10.17138/tgft(8)86-92
Chorom M, Rengasamy P (1997) Carbonate chemistry, pH, and physical properties of an alkaline sodic soil as affected by various amendments. Aust J Soil Res 35:149–161. https://doi.org/10.1071/S96034
Davey RS (2013) Soil-borne disease suppression to Rhizoctonia solani AG8 in agricultural soils from semi-arid region of South Australia. Doctor of Philosophy Thesis, The University of Adelaide, Australia
Davey RS, McNeill A, Barnett S, Gupta V (2021) Potential for suppression of Rhizoctonia root rot is influenced by nutrient (N and P) and carbon inputs in a highly calcareous coarse-textured topsoil. Soil Res 59:329–345. https://doi.org/10.1071/SR20247
Degryse F, McLaughlin MJ (2013) Phosphorus diffusion from fertilizer: visualization, chemical measurements, and modeling. Soil Sci Soc Am J 78:832–842. https://doi.org/10.2136/sssaj2013.07.0293
Deluca TH, Skogley EO, Engel RE (1989) Band-applied elemental sulfur to enhance the phytoavailability of phosphorus in alkaline calcareous soils. Biol Fertil Soils 7(4):346–350. https://doi.org/10.1007/BF00257831
Emsley J (1980). The phosphorus cycle. The natural environment and the biogeochemical cycles. Springer, Berlin, pp147–167
FAO (2020) Standard operative procedure for soil calcium carbonate equivalent: Titrimetric method. Food and Agriculture Organization of the United States, Rome. http://www.fao.org/3/ca8621en/ca8621en.pdf
Fertiliser Technology Research Centre (2020a) Technical bulletin: fertilisers and soil acidity. The University of Adelaide, Mosaic. https://sciences.adelaide.edu.au/fertiliser/system/files/media/documents/2020a-01/factsheet-fertilizers-and-soil-acidity.pdf. Accessed 22 Oct 2021
Fertiliser Technology Research Centre (2020b) Technical bulletin: oxidation of elemental sulfur in soils. The University of Adelaide, Mosaic. https://sciences.adelaide.edu.au/fertiliser/system/files/media/documents/2020b-01/factsheet-oxidation-of-elemental-sulfur-in-soils.pdf. Accessed 15 October 2021
Frischke BM, Holloway RE, McLaughlin MJ, Lombi E (2004) Phosphorus management and availability in highly calcareous soil. International Crop Science Congress, http://www.cropscience.org.au/icsc2004/poster/2/5/4/1263_frischkebm.htm#:~:text=In%20Australia%2C%20calcareous%20soils%20are,5%20to%2090%25%20calcium%20carbonate. Accessed 3 March 2021
Gupta VVSR (2016) Tips and tactics: Rhizoctonia southern region. Grains Research and Development Corporation, Canberra, ACT
Gustafsson JP (2021) Visual MINTEQ version 3.1. KTH Royal Institute of Technology, Stockholm, Sweden
Hall J (2002) Lab Manual for Zumdahl/Zumdahl’s Chemistry, edn. 6. Appendix 5. Brooks Cole. http://clas.sa.ucsb.edu/staff/Resource%20folder/Chem109ABC/Acid,%20Base%20Strength/Table%20of%20Acids%20w%20Kas%20and%20pKas.pdf. Accessed 27 Oct 2021
Hopkins B, Ellsworth J (2005) Phosphorus availability with alkaline/calcareous soil. Western nutrient management conference, 6. Salt Lake City, UT, pp 88–93
Janik LJ, Merry RH, Skjemstad JO (1998) Can mid infrared diffuse reflectance analysis replace soil extractions? Aust J Exp Agric 38(7):681–696. https://doi.org/10.1071/EA97144
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257. https://doi.org/10.1007/BF00008338
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behaviour in soils—misconceptions and knowledge gaps. Plant Soil 248:31–41. https://doi.org/10.1023/A:1022304332313
Khorsandi F (1994a) Phosphorus fractions in two calcareous soils as affected by sulfuric acid application. J Plant Nutr 17(9):1599–1609. https://doi.org/10.1080/01904169409364832
Khorsandi F (1994b) Sulfuric acid effects on iron and phosphorus availability in two calcareous soils. J Plant Nutr 17(9):1611–1623. https://doi.org/10.1080/01904169409364833
