Abstract
Winter oilseed rape (WOSR) is the major oil crop cultivated in Europe and the most important feedstock for biodiesel. Up to 90% of the greenhouse gas (GHG) emissions from biodiesel production can occur during oilseed rape cultivation. Therefore, mitigation strategies are required and need to focus on direct nitrous oxide (N2O) emission as one of the largest GHG contributors in biodiesel production. Earlier studies show that nitrification inhibitors (NIs) can reduce N2O emissions derived from N-fertilization. Since information on the effect of biogas digestates with or without NIs on N2O emissions from WOSR fields is scarce, the aim of this study was to evaluate their effects on N2O emissions, mineral N dynamics, and oil yield in WOSR production fertilized with digestate. The study was conducted at five sites across Germany over three years resulting in 15 full site-years data sets. Across all sites and years, N2O emission from WOSR fertilized with biogas digestate (180 kg NH4+-N ha−1yr−1) ranged between 0.2 and 3.5 kg N2O–N ha−1 yr−1. Due to the reduction of the nitrate concentrations following digestate application, application of NI significantly reduced annual N2O emission by 36%. Our results demonstrate that NI can be an effective measure for reducing N2O emissions from digestate application, but its effectiveness depends on soil and weather conditions, and ultimately on the site-specific potential for N2O production and release. There was no effect of NI application on grain and oil yield.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
During the last decade, demand of biofuel in Europe has been growing with rapeseed oil as it is the most important feedstock (Hamelinck et al. 2012; Aldhaidhawi et al. 2017). Oilseed rape (Brassica napus L.) production in the European Union increased by 38% between 2000 and 2014 (FAOSTAT 2017). However, there has been a long controversial discussion whether rapeseed cultivation for biofuel production is environmentally sound. The potent greenhouse gas nitrous oxide (N2O) is emitted during the cultivation of feedstock in the field, accounting for 75 to 90% of the total GHG emissions in biodiesel production (66.7–119.5 g CO2 MJfuel−1; Hoefnagels et al. 2010). Nitrous oxide contributes to both the greenhouse effect (e.g., 100-year Global Warming Potential of 298; Myhre et al. 2013) and to stratospheric ozone depletion (Crutzen 1981; Ravishankara et al. 2009). More than half of the entire anthropogenic N2O emission is originated from agricultural soils (IPCC 2006). Nitrification and biological denitrification are main sources for N2O production in soils (Bremner 1997). Apart from these two processes, the contribution of further microbial and chemical N transformations to the total N2O release from soils, such as i.e. nitrifier-denitrification or chemo-denitrification, is currently discussed (Shaw et al. 2006; Butterbach-Bahl et al. 2013). Since all processes of N2O production in soils rely on mineral N as a substrate, N-fertilization has frequently shown to enhance N2O emissions from agricultural soils (Stehfest and Bouwman 2006; Jungkunst et al. 2006; Kaiser and Ruser 2000).
In the European Union, primary energy production from biogas production has increased from 2.5 billion m3 methane equivalent in 2000 to 18 billion m3 in 2015, representing half of the global biogas production (Scarlat et al. 2018). The residual digestates after anaerobic digestion of the biogas feedstocks are valuable fertilizers which gain importance with increasing biogas production. To close the nutrient cycle within renewable energy production, digestate from biogas plants are promoted as a substitute for mineral N-fertilizer. Anaerobic digestion changes the chemical composition of the biogas substrate, resulting in higher NH4+ contents, higher pH values and lower carbon contents (Möller and Müller 2012; Wolf et al. 2014). The application of organic N-fertilizers such as digestates on soils might result in small-scale anaerobic zones with increased oxygen consumption due to the input of easily available C-sources favoring N2O release from denitrification (Flessa and Beese 1995) and thus increasing N2O emissions when compared to mineral N-fertilizer application (Jones et al. 2007).
Nitrification is one of the main sources of N2O formation in soils and it provides NO3− which serves as an initial substrate for N2O production via denitrification. Nitrification inhibitors (NIs) inhibit the enzyme ammonia monooxygenase (AMO) which catalyzes the first step of the nitrification process carried out by microorganisms (oxidation of NH4+ to hydroxylamine), thus stabilizing NH4+. It has often been reported that application of organic fertilizers with NIs reduces NO3− leaching, increases N-use efficiency and enhances yields (i.e. Di and Cameron 2007; Fangueiro et al. 2009). A meta-analysis from Abalos et al. (2014) showed an increase in productivity of approximately 5% when N-fertilizers were applied with an NI. However, this study included only one experiment with an organic fertilizer (cattle slurry) other than urine.
Due to the reduction of substrate availability for microbial N2O production, NIs are a promising tool for N2O mitigation. Akiyama et al. (2010) and Ruser and Schulz (2015) reported a N2O mitigation potential of approximately 35% by using NIs. The exploitation of this mitigation potential depends widely on site-specific conditions such as weather, soil properties and management practices (Volpi et al. 2017). To date, only few studies investigated the effect of NIs on N2O emissions from soils fertilized with biogas digestates (e.g. Severin et al. 2016; Wolf et al. 2014). Under laboratory conditions (50 days), N2O emission from a digestate treatment with 3,4-dimethylpyrazole phosphate (DMPP) was 70% lower than from a treatment without NI (Severin et al. 2016). Wolf et al. (2014) reported a similar reduction of the N2O emission between 37 and 62% in the first weeks following digestate application under field conditions. However, despite this high short-term mitigation, the annual N2O emission did not differ significantly between the treatments of Wolf et al. (2014).
Due to the paucity of information on the impacts of biogas digestates in WOSR production at the present, the aims of this study were to quantify the effects of a nitrification inhibitor added to digestate on N2O emission, mineral N dynamics, and WOSR oil yield. We further aimed to quantify whether it would be possible to reach the CO2 reduction goals of the European Directive on the promotion of the use of energy from renewable sources (EU-RED-II, Annex V: EC 2018) with biogas digestates as N-fertilizers in WOSR.
Material and methods
Study sites, experimental design and management
This 3 y study encompassed five experimental sites geographically located in areas representative of WOSR production across Germany (Table 1). A companion report by Ruser et al. (2017) provided further details. Three experimental sites where established in northern Germany (Hohenschulen, Dedelow and Berge), where is the main WOSR cultivation area. One site was located in central Germany (Merbitz) and one site in southern Germany (Ihinger Hof). Soil and environmental characteristics of each study sites are shown in Table 1.
A randomized split-plot design with four replicate blocks was established at each site. Crop rotation was winter oilseed rape (var. ‘Visby’)—winter wheat (Triticum aestivum L., var. ‘Julius’)—winter barley (Hordeum vulgare L., var. ‘Tenor’ in Berge, var. ‘Meridian’ in Hohenschulen and var. ‘Souleyka’ at all other sites) with the crops as main plot factor. The main plots were managed according to best management practices in Germany (e.g. the compliance of environmental standards such as the German Fertilizer Ordinance (DüV 2006) or the cross compliances of the common agricultural policy of the European Union (Regulation 73/2009). In each main plot of the WOSR seven treatments were established. Here, we report the results from the treatments biogas digestate without NI and biogas digestate with NI. Oilseed rape was the only crop within the crop rotation that received biogas digestates. Plot size varied slightly across study sites due to differences in the dimensions of the farming equipment; the minimum size was 3 m \(\times\) 9 m (27 m2).
