Abstract
In the aquaculture industry, silica nanoparticles (SiNPs) have great significance, mainly for confronting diseases. Therefore, the present study aims to assess the antibacterial efficiency of SiNPs as a versatile trial against Aeromonas veronii infection in African catfish (Clarias gariepinus). Further, we investigated the influence of SiNPs in palliating the immune-antioxidant stress biochemical, ethological, and histopathological alterations induced by A. veronii. The experiment was conducted for 10 days, and about 120 fish were distributed into four groups at random, with 30 fish each. The first group is a control that was neither exposed to infection nor SiNPs. The second group (SiNPs) was vulnerable to SiNPs at a concentration of 20 mg/L in water. The third group was experimentally infected with A. veronii at a concentration of 1.5 × 107 CFU/mL. The fourth group (A. veronii + SiNPs) was exposed to SiNPs and infected with A. veronii. Results outlined that A. veronii infection induced behavioral alterations and suppression of immune-antioxidant responses that appeared as a clear decline in protein profile indices, complement 3, lysozyme activity, glutathione peroxidase, and total antioxidant capacity. The kidney and liver function biomarkers (creatinine, urea, alkaline phosphatase, and alanine aminotransferase) and lipid peroxide (malondialdehyde) were substantially increased in the A. veronii group, with marked histopathological changes and immunohistochemical alterations in these tissues. Interestingly, the exposure to SiNPs resulted in a clear improvement in all measured biomarkers and a noticeable regeneration of the histopathological changes. Overall, it will establish that SiNPs are a new, successful tool for opposing immunological, antioxidant, physiological, and histopathological alterations induced by A. veronii infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquaculture industry supplies humans with a high source of animal protein, which is rich in nutrients (FAO 2022). Globally, the extensive production of edible fish puts fish under many stressors, particularly bacterial infections (Rajme-Manzur et al. 2021; Irshath et al. 2023). Aeromonas infection is one of the most devastating pathogenic bacteria associated with massive mortalities during fish culture (Soni et al. 2021). Aeromonas veronii is a Gram-negative bacterium responsible for huge losses in aquaculture production worldwide (Adhikary et al. 2023). In fish, previous reports address the hazards of A. veronii, including the occurrence of ulceration in Chinese long-snout catfish (Leiocassis longirostris Günther) (Cai et al. 2012), mass mortalities in dark sleeper (Odontobutis potamophila) (Liu et al. 2022), African catfish (Clarias gariepinus) (Li et al. 2019), and Nile tilapia (Oreochromis niloticus) (Reda et al. 2021).
Recently, extensive investigations have been carried out in the progress of innovative nanomaterials that are environmentally friendly, synthesized at low cost, and highly effective against pathogens (Ismail et al. 2021; Ibrahim et al. 2022; Abdel Rahman et al. 2023b). In comparison with other developed nanomaterials, silica nanoparticles (SiNPs) are characterized by higher stability chemical, mechanical, and thermal (up to 1500 °C) and contain higher contents in hydroxyl-containing groups (Singh et al. 2017). Also, SiNPs have been recorded in the food industry and agriculture owing to their safety application plus their promising effect on the development and growth of plants, particularly under stress conditions (Winkler et al. 2016; Bhat et al. 2021). They have been proven to have potent antibacterial activity as they are characterized by perfect synergic activity depending on reactive oxygen species (ROS) to destroy bacteria because of penetrating the microbial membrane (Karaman et al. 2018; Bernardos et al. 2019; Tabriz et al. 2023). In the aquaculture sector, SiNPs have a major verified role in fostering fish immunity and antioxidant response against heavy metal toxicity and thus improve the health of C. gariepinus (Mahboub et al. 2022b). More recently, SiNPs have been reported to stimulate immune parameters and enhance gene expression, and accordingly, antagonize the immune dysfunction and gene down-regulation elicited by A. veronii bacterial infection in C. gariepinus (Abdel Rahman et al. 2023a). Supplementing O. niloticus in SiNP-enriched diets boosts the ionic exchange mechanism and enhances growth efficacy and hematological picture indicated by augmenting growth indices and blood biomarkers (Alandiyjany et al. 2021; Bashar et al. 2021).
Hence, few studies assess the antibacterial effect of SiNPs. Therefore, the current perspective investigates the efficacy of the aqueous addition of SiNPs against A. veronii infection plus studying their pivotal role against immune-antioxidant suppression, hepatic-renal dysfunction, histopathological, and immunohistochemical alterations produced by A. veronii challenge in C. gariepinus.
Materials and methods
Ethical acceptance and bacterial strain (A. veronii)
By the approval number (ZU-IACUC/2/F/309/2022), the Ethical Committee for the Used Animals authorized the ongoing research at Zagazig University in Egypt. At the Aquatic Animal Medicine Department of the Faculty of Veterinary Medicine, Zagazig University, Egypt, A. veronii was recovered from naturally infected African catfish. Additionally, its pathogenicity was confirmed. A. veronii was cultivated for a day at 26 °C on tryptic soy agar (TSA) of HiMedia®. One colony was picked to incubate for an additional day at 26 °C in tryptic soy broth (TSB) of HiMedia®. The pellet from the A. veronii cultured broth was extracted using a centrifuge at 3000 rpm for 10 min, and it was then suspended in a sterile phosphate-buffered saline (PBS). The lethal dose (LD50) was previously determined by Abdel Rahman et al. (2023a), which was 8.7 × 108 CFU/mL, and 1.5 × 107 CFU/mL was employed as a sub-lethal dosage in the treatment assay.