Klute A (1986) Water retention: laboratory methods. Methods of soil analysis: part 1 physical and mineralogical methods, pp 635–662. https://doi.org/10.2136/sssabookser5.1.2ed.c26
Kouno K, Wu J, Brookes PC (2002) Turnover of biomass C and P in soil following incorporation of glucose or ryegrass. Soil Biol Biochem 34:617–622
Lombi E, McLaughlin MJ, Johnston C, Armstrong RD, Holloway RE (2004a) Mobility and lability of phosphorus from granular and fluid monoammonium phosphate differs in a calcareous soil. Plant Soil 68(2):682–689. https://doi.org/10.2136/sssaj2004.6820
Lombi E, McLaughlin MJ, Johnston C, Armstrong RD, Holloway RE (2004b) Mobility, solubility and lability of fluid and granular forms of P fertiliser in calcareous and non-calcareous soils under laboratory conditions. Plant Soil 269:25–34. https://doi.org/10.1007/s11104-004-0558-z
Mace JE, Amrhein C, Oster JD (2010) Comparison of gypsum and sulphuric acid for sodic soil reclamation. Arid Soil Res Rehabil 13(2):171–188. https://doi.org/10.1080/089030699263401
Macias-Benitez S, Garcia-Martinez AM, Caballero Jimenez P, Gonzalez JM, Tejada Moral M, Parrado Rubio J (2020) Rhizospheric organic acids as biostimulants: monitoring feedbacks on soil microorganisms and biochemical properties. Front Plant Sci 11(633):1. https://doi.org/10.3389/fpls.2020.00633
McBeath TM, McLaughlin MJ, Kirby JK, Armstrong RD (2012) The effect of soil water status on fertiliser, topsoil and subsoil phosphorus utilization by wheat. Plant Soil 358:337–348. https://doi.org/10.1007/s11104-012-1177-8
McLaughlin MJ, McBeath T, Smernik R, Stacey S, Ajiboye B, Guppy C (2011) The chemical nature of P accumulation in agricultural soils—implications for fertiliser management and design: an Australian perspective. Plant Soil 349:69–87. https://doi.org/10.1007/s11104-011-0907-7
McNeill A (2014) UA00103—DGT as the soil test of choice for predicting phosphorus requirements of grain crops. Grains Research and Development Corporation. https://grdc.com.au/research/reports/report?id=3771. Accessed 19 Feb 2022
Mengel K, Kirkby E (2001) Phosphorus. Principles Plant Nutrit 93(4):453–479. https://doi.org/10.1093/aob/mch063
Mihoub A, Bouhoun MD, Naeem A, Saker ML (2017) Low-molecular weight organic acids improve plant availability of phosphorus in different textured calcareous soils. Arch Agron Soil Sci 63(7):1023–1034. https://doi.org/10.1080/03650340.2016.1249477
Mitchell J, Dehm JE, Dion H (1952) The effect of small additions of elemental sulphur on the availability of phosphate fertilizers. Sci Agric 32:311–316. https://doi.org/10.4141/sa-1952-0044
Msimbira LA, Smith DL (2020) The Roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front Sustain Food Syst 4(106):1. https://doi.org/10.3389/fsufs.2020.00106
Oehl F, Oberson A, Probst M, Fliessbach A, Roth H-R, Frossard E (2001) Kinetics of microbial P uptake in cultivated soils. Biol Fertil Soils 34(1):31–41. https://doi.org/10.1007/s003740100362
Pierzynski GM, McDowell RW, Thomas Sims J (2005) Chemistry, cycling, and potential movement of inorganic phosphorus in soils. Phosphorus Agric. Environ. 1:53–86. https://doi.org/10.2134/agronmonogr42.c3
Rafiullah KM, Muhammad D, Fahad S, Adnan M, Wahid F, Alamri S, Khan F, Dawar K, Irshad I, Danish S, Arif M, Amanullah SS, Khan B, Mian I, Datta R, Zarei T, Shah A, Ramzan M, Zafar-ul-Hye M, Mussarat M, Siddiqui M (2020) Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants 9(10):1. https://doi.org/10.3390/plants9101389
Rayment GE, Lyon DJ (2011) Soil chemical methods: Australasia. CSIRO Publishing, Collingwood
Reuter DJ, Robinson JB (1997) Plant analysis: an interpretation manual, 2nd edn. CSIRO Publishing, Collingwood, p 241