Before seeding of the WOSR, soil was conventionally plowed with a moldboard plow (0–0.3 m). Oilseed rape was sown between end of August and early September at all sites and in all years (40 to 45 seeds m−2, at inter-row spacing of 0.36 m). As a common practice for WOSR production in Germany, N-fertilization was split into two doses. The first dose was applied at the beginning of the growing season in spring and the second approximately four weeks later. For each N-fertilization 90 kg NH4+–N ha−1 was applied as liquid-based digestate, resulting in a total of 180 kg NH4+–N ha−1 yr−1. Characteristics of the digestates are shown in Table S1. At the study site Dedelow the whole N amount was applied with only one single application in 2014.
Trailing-hose application method was used at all sites to apply the digestate between the plant rows. The width of the surface applied digestate was 0.12 m, corresponding to 1/3 of the whole area. In contrast, an injection technique was used for the first digestate application in 2013 at the site Berge.
To avoid sulfur deficiency, 90 kg S ha−1 were applied to all plots as kieserite (MgSO4) every spring. After harvest, soil was plowed at each site.
Piadin® (SKW, Piesteritz, Germany), which is a pyrazole derivate, was used as nitrification inhibitor. According to the producer`s recommendation we applied 5 L Piadin® solution ha−1. It was added to the digestates directly before application. The two active compounds in Piadin® (1H-1,2,4-triazole and 3-methylpyrazole) have been shown to inhibit nitrification efficiently (Aulakh et al. 2001; Barneze et al. 2014; Wu et al. 2017).
Flux measurement and calculations of flux rates and greenhouse gas (GHG) emission
Between 2013 and 2015, N2O flux rates were measured using the closed chamber method (Mosier and Hutchinson 1981). Briefly, chamber frame bases (0.71 m \(\times\) 0.27 m) were installed between the plant rows. We used the CO2 fluxes as indicator for soil respiration. Although the chamber frame bases did not include growing WOSR plants, we cannot exclude root respiration from WOSR also contributing to CO2 fluxes through diffusion into the chamber headspace from beneath the frames. This bias was not quantified for our chamber setting. Fluxes were measured at least once a week and supplemented with additional, event-oriented measurements after events which were frequently shown to increase N2O fluxes (i.e. N-fertilization, tillage, heavy rain, and frost-thaw cycling). During gas sampling, dark closed chamber covers were placed airtight on the chamber frame bases. Four gas samples were taken periodically (every 15–20 min) from the chamber headspace and transferred into pre-evacuated glass vials. The N2O and CO2 concentrations in the gas samples were analysed in the laboratories of the participating research groups by various gas chromatographs equipped with 63NI electron capture. Analytical laboratory inter-comparability was verified by conducting blind inter-comparison measurements between the laboratories involved in the study at the beginning of the experiment. Each laboratory achieved a coefficient of variance below 2% on ten repeated measurements of an ambient N2O standard gas (Ruser et al. 2017). The GC instrumentation of the laboratories is given in Table S5.
Flux rates were calculated using the R (R Core Team 2017) package gasfluxes. Measured fluxes were subjected to a rigorous quality check using CO2 accumulation above the freezing point since a large number of personnel was involved in the comprehensive gas sampling (e.g., about 60,000 gas samples were taken in the whole study during the three experimental years) and missing or discarded fluxes were filled by multiple imputation (Honaker et al. 2011).
Cumulative annual N2O emissions were calculated for the periods between 1 January and 31 December. This calendar year cycle was chosen since it covered all soil management and N-fertilization measures of the WOSR as well as it captures the time when increased soil mineral N contents were expected. Furthermore, cumulative N2O emission during the fertilization period was calculated for each year and each site separately. The fertilization periods started with the date of the first digestate application and ended four weeks after the second N application (Table S2).
For the calculation of the cumulative N2O emission, we linearly interpolated between two consecutive sampling dates. We also calculated the oil yield-related N2O emission by dividing the annual N2O-N emission by the oil yield.
We compared the GHG emissions including measured or calculated N2O emissions from soils fertilized with biogas digestate without NI against the default reduction value claimed by the European Directive on the promotion of the use of energy from renewable sources (EU-RED-II Annex V: EC 2018). Frequently GHG emissions from agricultural products are calculated with IPCC emissions factors. The IPCC emission factor (IPCC-EF) for calculating direct N2O emissions based on the N-input is globally applicable and assumes a linear relationship between N-input and N2O emissions. Therefore, the uncertainty of so calculated N2O emissions is high; the (IPCC-EF for direct N2O emissions) ranges between 0.1% and 1.8%. (IPCC 2019) The Global Nitrous Oxide Calculator (GNOC, see https://gnoc.jrc.ec.europa.eu/) takes the non-linearity of N2O flux response with varying N-fertilizer amount into account and allows for the calculation of site- und crop-specific N2O emissions with a lower uncertainty when compared to the IPCC-EF. Therefore, GNOC was used in this study for comparison with the measured N2O emissions at the experimental sites. The calculated direct N2O emissions were compared with measured data during the period from 2013 to 2015 at the various experimental sites.
Default input data from the latest report of the Joint Research Centre (JRC) of the European Commission were used for all activity data not measured in the study, such as diesel supply and consumption, pesticides, non-N-fertilizer, and others (Edwards et al. 2019). Moreover, default data of the EU-RED-II Annex V (EC 2018) were used for processing of rapeseed to biodiesel as well as for distribution and storage. Based on those default data for processing and distribution the required GHG savings from cultivation were calculated for 50%, 60%, and 65% total GHG savings, when digestate was used as N-fertilizer. The GHG savings were calculated based on default GHG emissions of fossil-based diesel. The revised EU-RED-II requires 50% GHG-savings for biofuel processing facilities installed before 2015, 60% GHG reduction for biofuels from facilities installed between 2015 and 2020, and 65% for biofuels from facilities installed from 2021 onwards.
We considered the following two scenarios:
-
Taking 50% of total–N in digestate into account to estimate N2O emission with the GNOC approach as recommended by JRC (Edwards et al. 2019).
-
Taking the average ammonium–N content (57%) of the digestates used in the field trials into account to estimate N2O emission by GNOC.
Environmental, soil, and plant analyzes
Weather stations were installed at each study site directly next to the experimental plots. We detected air temperature at 2 and 0.05 m height and daily precipitation. Data loggers (LogTag, TRIX-8, CIK solutions, Karlsruhe, Germany) were used to determine soil temperature in 0.05, 0.1 and 0.2 m soil depth. Soil samples were taken concurrently with each gas sampling from 0 to 0.3 m depth. We used soil augers with an inner diameter of 0.02 m. According to the share of surface area covered by digestate, two soil samples were taken aside and one in the middle of the digestate bands in each plot. We mixed the three samples of each plot to one homogenized sample. The four samples from the replicate plots were then pooled resulting in one composite sample per treatment and sampling date.
For further analysis, the samples were sieved (< 2 mm) and stored frozen. To determine the mineral N content, 80 g of fresh soil were extracted with 200 ml of a 1.25 10–2 M CaCl2 solution. Photometric analysis was used to measure the concentrations of NO3− and NH4+ in the extracts. Since these measurements were also done separately at the several research laboratories across Germany and thus with different analytical systems, we conducted an inter-laboratory comparison with all laboratories involved in the study. Detection limits were calculated based on DIN 32,645 (2008) and shown together with the manufacturer and model information of analyzers in Table S4.