Fish rearing and experimental design
Two hundred and twenty African catfish (90 ± 6.19 g) were purchased from a private fish farm (Al-Abbassa) in Sharkia Governorate, Egypt. For acclimatization, the fish were kept for 14 days in 100-L well-aerated aquaria (10 fish/aquarium), whereas the water was partially exchanged (25%). The fish were fed a commercial diet at a percentage of 3% of their body weight. Temperature, dissolved oxygen, pH, and ammonia of the acclimating water were all monitored every day during the acclimation and trial, and they recorded 25 ± 1.5 °C, 6.4 ± 0.6 mg/L, 6.3 ± 0.2, and 0.01 ± 0.02 mg/L respectively.
For 10 days (treatment trial), about 120 fish were divided into four groups in random with 30 fish each (3 replicate/group; 10 fish/replicate). The first group (control) was neither exposed to infection nor SiNPs. The second group (SiNPs) was exposed to SiNPs at a concentration of 20 mg/L in water (Abdel Rahman et al. 2023a). The third group (A. veronii) was experimentally intraperitoneally injected with 0.2 mL of A. veronii bacterial suspension at a concentration of 1.5 × 107 CFU/mL (Li et al. 2019). The fourth group (A. veronii + SiNPs) was challenged with A. veronii and exposed to SiNPs (the same doses as the second and third groups). The SiNPs were synthesized and characterized in a recent publication (Abdel Rahman et al. 2023a). SiNPs were introduced to the aquarium water on the second day of the trial (after the manifestation of clinical signs) and continued for 10 days. To get rid of waste, siphoning was done daily. To maintain the 20 mg/L SiNP concentration after water renewal (three times weekly), a freshly produced SiNP solution was added.
Behavioral and gross observations
For 10 days, clinical signs and fish behavior in all experimental groups were recorded in each aquarium twice daily from 09:00 a.m. to 03:00 p.m. using a controlled camera with an adjustable timer according to Altman’s (1974) approach. According to Ismail et al. (2009) technique, the spiral movement, activeness, and laterality were recorded. Spiral movement refers to the fish numbers that swim in the aquarium in a spiral pattern with jerks for 3 min each day. Activeness involved fish remaining motionless in a group at the aquarium’s bottom for 3 min each day. Laterality is measured by the fish numbers that shift to the lateral side at the bottom for 3 min each day.
Meanwhile, loss of equilibrium was recorded according to Calfee et al. (2016), which referred to a fish’s failure to consistently hold itself upright in the water column once daily. According to Abdel Rahman et al. (2022), hiding behavior was observed, referring to the fish numbers that remain hidden in the tank’s corners within 3 min per day.
Sampling
After the study (10 days), fish were chosen at random (9 fish/group) to drain blood samples. A 100 mg/L of benzocaine solution was used for anesthetizing fish (Neiffer and Stamper 2009), and blood was drawn from the caudal blood vessels using anticoagulant-devoid syringes. To obtain serum samples for biochemical, immunological, and antioxidant/oxidant assays, blood samples were centrifuged at 1750 × g for 10 min. Samples from kidney and liver tissue (9 fish/group) were collected for histopathological and immunohistochemical analysis.
Assessment of biochemical parameters
The serum concentration of creatinine was estimated at a wavelength of 340 nm as described by Bartels et al. (1972) using a spectrophotometric protocol depending on the manual Centromic Gmbit kit (German). The analysis of serum urea (Catalog No. MBS9374784), alkaline phosphatase (ALP) (Catalog No. E-EL-R1109), and alanine aminotransferase (ALT) (Catalog No. MBS038444) of MyBioSource Co., CA, USA, were assayed. Moreover, serum levels of total protein (TP) (Catalog No. MBS9917835) and albumin (ALB) (Catalog No. MBS019237) were investigated. The determination was spectrophotometrically according to the standard method of their specific pamphlets using a spectrophotometer (Lambda EZ201; Perkin Elmer). The total globulin level (GLO) was calculated by subtracting ALB from TP.
Immunological and antioxidant/oxidant assays
The immune indices, including lysozymes activity (LYZ) and complement-3 (C3), were estimated in the current study. The activity of LYZ was measured using inhibition zone protocol in agarose gel plates according to the method of Lee and Yang (2002) using ELISA Kit (Bio-diagnostics, Egypt). C3 level was evaluated by immuno-turbidimetry using separated Eastbiopharm ELISA kits (Hangzhou Eastbiopharm CO., LTD., Torrance, USA) following the method of Abdollahi et al. (2016).
The total antioxidant capacity (TAC) concentration was estimated spectrophotometrically in serum following the illustrated assay of Benzie and Strain (1996). The glutathione peroxidase (GPx) and malondialdehyde (MDA) levels were measured using Sigma (MAK085) assay kits depending on the assay of Hamed et al. (2004) and Ohkawa et al. (1979).
Histopathological and immunohistochemical investigations
Sections from kidney and liver tissues were taken from all groups, then exposed to fixation in 10% buffered neutral formalin, dehydration using ascending grades of alcohol, clearance in xylene, and eventually embedded in paraffin. About 5-μm-thick paraffin sections were collected and then stained with hematoxylin and eosin (H&E) and finally examined by AmScope microscope with a digital camera (Irvine, CA, USA) according to the method of Suvarna et al. (2018).