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906. https://doi.org/10.1071/PP01093
Ros MBH, Koopmans GF, van Groenigen KJ, Abalos D, Oenema O, Vos HMJ, van Groenigen J (2020) Towards optimal use of phosphorus fertiliser. Sci Rep 10(1):17804. https://doi.org/10.1038/s41598-020-74736-z
Ruellan A (1973) Morphology and distribution of calcareous soils in the mediterranean and desert regions. FAO Soil Bull: Calcareous Soils 21:7–15
Ryan J, Curtin D, Cheema MA (1985) Significance of iron oxides and calcium carbonate particle size in phosphate sorption by calcareous soils. Soil Sci Soc Am J 49:74–76. https://doi.org/10.2136/sssaj1985.03615995004900010014x
Ryan J, Stroehlein JL (1979) Sulfuric-acid treatment of calcareous soils: effects on phosphorus solubility, inorganic phosphorus forms, and plant-growth. Soil Sci Soc Am J 43(4):731–735. https://doi.org/10.2136/sssaj1979.03615995004300040022x
Schneider KD, Thiessen Martens JR, Zvomuya F, Reid DK, Fraser TD, Lynch DH, O’Halloran IP, Wilson HF (2019) Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J Environ Qual 48(5):1247–1264. https://doi.org/10.2134/jeq2019.02.0070
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156(3):997–1005. https://doi.org/10.1104/pp.111.175232
Shrivastava M, Srivastava P, D’Souza S (2018) Phosphate-solubilizing microbes: diversity and phosphates solubilization mechanism. Role Rhizospheric Microb Soil. https://doi.org/10.1007/978-981-13-0044-8_5
Taalab AS, Ageeb GW, Siam Hanan S, Mahmoud Safaa A (2019) Some characteristics of calcareous soils: a review. Middle East J Agric Res 8(1):96–105
United States Department of Agriculture (1999) Soil quality test kit guide. United States Department of Agriculture. https://efotg.sc.egov.usda.gov/references/public/WI/Soil_Quality_Test_Kit_Guide.pdf
Wahba M, Labib F, Zaghloul A (2019) Management of calcareous soils in arid region. Int J Environ Pollut Environ Model 2(5):248–258
Wang Y, Chen X, Whalen JK, Cao Y, Quan Z, Lu C, Shi Y (2015) Kinetics of inorganic and organic phosphorus release influenced by low molecular weight organic acids in calcareous, neutral and acidic soils. J Plant Nutr Soil Sci 178(4):555–566. https://doi.org/10.1002/jpln.201500047
Weeks JJ Jr, Hettiarachchi GM (2019) A review of the latest in phosphorus fertilizer technology: possibilities and pragmatism. J Environ Qual 5(48):1300–1313. https://doi.org/10.2134/jeq2019.02.0067
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal Method 3:2854–2863. https://doi.org/10.1039/C1AY05430A
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084
Zhao C, Gupta VVSR, Degryse F, McLaughlin MJ (2017) Effects of pH and ionic strength on elemental sulphur oxidation in soil. Biol Fertil Soils 53:247–256. https://doi.org/10.1007/s00374-016-1170-0
Acknowledgements
We would like to thank Bogumila Tomczak, Andrea Paparella and Ashleigh Panagaris for their technical support. We would also like to thank Dr Glenn McDonald, Professor Michael Hedley and Dr Ronald Smernik for their comments on an earlier version of this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. We acknowledge the University of Adelaide, the Commonwealth Scientific and Industrial Research Organisation and the Grains Research and Development Corporation (project CSP2009-003RTX) for funding this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no financial interests and no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brownrigg, S., McLaughlin, M.J., McBeath, T. et al. Effect of acidifying amendments on P availability in calcareous soils. Nutr Cycl Agroecosyst 124, 247–262 (2022). https://doi.org/10.1007/s10705-022-10241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-022-10241-1