Soil moisture was determined gravimetrically by drying soil at 105 °C for 24 h. Bulk density of the topsoil was determined using stainless steel cylinders (100 ml) before and after each tillage operation. Water-filled pore space (WFPS) in the Ap horizon (0–0.3 m depth) was calculated as follows:
with soil porosity calculated as
where 2.65 Mg m−3 (particle density of quartz) was the assumed particle density of the soil.
Plant biomass yield was measured on 1 m2 cuttings. Moisture content of the plant samples was determined by drying for three days at 60 °C (straw and pods separately). Composite subsamples of the crop straw and grain were analyzed for C and N using an elemental analyzer (vario Max CN, Elementar Analysensysteme, Hanau, Germany). Oil content of the WOSR seeds was measured using near-infrared spectroscopy (NIRSystem 5000, Foss, Hamburg, Germany).
In 2017 the German Fertilizer Ordinance, which regulates the maximum amount of N-fertilizer, was revised. In contrast to the preceding ordinance (DüV 2006), the maximum rate of N applied with digestate in arable land was reduced from 170 kg N ha−1 based on NH4-N to 170 kg N ha−1 based on total N (DüV 2017).
Meteorological conditions
The annual precipitation varied between 409 mm (Hohenschulen in 2014) and 923 mm (Ihinger Hof in 2013) (Table 1). Except for Hohenschulen, the long-term mean annual precipitation at each study site lay within the range of the annual precipitation in the three single experimental years, indicating that our measurements were conducted in a period with site typical precipitation characteristics at Dedelow, Ihinger Hof, Merbitz, and Berge. In contrast, precipitation during the whole study period in Hohenschulen was 35% lower than the long-term mean hinting on a potential water deficit for that region. At Ihinger Hof, Merbitz and Berge, precipitation in the first experimental year (2013) was 34%, 35%, and 22% higher when compared to the long-term mean. Particularly spring and summer 2015 was very dry at almost every study site.
Mean annual air temperature ranged between 8.1 °C (Hohenschulen in 2013) and 13.0 °C (Berge in 2014). Except for the study site Hohenschulen, annual temperature was predominately higher than the long-term mean at all remaining study sites and in all three experimental years.
Statistical methods
A mixed model approach using SAS PROC MIXED for the comparison of cumulative N2O emissions, oil yield and oil yield-related N2O emissions was used. The model can be described as follows:
where \(a_{h}\), \(l_{j}\) and \(\tau_{i}\) are the fixed main effects for the hth year, jth site and ith level of nitrification inhibitor (NI). \(\left( {al} \right)_{hj}\), \(\left( {a\tau } \right)_{hi}\), \(\left( {l\tau } \right)_{ji}\), and \(\left( {al\tau } \right)_{hij}\) are the fixed interaction effects of corresponding main effects. \(b_{hjkl}\) is random block effects. \(e_{hijkl}\) is the plot error effect with a site-by-year-specific variance. As data for N2O emissions were repeatedly taken, block effects and error effects were allowed to have a first order autocorrelation variance–covariance structure if this decrease the AIC (Wolfinger 1993). The assumptions of normally distributed residuals and a homogeneous variance (beside the heterogeneity accounted for) were checked graphically. After finding significant effects via F tests in the second stage of the analysis, least square means for corresponding effects were calculated and compared with a Tukey-test at α = 0.05. Additionally, least square means of cumulative N2O emission and their standard errors were calculated for each site-by-year-by-NI treatment combination using equation 3. These means were additionally compared using Fishers LSD test to show single site-by-year results.
Furthermore, for each site, a multiple regression analysis for N2O flux rates was performed in order to explore the main processes of N2O release. To include block and treatment effects within multiple regression approach, dummy variables were created and included per default within the model. The best model per site was selected via adjusted R2 after fitting all possible models. The following explaining variables were used: air temperature (2 m), soil extractable NO3− and NH4+ concentrations, water-filled pore space (WFPS) and CO2 flux rates. From this approach, the correlation was determined as the square root of the partial R2 value. Additionally, selected variables were tested via F-test and the result and their slope estimates were presented. Analyses were performed in SAS 9.4 (SAS Institute, Cary NC, USA). Plots and graphics were created with SigmaPlot 11.0 (Systat Software GmbH, Erkrath, Germany).
Results
N2O flux rates
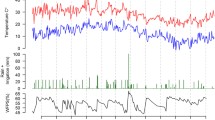
Nitrous oxide fluxes showed a high spatial and temporal variability (Fig. 1). Increased fluxes were often measured after digestate application in conjunction with precipitation events. With 458 µg N2O-N m−2 h−1 the highest flux rate during the entire experimental period was determined in the treatment without NI at the study site Merbitz on 8th May 2013. This peak occurred after the second fertilizer application one day after a heavy rainfall event (42 mm d−1). Similarly, high N2O flux rates after N-fertilization were also measured 2013 in Berge, 2015 in Dedelow, and 2014 and 2015 in Hohenschulen.
In cases where the application of digestates induced a considerable increase of the N2O fluxes (i.e. when flux rates after N-fertilization exceeded 50 µg N2O-N m−2 h−1), the flux rates were higher in the treatment without NI than in the treatment with NI (Fig. 1). On 8th May 2013, the mean N2O flux rate from the treatment with NI in Merbitz was by factor 8 lower (55 µg N2O-N m−2 h−1) when compared to the treatment without NI application.
Approximately two weeks after the second digestate application the N2O fluxes decreased and they were on background level four weeks after N-fertilization. Except for Berge and Ihinger Hof (both in 2014 and 2015) N2O flux rates increased after harvest and less frequently after soil tillage. Winter fluxes were generally low at all sites and in all years (Fig. 1).
For all experimental sites, N2O fluxes were significantly correlated (p < 0.001, Table 2) with CO2 fluxes. Except for the sandy site Berge, we also found a positive correlation between the N2O fluxes and soil moisture. Additionally, N2O fluxes in Dedelow, Hohenschulen and Merbitz were also positive correlated with soil nitrate contents (Table 2).
Annual N2O emission and N2O emission during the fertilization period
Annual N2O emission varied between 0.2 kg N2O-N ha−1 yr−1 and 3.5 kg N2O-N ha−1 yr−1. The study site Merbitz revealed the highest annual N2O emissions in every experimental year and in both treatments (-NI and + NI). Except for the treatment without NI in 2013 (measured in Hohenschulen), Berge showed the lowest annual N2O emissions.
When compared to the treatment without NI, the application of the NI significantly reduced the annual N2O emission over the whole data set by 36% (all sites and years, p = 0.0027, Table 3). With regard to the single years and study sites, the mean annual N2O emission in the treatment with NI was only significantly (p < 0.05, Table 4) lower for 3 of the 15 site-years: at Dedelow in 2014 (50% less than -NI) and in 2015 (73% less than -NI), and at Merbitz in 2013 (49% less than -NI). Cumulative N2O emission during the fertilization period varied between 0.01 and 1.82 kg N2O-N ha−1 period−1 (Table 4). During the fertilization period, the mean N2O emission in the treatment with NI tended to be lower in 12 out of 15 observations with 6 observations being statistically significant. The N2O mitigation in this period varied between 65% (Hohenschulen in 2015) and 97% (Dedelow in 2015) (Table S6).