Following the ABC method (Hsu et al. 1981), the immune-histochemical (IHC) identification of B-cell lymphoma 2 (BCL-2) and cysteine-aspartic proteases (caspase-3) proteins was carried out. An HRP/DAB detection IHC kit (ab80436 Abcam, China) was used according to the manufacturer’s approach. The tissue sections (5 μm thick) that had been formalin fixed and paraffin embedded were then dewaxed and rehydrated. The slices were covered with a 3% hydrogen peroxide solution for 10 min to suppress endogenous peroxidase. Sections were boiled in a solution containing 10 mM sodium citrate buffer (pH 6.0) for 25 min in a microwave to retrieve the antigen (ab64236, Abcam, China).
The non-specific binding was prevented for 60 min using 2% bovine serum albumin in PBS. The sections were incubated with 10 μg/mL primary antibodies against caspase-3 (purified rabbit polyclonal anti-caspase-3 antibody at a dilution of 1:750 of Cell Signaling Technology, Danvers, MA, USA; 9661) and BCL-2 (a mouse anti-human BCL-2 antibody at a dilution of 1:50 of DAKO, Glostrup, Denmark; M0887) overnight at 4 °C. The conjugate was then treated with mouse-specific HRP for 15 min at room temperature. Sections from tissues were counterstained with DAB and Mayer’s hematoxylin to verify the occurrence of an immunostain reaction. The sections were negatively controlled by being dipped in PBS to replace the specific antibody. According to Metwally et al. (2018) protocol, the lesion was graded by computing the percentage of positive cells relative to the total number of cells in the image.
Data analysis
Firstly, the obtained data were checked for normality using Shapiro-Wilk’s test. One-way analysis of variance (ANOVA) was applied to examine the results of ethological, biochemical, and immuno-antioxidant variables using SPSS version 21 of IBM Corp. (Armonk, USA). Duncan’s multiple range tests were used at a significance level of 0.05 to record the variances between means.
Results
Behavioral alterations and clinical signs
Table 1 shows the recorded behaviors where no significant changes (p > 0.05) were noted in the hiding and activeness between the SiNP group and the control. The spiral movement, loss of equilibrium, and laterality were not recorded in the SiNPs and the control groups. A. veronii infection induced a significant appearance and increased these behaviors. Meanwhile, marked minimizing in these alterations was obvious in the A. veronii + SiNP group relative to the A. veronii group.
No abnormal signs were seen in the control or SiNP group (Fig. 1A). In contrast, several clinical observations in the A. veronii group included dark body coloration, excess mucus section, fin rot, and body hemorrhages, especially at the barbles and fins (Fig. 1B–E). SiNP exposure to A. veronii-infected fish recovered the prior clinical observations except for moderate fin rot at the caudal fin, which is exhibited in some fish (Fig. 1F).
Effect of A. veronii infection and/or SiNPs (20 mg/L) exposure on the clinical observation of African catfish for 10 days. A Fish of the control or SiNP groups exhibiting no abnormal signs. B–E The fish of the A. veronii group exhibiting dark body coloration, fin rot (dark arrows), and body hemorrhages, especially at the barbles and fins (yellow arrows). F Fish of the A. veronii + SiNP group exhibiting fin rot at the caudal fin (dark arrow)
Kidney and liver function biomarkers
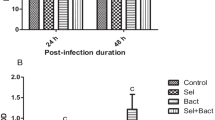
Figure 2A–D shows the serum creatinine, urea, ALP, and ALT results, with a non-significant alteration (p > 0.05) between the SiNP group and the control. These variables showed the greatest significant values (p < 0.05) in the A. veronii group. Contrarily, these biomarkers noticeably decreased in the A. veronii + SiNP group compared to the A. veronii group.
Effect of A. veronii infection and/or SiNPs (20 mg/L) exposure on the kidney and liver function biomarkers of African catfish for 10 days (n = 9/group). A Creatinine level (p < 0.0001). B Urea level (p = 0.002). C Alkaline phosphatase level (ALP; p < 0.0001). D Alanine aminotransferase level (ALT; p < 0.0001). Values (mean ± SE) that do not share the same superscripts differ significantly
Protein profile and immune response
Table 2 reveals that protein profile indices (TP, ALB, and GLO) did not significantly (p > 0.05) change in the SiNP group compared to the control; however, the immune response (LYZ and C3) was notably augmented. These biomarkers’ lowest significant (p < 0.05) values were obvious in the A. veronii group. These biomarkers of the A. veronii + SiNP group were substantially enhanced (p < 0.05) relative to the A. veronii group.
Antioxidant/oxidant response
Figure 3A–C shows a marked elevation (p < 0.05) in GPx and TAC values of the SiNP group compared to the control. Meanwhile, no marked changes were noted for MDA value. A. veronii infection induced a noticeable decline (p < 0.05) in the GPx and TAC values with an increase of MDA compared to a control group. In contrast, SiNP exposure to A. veronii-challenged fish caused a significant augmentation (p < 0.05) in GPx and TAC and decreased MDA levels compared to the A. veronii group.
Effect of A. veronii infection and/or SiNPs (20 mg/L) exposure on the antioxidant/oxidant biomarkers of African catfish for 10 days (n = 9/group). A Glutathione peroxidase level (GPx; p < 0.0001). B Total antioxidant capacity level (TAC; p < 0.0001). C Malondialdehyde level (MDA; p < 0.0001). Values (mean ± SE) that do not share the same superscripts differ significantly
Histopathological findings
The kidneys of the control and SiNP groups exhibited normal histological pictures of glomeruli and renal tubules (Fig. 4A and B). A. veronii infection induced hypo-cellular glomerular tufts, oncotic necrosis of a large number of renal tubular epithelium, and interstitial infiltration of hemopoietic tissues primarily lymphocytes and erythrocytes (Fig. 4C). On the contrary, kidney of A. veronii + SiNP group exhibited normal configuration of most glomerular and tubular structures beside the presence of hemopoietic tissues in between (Fig. 4D).