Outside the fertilization periods, the mean N2O emission varied between 0.16 and 1.63 kg N2O-N ha−1 (Table 4). The emission in this period accounted for between 48 and 99% of the annual N2O emissions (Table S6). Except for Dedelow in 2014, NI application did not affect N2O emissions outside the fertilization periods. The higher N2O emissions in the + NI treatment outside the fertilization period in Dedelow 2014 were mainly the result of higher N2O fluxes following heavy rainfall events at the begin of June and August.
Mineral N in the topsoil
Digestate application often increased NO3− concentrations in the topsoil (Fig. 2). With 19.4 mg NO3−–N kg−1 (corresponding to approximately 75 kg N ha−1) highest concentration after digestate application was measured mid-March 2015 at the study site Ihinger Hof. Following digestate applications NO3− concentrations decreased with increasing N demand of the growing WOSR. Nitrate concentrations increased again either shortly before or directly after harvest of the WOSR. At all study sites and in nearly every experimental year highest NO3−-concentrations of up to 31.5 mg NO3−–N kg−1 (Dedelow September 2014) were measured in this post-harvest period.
When compared to digestate application without NI, usage of the NI significantly decreased the NO3−-concentrations in the topsoil during the fertilization period (Fig. 3). The median NO3−-concentration in this period was 32% lower in the + NI treatment. This effect of the NI on lowering NO3−-concentration in the fertilization period was highly significant with a high coefficient of determination (R2 = 0.79).
The NH4+ concentrations in the plow layer increased after digestate application at all sites (Table S2) whereas their contribution to total mineral N outside the fertilization period was negligible (< 5 kg NH4+-N ha−1, data not shown). We did not find any correlation between the NH4+-N concentrations and the N2O flux rates, neither for the whole data set nor for a single site or year.
Following digestate application the NH4+-N/NO3−-N ratio in the treatment + NI at the study sites Dedelow, Ihinger Hof and Hohenschulen was higher than in the treatment without NI, this was also measured at the site Berge in the third year (Figure S1). For the study site Merbitz (silty loam texture), this effect was not observed.
Effect of NI on grain yield, oil yield and oil yield-related N2O emission
The WOSR grain yield was not affected by NI application, over the whole experimental period and all sites it varied between 2.8 Mg ha−1 yr−1 and 5.7 Mg ha−1 yr−1 (Table S3). The corresponding mean WOSR oil yield was 2.0 Mg ha−1 yr−1, it ranged between 1.2 and 2.7 Mg ha−1 yr−1 (Table 5). Oil yield was not affected by NI application, neither in one single experimental year nor at any of the study sites.
Oil yield-related emission varied over the experimental sites and years between 0.1 and 1.9 kg N2O-N Mg−1 oil ha−1 (Table 5). Oil yields were not affected by NI application. NI application reduced oil yield-related N2O emissions in only 3 of the 15 tested site-years (Dedelow in 2014 and 2015 and Merbitz in 2013).
GHG emissions from digestate application
Calculated direct N2O emissions with GNOC were generally higher than the measured N2O emissions at the experimental sites (Table 6). The only exception was the study site Merbitz with lower GHG emissions in the GNOC calculation.
Newly established facilities for biodiesel production have to prove that 65% GHG reduction is achieved when replacing fossil fuel by biodiesel. This target could be achieved at all experimental sites except for Merbitz, if measured direct N2O emissions and calculated indirect N2O emissions were used. When N2O emissions were calculated with GNOC taking 50% of the total N in digestate into account as recommended by JRC, all experimental sites would meet the 65% GHG reduction target.
Using the average NH4+-N content of the applied digestate (57%) instead of 50% of the total N for the GNOC calculation did not change the overall picture. A GHG reduction of 65% could be achieved at all experimental sites, although Hohenschulen just met the requirement.
Discussion
N2O flux rates as affected by environmental conditions and soil drivers
Nitrous oxide release at our five study sites showed a high temporal variability with increased flux rates after N-fertilization in conjunction with rainfall. Several studies in arable fields reported similar N2O flux patterns and mainly explained the increased flux rates with rainfall enhancing denitrification (Ambus and Christensen 1994; Flessa et al. 1995; Kaiser et al. 1996). After fertilization, ammonium in liquid manure stripes is rapidly oxidized to nitrate (Delin and Strömberg 2011), thus increasing substrate availability for denitrification. Oxygen (O2) diffusion in soil water is approximately 104 times lower than in soil air (Heincke and Kaupenjohann 1999). Consequently, high soil moisture reduced O2 diffusion from the atmosphere into and within the moist soil after rainfall. Additionally, high microbial O2 demand during C turnover of easily available C from cattle slurry further stimulated formation of anaerobic soil conditions and thus promoted denitrification (Flessa and Beese 1995, 2000). As shown for cattle slurry, oxygen depletion in the upper soil centimeters during intense nitrification of the NH4+ can further enhance the development of anaerobic conditions after surface application (Van Nguyen et al. 2017), this might also hold true for the application of digestates.
Besides high N2O flux rates measured after digestate applications, we also found increased N2O release after harvest of WOSR which, depending on study site and year, lasted up to six weeks. These high fluxes coincided with increased NO3− contents of the topsoil post-harvest. Consistently, Ruser et al. (2017) reported enhanced post-harvest N2O fluxes from WOSR plots in the same field experiment from treatments fertilized with mineral N. We found a significant correlation between N2O and CO2 fluxes, which was also confirmed by Ruser et al. (2017). This indicates a positive relationship between C-heterotrophic microbial activity, including activity of the denitrifying community, and N2O release. Our results are in good agreement with Walter et al. (2015) who also explained the higher post-harvest N2O emissions from WOSR fields in comparison to winter wheat fields with a higher NO3− availability as substrate for denitrification under WOSR.
The output of the explorative multiple linear regression analysis indicated that (i) CO2 flux was the main explanatory variable in the regression model at all study sites and that (ii) further significant parameters such as soil moisture and NO3− concentration at some sites also hint on denitrification as the main N2O source in our experiment. Michaelis–Menten kinetic studies for denitrification in agricultural soils derived KM values in the range between 4 and 13 mg NO3−-N kg−1 soil (Klemedtsson et al. 1977; Limmer and Steel 1982; Mosier et al. 1983). In our experiment, NO3− concentrations measured during periods with enhanced N2O fluxes were well within this KM range indicating that NO3− as substrate for denitrification was never limiting at all study sites.
In a lab study with 20 different soils, Gödde and Conrad (2000) reported a significant relationship between the N2O production rate and the CO2 release rate, whereas N2O production during nitrification was not correlated with the CO2 flux. A low degree of the coefficient of determination (R2 = 0.06) between N2O and CO2 flux rates and no further correlating soil driver (e.g., NO3− or soil moisture) implies different main sources such as nitrification at this site. Note that the presented multiple linear regression analysis was performed for each site separately and thus the influence of site-specific variables was not considered. Further, static parameters such as clay contents or soil classification do not change over short periods. Although they might be helpful for the estimation of a site-specific potential for N2O release, they are unhelpful to analyze the high temporal dynamics of N2O flux rates which are mainly driven by events such as N-fertilization or precipitation occurrence.
N2O emission after digestate application as affected by the study site
Over all sites and experimental years, cumulative N2O emission ranged between 0.2 and 3.5 kg N2O-N ha−1 yr−1. Walter et al. (2015) summarized the results from N2O measurements in WOSR fields in Germany, Spain, France and UK. The emissions reported by Walter et al. (2015) were higher than the emissions measured in our experiment. For mineral N-fertilizer amounts between 172 and 195 kg N ha−1 yr−1 (n = 6) the annual N2O emissions varied between 1.71 and 5.69 kg N2O-N ha−1 yr−1 with a mean emission of 3.42 kg N2O-N ha−1 yr−1. This mean emission value was close to our highest annual N2O emission (3.5 kg N2O-N ha−1 yr−1 in Merbitz in 2013). In agreement with our lower emissions, measurements of Ruser et al. (2017) in the same experiment in treatments receiving different amounts of mineral N-fertilizer also showed lower emissions when compared to the whole data set of Walter et al. (2015).
The mean annual N2O emission in the treatments without NI decreased in the following order: Merbitz > Hohenschulen > Dedelow > Ihinger Hof > Berge. Except for the site Ihinger Hof, annual N2O emissions decreased from loamy to sandy textures of the soils. Sandy soil texture and low Corg contents (Berge and Dedelow) result in a low water holding capacity and thus in good soil aeration limiting denitrification (Bouwman et al. 2002). Pelster et al. (2012), Stehfest and Bouwman (2006) and Leip et al. (2011) reported similar results for N2O emissions from soils with varying soil texture. The Dedelow and Hohenschulen sites had more or less a similar soil texture. The twofold higher Corg content in the top soil of Hohenschulen compared to the topsoil in Dedelow might be one reason for the higher N2O emission in Hohenschulen. Further, the lower pH values at Hohenschulen (pH: 5.9) might also be a reason for the higher N2O emission when compared to the site Dedelow (pH: 7.4). As shown by Russenes et al. (2016) even small differences in soil pH affect N2O emission in periods with high denitrification losses with an increasing share of N2O to the total product ratio of denitrification with decreasing soil pH. When compared to Hohenschulen, the higher pH and the lower Corg content at the site Ihinger Hof might also have been a reason for the lower N2O emission at Ihinger Hof.
In keeping with Ruser et al. (2017), highest annual N2O emission in both treatments within every site was measured in the experimental year with the highest precipitation. This would as well confirm our assumption of denitrification as the major N2O source.
Between 48.4% and 98.8% of the annual N2O emission occurred outside the fertilization period. A major part of these emissions stemmed from the post-harvest period, indicating the importance of this period for annual N2O budgets from WOSR fields. As already pointed out by Ruser et al. (2017) frost periods during our experiment were short and the air temperature only slightly below 0 °C. Therefore, frost-thaw cycles did not significantly contribute to the annual N2O emissions.
Effect of NI on N2O emissions after digestate application
Over the whole data set, annual N2O emission from WOSR fields fertilized with biogas digestate was reduced by 36% when a NI was applied (p = 0.0027). For single sites and years, the reduction was very efficient (> 45%) and thus statistically significantly in 3 data sets with comparably high annual emissions and with low emissions outside the fertilization period. When compared to the -NI treatment, this decrease in N2O release is in good agreement with reduction potentials reported by Akiyama et al. (2010) and by Ruser and Schulz (2015) who both calculated a N2O mitigation of approximately 35% from publications that had tested a range of available NIs.
Nitrification inhibitors inhibit the ammonia monooxygenase (AMO) as the first step during nitrification, and therefore, directly decrease the release of N2O from nitrification. Due to lower NO3− concentration as substrate for denitrifiers, NIs can also reduce N2O emissions from denitrification (Ruser and Schulz 2015). Additionally, the N2/N2O ratio increases with decreasing NO3− concentration as a result of a competitive effect of NO3− and N2O as a terminal electron acceptor during denitrification (Blackmer and Bremner 1978; Yamulki et al. 1995). This effect would further decrease N2O release from denitrification due to lower NO3− concentrations.
The lower NO3− concentrations in the + NI treatment during the fertilization period clearly show the effect of reduced NO3− availability after NI application. This effect was even observed on an annual base, although the differences in annual median NO3− concentrations between + NI and –NI treatment were only low. The inhibitory effect of NI on NH4+ oxidation after digestate application was also confirmed by higher NH4+-N/NO3−-N ratios at most study sites (Figure S1). This ratio did not differ between + NI and –NI at Merbitz, the study site with the highest clay content within our experiment. Since the NO3− concentrations during the fertilization period at Merbitz were higher in the treatment without NI, we infer that the NH4+-N/NO3−-N ratio did not respond to NI application as a result of low NH4+ extraction recoveries for soils with considerably high clay contents when 0.01 M CaCl2 solution is used as extraction solution (Li et al. 2012).
Except for the site Ihinger Hof, we found significant lower cumulative N2O emission during the fertilizer period for study sites where we also found a positive correlation between N2O flux rates and NO3− concentration in the topsoil. For the Berge site, this also shows that despite a lower NO3− availability in the + NI treatment, the potential to decrease N2O emissions was not realized because good aeration as a result of the low water-holding capacity limited denitrification. Similar results with almost no effect of NI after slurry application under dry conditions and distinct reduction of the N2O emission at higher soil moisture conditions were also reported by Lin and Hernandez-Ramirez (2020).
Comparing single years and sites, mean cumulative N2O emission in the -NI treatment during the fertilization period was significantly higher than in the + NI treatment in 6 of the 15 data sets. While not statistically significant, the mean N2O emission from the -NI treatment in the fertilization period was higher than in + NI in further 6 data sets. Although the mixed model revealed a significant N2O mitigation for NI application on an annual basis, only 3 annual data sets showed significances in single years and at single sites. Besides the well-known high spatial variability of N2O fluxes (i.e., Hénault et al. 2012; Röver et al. 1999), this discrepancy could also be explained in part by the high post-harvest N2O emissions masking the beneficial NI effect during the fertilization period. Indeed, all sites with a significant N2O reduction during the fertilization period, but without significant annual effects, showed between two- and threefold higher N2O emissions outside the fertilization period. This result clearly demonstrates the need of further investigations with the aim of reducing post-harvest N2O emissions from WOSR.
Dedelow 2014 was an exceptional data set because in contrast to all other study sites and also to Dedelow in 2013 and 2015, it showed an effect of the NI also outside the fertilization period. In 2014 digestate application was applied in Dedelow with only one single dose and the fertilization period (defined as period between day of first digestate application and four weeks after last application) was therefore very short (28 days). At the beginning of June, the N2O flux rates following rainfall were higher in the –NI treatment than in the + NI treatment indicating that the NI was still active approximately ten weeks after application. This flux event occurred outside the short fertilization period and was one reason for the higher N2O emission in the -NI treatment outside the fertilization period. The second reason was an emission event after harvest with higher N2O fluxes from the -NI treatment. The reason for these higher fluxes remains unclear because one could expect that the NI was completely degraded after more than 18 weeks in soil. However, some indication for a longer activity has been shown by Pfab et al. (2012) who reported an inhibitory effect of 3,4 dimethylpyrazol phosphate (a similar compound as 3-methylpyrazol in Piadin®) 15 weeks after application to cauliflower fields.