Representative photomicrographs of kidney sections (H&E). A, B The kidney of the control and SiNPs groups, respectively, exhibiting normal histological structures of glomeruli (arrowheads) and renal tubules (arrows). C The kidney of the A. veronii group exhibiting hypo-cellular glomerular tufts (arrowhead), oncotic necrosis of a large number of the renal tubular epithelium (arrow), and interstitial infiltration of hemopoietic tissues (star). D The kidney of the A. veronii + SiNP group exhibiting normal configuration of most glomerular (arrowhead) and tubular structures (arrow) beside the presence of hemopoietic tissues in between (star). Scale bar 20 μm
The liver of the control and SiNP groups exhibited normal histo-architectures of hepatocytes, sinusoids, central veins, and portal areas (Fig. 5A and B). In contrast, the liver of the A. veronii group exhibited degenerative changes of the most hepatic parenchyma, few necrotic cells, congested portal vein, and chronic cholangitis. Additionally, moderate fibrosis and inflammatory cells infiltrate within the portal area beside the presence of melanomacrophage centers were also seen (Fig. 5C). These alterations were found to be lessened in the A. veronii + SiNP group, where mildly congested central vein and prominent vacuolization were frequently seen (Fig. 5D).
Representative photomicrographs of liver sections (H&E). A, B The liver of the control and SiNP group, respectively, exhibiting normal histo-architectures of hepatocytes (arrows), sinusoids, and central vein (arrowheads). C The liver of the A. veronii group exhibiting degenerative changes of the most hepatic parenchyma, few necrotic cells (arrow), congested portal vein (arrowhead), moderate fibrosis, and inflammatory cells infiltrates within the bile duct wall (red arrow) beside the presence of melanomacrophage centers (star). D The liver of the A. veronii + SiNP group exhibiting mildly congested central vein (arrowhead) and prominent vacuolization (arrow). Scale bar 20 μm
Immunohistochemical findings
The kidney and liver tissues of the control and SiNPs groups showed potent BCL-2 immunoreactivity that appeared as brown granules inside the renal tubules (Fig. 6A and B) and hepatic cells (Fig. 7A and B), respectively. A. veronii infection caused a very weak BCL-2 immunoreactivity in the kidney tissue (Fig. 6C) and a negative immunoreactivity in the liver (Fig. 7C). A moderate immunoreactivity was noted in the kidney of A. veronii + SiNP group (Fig. 6D). In contrast, the immunoreactivity was weak in the liver (Fig. 7D).
Representative photomicrographs of immunostained kidney sections for BCL-2 immunoreactivity. A, B The kidney of the control and SiNP groups exhibiting strong immunoreactivity that appeared as brown granules inside the renal tubule cells. C The kidney of the A. veronii group exhibiting a very weak immunoreactivity. D The kidney of the A. veronii + SiNP group exhibiting moderate immunoreactivity. Scale bar 20 μm
Representative photomicrographs of immunostained liver sections for BCL-2 immunoreactivity. A, B The liver of the control and SiNPs groups exhibiting strong immunoreactivity that appeared as brown granules inside the hepatic cells. C The liver of the A. veronii group exhibiting negative immunoreactivity. D The liver of the A. veronii + SiNP group exhibiting weak immunoreactivity. Scale bar 20 μm
Figures 8 and 9 show a negative immunoreactivity of caspase-3 in the kidney and liver tissues of the control and SiNP groups (Fig. 8A and B, and Fig. 9A and B, respectively). A strong caspase-3 immunoreactivity caused by A. veronii infection was observed that appeared as brown granules inside the renal tubules cells (Fig. 8C) and hepatic cells (Fig. 9C). Contrarily, the tissues of the kidney (Fig. 8D) and liver (Fig. 9D) in the A. veronii + SiNP group were exhibited mild immunoreactivity.
Representative photomicrographs of immunostained kidney sections for caspase-3 immunoreactivity. A, B The kidney of the control and SiNP groups exhibiting negative immunoreactivity. C The kidney of the A. veronii group exhibiting strong immunoreactivity that appeared as brown granules inside the cells of the renal tubules. D The kidney of the A. veronii + SiNP group exhibiting mild immunoreactivity. Scale bar 20 μm
Representative photomicrographs of immunostained liver sections for caspase-3 immunoreactivity. A, B The liver of the control and SiNPs groups exhibiting negative immunoreactivity. C The liver of the A. veronii group exhibiting strong immunoreactivity that appeared as brown granules inside the hepatic cells. D The liver of the A. veronii + SiNP group exhibiting mild immunoreactivity. Scale bar 20 μm
Discussion
Due to the huge production in the aquaculture industry, many pathogenic infectious diseases arise that threaten fish life, specifically bacterial infections (Rajme-Manzur et al. 2021). Nowadays, there is extensive use of nanomaterials in the aquaculture sector. Nanoparticles have greatly succeeded in broad use as dietary supplements, water treatment, drug delivery, and disease control (Elabd et al. 2022). Until now, a few reports have addressed the influence of SiNPs against bacterium in African catfish. Therefore, the present work aimed to investigate the potential role of the aqueous addition of SiNPs for mitigating immune-antioxidant suppression, hepato-renal dysfunction, and histopathological and immunohistochemical alterations of African catfish experimentally infected with A. veronii.