Application of NIs with organic fertilizers can decrease NO3− leaching and lower N2O and NO emissions from soils, but it also bears the risk of increasing NH3 losses (Qiao et al. 2015). For a full environmental assessment of NIs and their potential trade-offs, future studies must therefore also consider NH3 losses.
NI effects on grain yield, oil yield and oil yield-related N2O emission
The yields measured at the study sites were in the same range as yields published from the official German WOSR yield statistics; 4.0, 4.5, and 3.9 Mg ha−1 yr−1 in 2013, 2014, and 2015, respectively (German Federal Statistical Office 2017) indicating that the selection of our study sites fulfilled the claim of representability for WOSR production in Germany. Application of the NI neither showed any effect on grain yield nor on oil yield. Consistent with our study, Wolf et al. (2014) also found no effect of NI on the yield of maize fertilized with biogas digestate. Generally, NIs were shown to be efficient in increasing crop yield, N uptake or N use efficiency when applied on sandy soils or in systems with high precipitation or irrigation (Pasda et al. 2001). Wolf et al. (2014) measured at a study site with a high sand content (62%) where an increase in N uptake or maize yield could have been expected. However, they also reported the need for irrigation due to water shortage, and hence, it can be presumed that most of NO3− was not leached and thus it was still available for plant uptake even in the treatment without NI. Further, N-fertilization and oil yield were shown to be negatively correlated. Hegewald et al. (2016) reported only minor increases in oil yield (0.04 Mg ha−1 yr−1) when N-fertilization was increased from 120 to 180 kg N ha−1 yr−1. As pointed by Rathke et al. (2006), N-fertilization increases the crude protein content of rapeseeds at the expense of oil concentration. Since we fertilized with 180 kg N ha−1 yr−1 in our study, we considerably exceeded the N requirements for maximum oil yield, and therefore, an effect of NI on oil yield could not be expected.
For their data set with mineral N-fertilization in WOSR, Walter et al. (2015) reported oil yield-related N2O emissions ranging between 0.09 and 53.3 kg N2O-N Mg−1 oil ha−1 yr−1 with a median emission of 2.7 kg N2O-N Mg−1 oil ha−1 yr−1. The upper boundary of their data (53.3 kg N2O-N Mg−1 oil ha−1 yr−1) was clearly marked as an outlier which was the result of complete yield loss. Similar to the area-related N2O emissions, oil yield-related N2O emissions in our study were also lower because N2O emissions were lower and oil yields were similar to the yields reported by Walter et al. (2015). The application of NI in our study reduced oil yield-related N2O emissions in the same data sets as in the area-related annual N2O emission data. This was the result of lower N2O emissions in the –NI treatments whereas oil yields did not differ.
GHG emission from digestate application
The digestate related N2O emission factor was 0.45 (N2O emission from fertilized treatment corrected for N2O emission from an unfertilized control and related to total N from the digestates. Mean over all digestate treatments without NI; range: 0.01—1.23; data not shown). It was lower than the emission factor calculated for mineral N-fertilization at the same study site (0.6; Ruser et al. 2017). The corresponding emission was distinctively lower when compared to the emission modelled with the GNOC tool (except for study site Merbitz). For treatments fertilized with mineral N in the same experiment, Ruser et al. (2017) also reported higher N2O emissions calculated with GNOC when compared to the measured data.
As mentioned above, fertilization regulation in Germany has changed after the completion of our study, and digestates now belong to the category of “organic fertilizers”, resulting in a mandatory cap of 170 kg total N ha−1yr−1. If it would be possible to fertilize digestate rates as in our study, 65% savings (relative GHG compared to fossil fuels, here biodiesel from rapeseed) would be achieved at every site (assuming 50% NH4+-N of total N). The contrasting GHG emission resulting from data calculated with GNOC or with IPCC Tier 1 emission factors and measured data (Table 6) clearly shows the necessity of more reliable regional-specific estimation of direct N2O emissions.
Styles et al. (2015) also reported the avoidance of fossil resource depletion from a broad consortium of biofuel crops, including WOSR. However, it is difficult to compare their GHG data directly with ours because they used the IPCC Tier 1 emission factor to calculate N2O emissions and considered indirect land use effects. Further studies either with digestates of another origin (biowaste and sewage sludge), different crop fertilized with digestate or with different production target (vehicle fuel) showed that substitution of mineral N-fertilizer through digestates result in a distinct reduction of the GHG emissions (Junker et al. 2015), but other reports also stressed the ample uncertainty of total GHG emissions due to the high share of soil borne N2O emissions and their challenging estimation (Havukainen et al. 2018; Börjesson et al. 2015; Smeets et al. 2009). Moreover, GHG calculations for biofuels are based on annual N2O emissions and they do not account for future N2O emissions due to the mineralization of organic N of the applied digestates.
Conclusion
Nitrification inhibitors can as effectively mitigate direct N2O emissions from biogas digestates as from synthetic ammonium-based fertilizers. However, our results show that the mitigation potential strongly depends on site characteristics: reduction of annual N2O emissions is strongest if N2O emission potential of the site is high and if a large proportion of emissions occurs during the fertilization period. The fact that 80% of our analyzed site-years did not show a significant effect of NI application on annual N2O emission hampers general recommendations. The NIs may efficiently reduce direct N2O emissions if specifically applied to high emitting sites and high emitting crops (e.g., vegetables), but we found no evidence of a general reduction of annual N2O emission. We did not determine NI effects on nitrate leaching and indirect N2O emission. In particular, at sandy sites and for crops with high risk of nitrate leaching, NI effects might become more important on indirect N2O emission than direct N2O emission.
There was no positive effect of NI application on WOSR yield. This result was probably influenced by the generally high fertilization rate. The effects might be different in ground water protection areas where farmers have to reduce nitrogen application rates to avoid nitrate leaching. Overall, our results indicate that application of organic fertilizers such as digestate can help to save greenhouse gas emissions from industrial fabrication of synthetic N-fertilizers based primarily on fossil fuels. We found no evidence that N2O emission from N application is higher for digestate than synthetic nitrogen fertilizer. The value of NIs with respect to mitigation of direct N2O emission is probably restricted to sites and crops with high N2O emission.
Future studies on the effects of NIs applied to WOSR fields fertilized with digestates should also focus on determining the magnitudes and trade-offs of indirect N2O emissions associated with i) a potential increase in NH3 volatilization caused by using NIs as well as ii) a decreased risk for NO3− leaching due to reduced soil NO3− concentrations over the fertilization period as also caused by using NIs. The latter seems particularly important for a proper evaluation of GHG release from sandy soils that showed a low potential for direct N2O emissions. Further investigation should also consider the development of mitigation strategies for high N2O emissions that can happen following WOSR harvest. This is crucially needed as such postharvest N2O emissions can negate and even reverse the significant mitigating effect of NIs that occurred following digestate application over the early vegetative period of WOSR.