In assessing ethological changes, the present study clarified that the infection with A. veronii induced different behavioral alterations, including spiral movement, loss of equilibrium, and laterality, as well as various clinical signs. It is assumed that the virulence genes of A. veronii plus its toxic products are responsible for its pathogenicity, which negatively affects the health and performance of fish, resulting in these abnormalities. As reported by Youssef et al. (2023), A. veronii has alt, fla, lipase, aerolysin, and act genes, identifying them as the primary cause of its pathogenicity. Another document verified virulence factors for A. veronii, including outer membrane proteins, proteases, toxins, secretory enzymes, and hemolytic and cytotoxic activities (Chen et al. 2019). Likewise, a recent study by Said et al. (2023) found that the infection of Nile tilapia in A. veronii resulted in equilibrium loss, hemorrhagic spots, loss of the scales, and fin rot.
Contrarily, the aqueous addition of SiNPs (20 mg/L) to the A. veronii challenged group regenerated these alterations and enhanced the fish behavior. It is suggested that the nano-sized SiNPs can easily penetrate cells, resulting in a direct immunomodulatory effect on the fish immune system via elevating values of immune and immunohistochemical biomarkers, as confirmed in our study, which in turn enhanced the health status of fish and, as a consequence, modulated the fish behavior.
The head kidney (anterior kidney) is a powerful hematopoietic organ in fish, while the other part of the kidney (posterior kidney) is responsible for excretion (Bates et al. 2018). The liver is the main organ that plays a pivotal role in the binding, storage, and detoxification (Mahboub and Shaheen 2021). The hepatic enzymes are crucial indicators that reflect the liver’s health status by boosting the host’s antioxidant status against pathogens. Urea and creatinine indicate kidney function (Giri et al. 2018). The current study demonstrated a dysfunction in the hepato-renal organs represented by elevation in renal biomarkers and liver enzymes upon exposure to A. veronii plus histopathological alterations in liver and kidney tissues. Such alterations induced by A. veronii could be dominated by the release of this bacterium to some virulence factors, including lipopolysaccharide (LPS), proteins, and extracellular factors hemolysins, flagella, lipases (Lip), and proteases (Ser) as previously mentioned by Hossain and Heo (2021). It is opined that these virulence factors could produce oxidative damage in the hepato-renal tissues and reflect noticeable increases in the ALP, ALT, urea, and creatinine levels, as well as histopathological alterations. Concurrently, Adhikary et al. (2023) reported severe histopathological changes in the liver, kidneys, and gills of the Indian major carp (Cirrhinus mrigala) post-exposure to A. veronii infection. Likely, Said et al. (2023) revealed that A. veronii negatively interfered with hepato-renal functions, indicated by elevated levels of ALT, ALP, creatinine, and urea in Nile tilapia as well as clear histopathological lesions in kidneys and liver tissues.
Conversely, exposure to SiNPs in A. veronii-challenged fish mitigated the histopathological alterations, regenerated the tissue picture, and improved hepato-renal function biomarkers, implying their protective role as antioxidants. SiNPs could probably lessen the A. veronii–induced ROS by penetrating smoothly through renal and hepatic cells, reflecting antioxidant protection. These findings were supported by Ravelo-Nieto et al. (2023), who declared that SiNPs are safe, perfect penetrating agent that can easily pass through biological membranes without influencing the viability of cells. A similar report by Mahboub et al. (2022b) found that SiNPs can alleviate the histopathological changes induced by heavy metal toxicity in C. gariepinus.
TP and GLO are active indicators for stimulating the humoral immune response in fish (Castro and Tafalla 2015). LYZ and complement-Cs are components of the non-specific immune system that fish mainly rely on as a primary defense mechanism after bacterial challenge (Giri et al. 2018). The current study revealed that A. veronii suppressed the immune response by lessening levels of immune biomarkers. It could be dominated by the pathogenicity of A. veronii and secretion of enterotoxins, which depress the capacity of phagocytes to exert their activity on engulfing bacterium and, accordingly, suppress the immune system as documented by Arslan and Küçüksari (2015). In line with Said et al. (2023), A. veronii suppressed the immune response of Nile tilapia by inducing a clear reduction in nitric oxide and LYZ activity. Furthermore, Reyes-Becerril et al. (2015) mentioned that A. veronii is a pathogenic species to the Pacific red snapper (Lutjanus peru) by inducing a state of immune depression.
Herein, we report the immuno-stimulatory effect for SiNPs via augmenting LYZ, C3, and TP levels. It is opined that SiNPs suppress bacterial activity and minimize the dissemination of A. veronii through their promising role in enhancing the immune response. The mechanism of action of SiNPs on innate immunity was recently described by Ganesan et al. (2022), which represented the activating of macrophages and T and B cells and enhancing the release of immunoglobulins. Concurrent with a new report, Abdel Rahman et al. (2023a) verified the immunomodulatory role of SiNPs through elevating levels of nitric oxide and immunoglobulin plus up-regulation of the immune-associated genes as interleukins (IL-8 and IL-1β) in C. gariepinus. Also, Mahboub et al. (2022b) reported that the aqueous exposure of C. gariepinus to SiNPs up-regulated the cytokines IL-1β and IL-8, which play a key role in regulating the immune response.
GPx is a crucial enzyme responsible for ROS detoxifying and protecting the living organism from oxidative stress (Malandrakis et al. 2014). TAC indicates oxidative damage or increased vulnerability to oxidative stress (Young 2001). MDA is one of the most substantial products of oxidation and is mainly monitored as a marker of oxidative stress (Mendes et al. 2009; Mahboub et al. 2022a). Herein, we report the dangerous impact of A. veronii on the antioxidant defense mechanism via altering oxidative/antioxidant biomarkers (elevating MDA and decreasing GPx and TAC). It is assumed that the oxidative stress produced by A. veronii is because it produces extracellular enzymes that suppress the antioxidant system. These findings were supported by Yang et al. (2020) and Zhu et al. (2022), who clarified that the oxidative stress produced by A. veronii is because of the elevated levels of ROS-generating cytotoxicity. Our study underlines the promising influence of the aqueous addition of SiNPs on antagonizing oxidative damage induced by A. veronii via modulating the biomarkers mentioned above, which confirms the potential role of SiNPs as a potent antioxidant. Likewise, a recent document by Abdel Rahman et al. (2023a) mentioned the occurrence of oxidative damage following exposure of C. gariepinus to A. veronii manifested by a reduction in the catalase, superoxide dismutase, and reduced glutathione content and modulation in these biomarkers in the exposed group to SiNPs. The promising influence of SiNPs could be returned to their effect on sustained cell viability because their nano-scale size enables them to penetrate cells and exert their direct effect against the bacterium. In the same manner, Abdel Rahman et al. (2023b) reported the antioxidant efficacy of magnetite nanoparticles in eliminating oxidative damage induced by Aeromonas sobria in C. gariepinus.
Apoptosis represents a programmed cell death essential to tissue homeostasis by removing the damaged cells (Raducka-Jaszul et al. 2020). It is monitored by various cellular signaling mechanisms (Edinger and Thompson 2004) and biochemical pathways, which induce cellular alterations involving shrinkage, fragmentation of the nucleus, condensation of chromatin, and fragmentation of DNA, and eventually cause the death of cells (Elmore 2007). Caspase-3 is an important apoptotic marker stimulated when apoptosis is commenced (Wong 2011). Intrinsic apoptosis represents an interaction of the BCL-2 and its membranes (Banjara et al. 2020). Herein, we report a strong apoptosis that appeared as a disturbance of BCL-2 and an upregulation of caspase-3 immuno-historeaction in the kidney and liver tissue of the A. veronii-challenged group. This finding was supported by previous studies that linked pathogen apoptosis, cytotoxicity, and DNA damage to the expression of related genes that contribute to hosting adherence, colonization, and occurrence of infection (Hoel et al. 2017; Srivastava et al. 2017). Another report supported our findings and clarified that A. veronii up-regulated many inflammatory genes and down-regulated 52 protein-coding genes influencing chromatin regulation and numerous minute nuclear RNAs, plus 55 cytoplasmic tRNAs. Such impacts induced apoptosis via intrinsic pathways (Lee and Lio 2023).
Contrarily, adding SiNPs generated the BCL-2 immunoreactivity and produced weak apoptosis as a down-regulation of the caspase-3 in the A. veronii-infected group. This could be an outcome of the protective impact of the nano-sized SiNPs that can easily penetrate cells and protect tissue against the bacterium, resulting in an elevation in the resistance of cells against apoptosis via mitigating apoptotic signaling induced by A. veronii reflecting a potent BCL-2 immunoreactivity. Also, the protecting effect of SiNPs against apoptosis could be returned to the reduction of ROS generation, which, in turn, SiNPs can protect cells, as recently documented by Kretowski et al. (2021).
Conclusion
Building on the outcomes, SiNPs are a new, successful tool to avoid the drawbacks of A. veronii bacterial infection in C. gariepinus. The aqueous SiNPs (20 mg/L) have a potent antibacterial agent that lessens behavioral changes and improves health status. Moreover, SiNPs noticeably boost the hepato-renal function, immune-antioxidant capacity, and histological parameters after exposure to the A. veronii challenge. Further studies are needed to investigate other antimicrobial roles of SiNPs and assess their efficacy on other fish species.
Data availability
All data generated or analyzed during this study are included in this article.
References
Abdel Rahman AN, Mohamed AA, Dahran N, Farag MFM, Alqahtani LS, Nassan MA, AlThobaiti SA, El-Naseery NI (2022) Appraisal of sub-chronic exposure to lambada-cyhalothrin and/or methomyl on the behavior and hepato-renal functioning in Oreochromis niloticus: supportive role of taurine-supplemented feed. Aquat Toxicol 250:106257. https://doi.org/10.1016/j.aquatox.2022.106257