Change history
27 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10705-021-10138-5
References
Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric Ecosyst Environ 189:136–144
Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced–efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils. Meta-analysis Glob Change Biol 16:1837–1846
Aldhaidhawi M, Chiriac R, Badescu V (2017) Ignition delay, combustion and emission characteristics of Diesel engine fueled with rapeseed biodiesel – a literature review. Renew Sust Energ Rev 73:178–186
Ambus P, Christensen S (1994) Measurement of N2O emission from a fertilized grassland: an analysis of spatial variability. J Geophys Res 99:16549–16555
Aulakh MS, Kuldip-Singh DJ (2001) Effects of 4–amino 1,2,4–triazole, dicyandiamide and encapsulated calcium carbide on nitrification inhibition in a subtropical soil under upland and flooded conditions. Biol Fertil Soils 33:258–263
Barneze AS, Minet EP, Cerri CC, Misselbrook T (2014) The effect of nitrification inhibitors on nitrous oxide emissions from cattle urine depositions to grassland under summer conditions in the UK. Chemosphere 119:122–129
Blackmer AM, Bremner JM (1978) Inhibitory effect of nitrate on reduction of N2O to N2 by soil microorganisms. Soil Biol Biochem 10:187–191
BMEL (2017) Novelle der Düngeverordnung: Düngen nach guter fachlicher Praxis. https://www.gesetze-im-internet.de/d_v_2017/DÜV.pdf. Accessed 5th Aug 2020
Börjesson P, Prade T, Lantz M, Björnsson L (2015) Energy crop-based biogas as vehicle fuel. The impact of crop selection on energy efficiency and greenhouse gas performance. Energies 8:6033–6058
Bouwman AF, Boumans LJM, Batjes NH (2002) Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob Biogeochem Cycles 16:1058
Bremner JM (1997) Sources of nitrous oxide in soils. Nutr Cycl Agroecosyst 49:7–16
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils. How well do we understand the processes and their controls? Philos T Roy Soc B 368:20130122
Crutzen PJ (1981) Atmospheric chemical processes of the oxides of nitrogen including nitrous oxide. In: Delwiche CC (ed) denitrification, nitrification and atmospheric N2O. Wiley, Chichester, pp 17–44
Damesa TM, Möhring J, Worku M, Piepho H-P (2019) One step at a time: stage-wise analysis of a series of xperiments. Agron J 109:845–857
Delin S, Strömberg N (2011) Imaging-optode measurements of ammonium distribution in soil after different manure amendments. Eur J Soil Sci 62:295–304
Di HJ, Cameron KC (2007) Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor–a lysimeter study. Nutr Cycl Agroecosyst 79:281–290
DIN 32645 (2008) Chemical analysis - Decision limit, detection limit and determination limit under repeatability conditions - Terms, methods, evaluation. German Institute for Standardization – in German. Beuth publishing, Berlin
DüV (2006) Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen (Düngeverordnung – DüV, German legislation on fertilization). Bundesgesetzblatt Jahrgang 2006 Teil I Nr. 2
DüV (2017) Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen (Düngeverordnung – DüV, German legislation on fertilization). Bundesgesetzblatt Jahrgang 2017 (BGBl. I S. 1305)
EC, (2018) DIRECTIVE 2018/2001 on the promotion of the use of energy from renewable sources EC. Official J. Europ, Union L, p 328
Edwards R, O’Connel A, Giuntoli J, Bulgheroni C, Koeble R, Marelli L, Padella M, Lonza L (2019) Definition of input data to assess GHG default emissions from biofuels in EU legislation -version 1d. Luxembourg, JRC-Ispra
Fangueiro D, Fernandes A, Coutinho J, Moreira N, Trindade H (2009) Influence of two nitrification inhibitors (DCD and DMPP) on annual ryegrass yield and soil mineral N dynamics after incorporation with cattle slurry. Commun Soil Sci Plant Anal 40:3387–3398
FAOSTAT (2017) http://www.fao.org/faostat/en/#data. Accessed 18 Aug 2017
Flessa H, Beese F (1995) Effects of sugar beet residues on soil redox potential and nitrous oxide emission. Soil Sci Soc Am J 59:1044–1051
Flessa H, Beese F (2000) Laboratory estimates of trace gas emissions following surface application and injections of cattle slurry. J Environ Qual 29:262–268
Flessa H, Dörsch P, Beese F (1995) Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J Geophys Res 100:23115–23124
Gödde M, Conrad R (2000) Influence of soil properties on the turnover of nitric oxide and nitrous oxide by nitrification and denitrification at constant temperature and moisture. Biol Fertil Soils 32:120–128
Hamelinck C, De Loveinfosse I, Koper M (2012) Renewable energy progress and biofuels sustainability. Ecofys, London, p 450
Havukainen J, Uusitalo V, Koistinen K, Liikanen M, Horttanainen M (2018) Carbon footprint evaluation of biofertilizers. Int J Sus Dev Plann 13:1050–1060
Hegewald H, Koblenz B, Wensch-Dorendorf M, Christen O (2016) Impacts of high intensity crop rotation and N management on oilseed rape productivity in Germany. Crop Pasture Sci 67:439–449
Heincke M, Kaupenjohann M (1999) Effects of soil solution on the dynamics of N2O emissions: a review. Nutr Cycl Agroecosys 55:133–157
Hénault C, Grossel A, Mary B, Roussel M, Léonard J (2012) Nitrous oxide emission by agricultural soils: a review of spatial and temporal variability for mitigation. Pedosphere 22:426–433
Hoefnagels R, Smeets E, Faaij A (2010) Greenhouse gas footprints of different biofuel production systems. Renew Sustainable Energy Rev 14:1661–1694
Honaker J, King G, Blackwell M (2011) Amelia II: a program for missing data. J Stat 772 Softw 45:1–47
IPCC (2019) 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. In ed. S. Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J Fukuda, M, Ngarize S, Osako A, Pyrozhenko, Y.Shermanau, P, Federici, Switzerland: IPCC
IPCC–Intergovernmental Panel on Climate Change (2006) Guidelines for national greenhouse gas inventories. Available at http://www.ipcc-nggip.iges.or.jp/public/2006gl. Geneva, Switzerland. Accessed 6th July 2020
IUSS Working Group (2015) World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106. FAO, Rome.