Abdel Rahman AN, Elsheshtawy HM, Yassin EMM, Omran A, Hashem MA, Eltanahy A, Khamis T, Ismail SH, Yousefi M, Mahboub HH (2023a) Hematological, immuno-antioxidant disruptions, and genes down-regulation induced by Aeromonas veronii challenge in Clarias gariepinus: the ameliorative role of silica nanoparticles. Fish Shellfish Immunol 18:108842. https://doi.org/10.1016/j.fsi.2023.108842
Abdel Rahman AN, Masoud SR, Fouda MMS, Abdelwarith AA, Younis EM, Khalil SS, Zaki HT, Mohammed E, Davies SJ, Ibrahim RE (2023b) Antimicrobial efficacy of magnetite nanoparticles against Aeromonas sobria challenge in African catfish: biochemical, protein profile, and immuno-antioxidant indices. Aquac Rep 32:101692. https://doi.org/10.1016/j.aqrep.2023.101692
Abdollahi R, Heidari B, Aghamaali M (2016) Evaluation of lysozyme, complement C3, and total protein in different developmental stages of Caspian kutum (Rutilus frisii kutum K.). Arch Pol Fish 24:15–22. https://doi.org/10.1515/aopf-2016-0002
Adhikary A, Hazarika G, Das R (2023) Isolation, identification and characterisation of Aeromonas veronii from diseased Indian major carp, Cirrhinus mrigala. Aquacult Int 31:997–1010. https://doi.org/10.1007/s10499-022-01012-4
Alandiyjany MN, Kishawy ATY, Hassan AA, Eldoumani H, Elazab ST, El-Mandrawy SAM, Saleh AA, ElSawy NA, Attia YA, Arisha AH, Ibrahim D (2021) Nano-silica and magnetized-silica mitigated lead toxicity: their efficacy on bioaccumulation risk, performance, and apoptotic targeted genes in Nile tilapia (Oreochromis niloticus). Aquat Toxicol 242:106054. https://doi.org/10.1016/j.aquatox.2021.106054
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–266. http://www.jstor.org/stable/4533591
Arslan S, Küçüksari R (2015) Phenotypic and genotypic virulence factors and antimicrobial resistance of motile Aeromonas spp. from fish and ground beef. J Food Saf 35:551–559. https://doi.org/10.1111/jfs.12205
Banjara S, Suraweera CD, Hinds MG, Kvansakul M (2020) The Bcl-2 family: ancient origins, conserved structures, and divergent mechanisms. Review Biomolecules 10:128. https://doi.org/10.3390/biom10010128
Bartels H, Bohmer M, Heierli C (1972) Serum creatinine determination without protein precipitation. Clinica Chimica Acta 37:193–197. https://doi.org/10.1016/0009-8981(72)90432-9
Bashar A, Hasan NA, Haque MM, Rohani F, Hossain S (2021) Effects of dietary silica nanoparticle on growth performance, protein digestibility, hematology, digestive morphology, and muscle composition of Nile tilapia, Oreochromis niloticus. Front Mar Sci 8:706179. https://doi.org/10.3389/fmars.2021.706179
Bates T, Naumann U, Hoppe B (2018) Kidney regeneration in fish. J Dev Biol 62:419–429. https://doi.org/10.1387/ijdb.170335ce
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Bernardos A, Piacenza E, Sancenón F, Hamidi M, Maleki A, Turner RJ, Martínez Máñez R (2019) Mesoporous silica-based materials with bactericidal properties. Small 15:1900669. https://doi.org/10.1002/smll.201900669
Bhat JA, Rajora N, Raturi G, Sharma S, Dhiman P, Sanand S, Shivaraj SM, Sonah H, Deshmukh R (2021) Silicon nanoparticles (SiNPs) in sustainable agriculture: major emphasis on the practicality, efficacy and concerns. Nanoscale Adv 3:4019–4028. https://doi.org/10.1039/d1na00233c
Cai SH, Wu ZH, Jian JC, Lu YS, Tang JF (2012) Characterization of pathogenic Aeromonas veronii bv. veronii associated with ulcer-ative syndrome from Chinese longsnout catfish (Leiocassis longirostris Günther). Braz J Microbiol 43:382–388. https://doi.org/10.1590/S1517-83822012000100046
Calfee RD, Puglis HJ, Little EE, Brumbaugh WG, Mebane (2016) Quantifying fish swimming behavior in response to acute exposure of aqueous copper using computer assisted video and digital image analysis. J Vis Exp:e53477. https://doi.org/10.3791/53477
Castro R, Tafalla C (2015) Overview of fish immunity. Mucosal Health in Aquaculture 3–54. https://doi.org/10.1016/B978-0-12-417186-2.00002-9
Chen F, Sun J, Han Z, Yang X, Xian J-a, L-v A, Hu X, Shi H (2019) Isolation, identification and characteristics of Aeromonas veronii from diseased crucian carp (Carassius auratus gibelio). Front Microbiol 10:2742. https://doi.org/10.3389/fmicb.2019.02742
Edinger AL, Thompson CB (2004) Death bydesign: apopto-sis, necrosis, and autophagy. Curr Opin Cell Biol 16:663–669. https://doi.org/10.1016/j.ceb.2004.09.011
Elabd H, Youssuf H, Mahboub HH, Salem SMR, Husseiny WA, Khalid A, El-Desouky HS, Faggio C (2022) Growth, hemato-biochemical, immune-antioxidant response, and gene expression in Nile tilapia (Oreochromis niloticus) received nano iron oxide-incorporated diets. Fish Shellfish Immunol 128:574–581. https://doi.org/10.1016/j.fsi.2022.07.051
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
FAO (Food and Agriculture Organization) (2022) The state of world fisheries and aquaculture (SOFIA). Rome, Italy, pp 266. https://doi.org/10.4060/cc0461en
Ganesan N, Ronsmans S, Hoet P (2022) Differential immunological effects of silica nanoparticles on peripheral blood mononuclear cells of silicosis patients and controls. Front Immunol 13:1025028. https://doi.org/10.3389/fimmu.2022.1025028
Giri SS, Yun S, Jun JW, Kim HJ, Kim SG, Kang JW, Kim SW, Han SJ, Sukumaran V, Park SC (2018) Therapeutic effect of intestinal autochthonous Lactobacillus reuteri P16 against waterborne lead toxicity in Cyprinus carpio. Front Immunol 7:1824. https://doi.org/10.3389/fimmu.2018.01824