Jones SK, Rees RM, Skiba UM, Ball BC (2007) Influence of organic and mineral N fertiliser on N2O fluxes from a temperate grassland. Agric Ecosyst Environ 121:74–83
Jungkunst HF, Freibauer A, Neufeldt H, Bareth G (2006) Nitrous oxide emissions from agricultural land use in Germany – a synthesis of available annual field data. J Plant Nutr Soil Sci 169:341–351
Junker FGA, Marquardt S, Osterburg B, Stichnothe H (2015) Biofuel sustainability requirements - the case of rapeseed biodiesel.". Germ J Agric Econ 64:274–285
Kaiser EA, Ruser R (2000) Nitrous oxide emissions from arable soils in Germany – an evaluation of six long-term field experiments. J Plant Nutr Soil Sci 163:249–259
Kaiser EA, Eiland F, Germon JC, Gispert MA, Heinemeyer O, Henault C, Lind AM, Maag E, Saguer E, Van Cleemput O, Vermoesen A, Webster C (1996) What predicts nitrous oxide emissions and denitrification N–loss from European soils? J Plant Nutr Soil Sci 159:541–547
Klemedtsson L, Svensson BH, Lindberg T, Rosswall T (1977) The use of acetylene inhibition of nitrous oxide reductase in quantifying denitrification in soils. Swed J Agric Res 7:179–185
Leip A, Busto M, Winiwarter W (2011) Developing spatially stratified N2O emission factors for Europe. Environ Poll 159:3223–3232
Li KY, Zhao YY, Yuan XL, Zhao HB, Wang ZH, Li SX, Malhi SS (2012) Comparison of factors affecting soil nitrate nitrogen and ammonium nitrogen extraction. Comm Soil Sci Plant Anal 43:571–588
Limmer AW, Steele KW (1982) Denitrification potentials: measurement of seasonal variation using a short-term anaerobic incubation technique. Soil Biol Biochem 14:179–184
Lin S, Hernandez-Ramirez G (2020) Nitrous oxide emissions from manured soils as a function of various nitrification inhibitor rates and soil moisture contents. Sci Total Environ 738:139669
Möhring J, Piepho H-P (2009) Comparison of weighting methods in two-stage analysis of plant breeding trials. Crop Sci 49:1977–1988
Möller K, Müller T (2012) Effects of anaerobic digestion on digestate nutrient availability and crop growth. A review Eng Life Sci 12:242–257
Mosier AR, Hutchinson GL (1981) Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J 45:311–316
Mosier AR, Parton WJ, Hutchinson GL (1983) Modelling nitrous oxide evolution from cropped and native soils. Ecol Bull 35:229–241
Myhre G, Shindell D, Bréon FM, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque JF, Lee D, Mendoza B, Nakajima T, Robock A, Stephens G, Takemura T and Zhang H (2013) Anthropogenic and Natural Radiative Forcing. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker TF, Qin D, Plattner GK, Tignor M. Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Pasda G, Hähndel R, Zerulla W (2001) Effect of fertilizers with the new nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biol Fertil Soils 34:85–97
Pelster DE, Chatigny MH, Rochette P, Angers DA, Rieux C, Vanasse A (2012) Nitrous oxide emissions respond differently to mineral and organic nitrogen sources in contrasting soil types. J Environ Qual 41:427–435
Pfab H, Palmer I, Buegger F, Fielder S, Müller T, Ruser R (2012) Influence of a nitrification inhibitor and of placed N-fertilization on N2O fluxes from a vegetable cropped loamy soil. Agric Ecosys Environ 150:91–101
Qiao C, Liu L, Hu S, Compton JE, Greaver TL, Li Q (2015) How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob Chang Biol 21:1249–1257
R Core Team (2017) R: a language and environment for statistical computing. https://www.R-project.org/
Rathke GW, Behrens T, Diepenbrock W (2006) Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): a review. Agric Ecosyst Environ 117:80–108
Ravishankara AR, Daniel JS, Portman RW (2009) Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Regulation (2009): Council Regulation (EC) No 73/2009 of 19 January 2009 establishing common rules for direct support schemes for farmers under the common agricultural policy and establishing certain support schemes for farmers, amending Regulations (EC) No 1290/2005, (EC) No 247/2006, (EC) No 378/2007 and repealing Regulation (EC) No 1782/2003
Röver M, Heinemeyer O, Munch JC, Kaiser EA (1999) Spatial heterogeneity within the plough layer: high variability of N2O emissions rates. Soil Biol Biochem 31:167–173
Ruser R, Schulz R (2015) The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils–a review. J Plant Nutr Soil Sc 178:171–188
Ruser R, Fuß R, Andres M, Hegewald H, Kesenheimer K, Köbke S, Räbiger T, Suarez Quinones T, Augustin J, Christen O, Dittert K, Kage H, Lewandowski I, Prochnow A, Stichnothe H, Flessa H (2017) Nitrous oxide emissions from winter oilseed rape cultivation. Agric Ecosyst Environ 249:57–69
Russenes AL, Korsaeth A, Bakken L, Dörsch P (2016) Spatial variation in soil pH controls off-season N2O emission in an agricultural soil. Soil Biol Biochem 99:36–46
Scarlat N, Dallemand J-F, Fahl F (2018) Biogas: Developments and perspectives in Europe. Renewable Energy 129:457–472
Severin M, Fuß R, Well R, Hähndel R, Van den Weghe H (2016) Greenhouse gas emissions after application of digestate: short-term effects of nitrification inhibitor and application technique effects. Arch Agron Soil Sci 62:1007–1020
Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM (2006) Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbiol 8:214–222
Smeets EMW, Bouwman LF, Stehfest E, Van Vuuren DP, Posthuma A (2009) Contribution of N2O to the greenhouse gas balance of first-generation biofuels. Glob Change Biol 15:1–23
Smith AB, Cullis BR, Thompson R (2005) The analysis of crop cultivar breeding and evaluation trials: an overview of current mixed model approaches. J Agric Sci 143:449–462
Stehfest E, Bouwman AL (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosyst 74:207–228
Styles D, Gibbsons J, Williams AP, Dauber J, Stichnothe H, Urban B, Chadwick DR, Jones DL (2015) Consequential life cycle assessment of biogas, biofuel and biomass energy options within an arable crop rotation. GCB Bioenergy 7:1305–1320
Van Nguyen Q, Wu D, Kong X, Bol R, Petersen SO, Jensen LS, Liu S, Brüggemann N, Glud RN, Larsen M, Bruun S (2017) Effects of cattle slurry and nitrification inhibitor application on spatial soil O2 dynamics and N2O production pathways. Soil Biol Biochem 114:200–209
Volpi I, Laville P, Bonari E, Di Nasso N, Bosco S (2017) Improving the management of mineral fertilizers for nitrous oxide mitigation. The effect of nitrogen fertilizer type, urease and nitrification inhibitors in two different textured soils. Geoderma 307:181–188
Walter K, Don A, Fuß R, Kern J, Drewer J, Flessa H (2015) Direct nitrous oxide emissions from oilseed rape cropping – a meta-analysis. GCB Bioenergy 7:1260–1271
Wolf U, Fuß R, Höppner F, Flessa H (2014) Contribution of N2O and NH3 to total greenhouse gas emission from fertilization. Results from a sandy soil fertilized with nitrate and biogas digestate with and without nitrification inhibitor. Nutr Cycl Agroecosyst 100:121–134
Wolfinger R (1993) Covariance structure selection in general mixed models. Commun Statist-Simulat Comput 22:1079–1106
Wu D, Senbayram M, Well R, Brüggemann N, Pfeiffer B, Loick N, Stempfhuber B, Dittert K, Bol R (2017) Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol Biochem 104:197–207
Yamulki S, Harrison RM, Goulding KWT, Webster CP (1995) Effect of fertilizer application on NO and N2O fluxes from agricultural fields. J Geophys Res 100:923–925
Acknowledgements
The authors are deeply grateful to the staff of the experimental farms, the numerous technical staff and students. The study was funded by the German Federal Ministry of Food and Agriculture and managed by the Agency for Renewable Resources under grants 22403212, 22403312, 22403412, 22403512, 22403712, 22403812, and 22403912. We also acknowledge the financial support of the Union for the Promotion of Oil and Protein Plants, Germany. We also thank Guillermo Hernandez-Ramirez for reading and commenting on the drafted manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original publication of the article headings in Table 6 were incorrectly published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kesenheimer, K., Augustin, J., Hegewald, H. et al. Nitrification inhibitors reduce N2O emissions induced by application of biogas digestate to oilseed rape. Nutr Cycl Agroecosyst 120, 99–118 (2021). https://doi.org/10.1007/s10705-021-10127-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-021-10127-8