Hamed RR, Maharem TM, Guinidi RAM (2004) Glutathione and its related enzymes in the Nile fish. Fish Physiol Biochem 30:189–199. https://doi.org/10.1007/s10695-005-3259-5
Hoel S, Vadstein O, Jakobsen AN (2017) Species distribution and prevalence of putative virulence factors in mesophilic Aeromonas spp. isolated from fresh retail sushi. Front microbiol 8:931. https://doi.org/10.3389/fmicb.2017.00931
Hossain S, Heo GJ (2021) Ornamental fish: a potential source of pathogenic and multidrug resistant motile Aeromonas spp. Lett Appl Microbiol 72:2–12. https://doi.org/10.1111/lam.13373
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580. https://doi.org/10.1177/29.4.6166661
Ibrahim RE, Elshopakey GE, Abd El-Rahman GI, Ahmed AI, Altohamy DE, Zaglool AW, Younis EM, Abdelwarith AA, Davies SJ, Al-Harthi HF, Abdel Rahman AN (2022) Palliative role of colloidal silver nanoparticles synthetized by moringa against Saprolegnia spp. infection in Nile tilapia: biochemical, immuno-antioxidant response, gene expression, and histopathological investigation. Aquacult Rep 26:101318. https://doi.org/10.1016/j.aqrep.2022.101318
Irshath AA, Rajan AP, Vimal S, Prabhakaran VS, Ganesan R (2023) Bacterial pathogenesis in various fish diseases: recent advances and specific challenges in vaccine development. Vaccines 11:470. https://doi.org/10.3390/vaccines11020470
Ismail M, Ali R, Ali T, Waheed U, Boec KQMJ (2009) Evaluation of the acute toxicity of profenofos and its effects on the behavioral pattern of fingerling common carp (Cyprinus carpio L., 1758). Toxicology 82:569–573. https://doi.org/10.1007/s00128-009-9670-3
Ismail SH, Hamdy A, Ismail TA, Mahboub HH, Mahmoud WH, Daoush WM (2021) Synthesis and characterization of antibacterial carbopol/ZnO hybrid nanoparticles gel. Crystals 11:1092. https://doi.org/10.3390/cryst11091092
Karaman DŞ, Manner S, Rosenholm JM (2018) Mesoporous silica nanoparticles as diagnostic and therapeutic tools: How can they combat bacterial infection? Ther Deliv 9:241–244. https://doi.org/10.4155/tde-2017-0111
Kretowski R, Kusaczuk M, Naumowicz M, Cechowska-Pasko M (2021) The pro-apoptotic effect of silica nanoparticles depends on their size and dose, as well as the type of glioblastoma cells. Int J Mol Sci 22:3564. https://doi.org/10.3390/ijms22073564
Lee SA, Lio F (2023) Emerging Aeromonas enteric infections: their association with inflammatory bowel disease and novel pathogenic mechanisms. Microbiol Spectr 11. https://doi.org/10.1128/spectrum.01088-23
Lee YC, Yang D (2002) Determination of lysozyme activities in a microplate format. Anal Biochem 310:223. https://doi.org/10.1016/s0003-2697(02)00320-2
Li Z, Wang X, Chen C, Gao J, Lv A (2019) Transcriptome profiles in the spleen of African catfish (Clarias gariepinus) challenged with Aeromonas veronii. Fish Shellfish Immunol 86:858–867. https://doi.org/10.1016/j.fsi.2018.12.029
Liu G, Li J, Jiang Z, Zhu X, Gao X, Jiang Q, Wang J, Wei W, Zhang X (2022) Pathogenicity of Aeromonas veronii causing mass mortalities of Odontobutis potamophila and its induced host immune response. Fish Shellfish Immunol 125:180–189. https://doi.org/10.1016/j.fsi.2022.05.009
Mahboub HH, Shaheen AA (2021) Mycological and histopathological identification of potential fish pathogens in Nile tilapia. Aquaculture 530:735849. https://doi.org/10.1016/j.aquaculture.2
Mahboub HH, Nada HS, Abdel-Ghany HM, Ghanem R, Ismail TA, Abdel Rahman AN (2022a) Detection, diagnosis, Koch’s postulate, hepatorenal and antioxidant indicators for some systemic pathogenic fungi invading the liver and kidneys of African catfish (Clarias gariepinus) in Egypt with a histopathological approach. Aquac Res 53:2670–2685. https://doi.org/10.1111/are.15783
Mahboub HH, Shahin K, Mahmoud SM, Altohamy DE, Husseiny WA, Mansour DA, Shalaby SI, Gaballa MMS, Shaalan M, Alkafafy M, Abdel Rahman AN (2022b) Silica nanoparticles are novel aqueous additive mitigating heavy metals toxicity and improving the health of African catfish, Clarias gariepinus. Aquat Toxicol 249:106238. https://doi.org/10.3354/dao03531
Malandrakis EE, Exadactylos A, Dadali O, Golomazou E, Klaoudatos S, Pan-agiotaki P (2014) Molecular cloning of four glutathione peroxidase (GPx) homologs and expression analysis during stress exposure of the marine teleost Sparus aurata. Comp Biochem Physiol B Biochem Mol Biol 168:53–61. https://doi.org/10.1016/j.cbpb.2013.11.005
Mendes R, Cardoso C, Pestana C (2009) Measurement of malondialdehyde in fish: a comparison on study between HPLC methods and the traditional spectrophotometric test. Food Chem 112:1038–1045. https://doi.org/10.1016/j.foodchem.2008.06.052
Metwally MM, Ebraheim LL, Galal AA (2018) Potential therapeutic role of melatonin on STZ-induced diabetic central neuropathy: a biochemical, histopathological, immunohistochemical and ultrastructural study. Acta Histochem 120:828–836. https://doi.org/10.1016/j.acthis.2018.09.008
Neiffer DL, Stamper MA (2009) Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J 50:343–360. https://doi.org/10.1093/ilar.50.4.343
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–335
Raducka-Jaszul O, Bogusławska DM, Edruchniewicz NJ, Sikorski AF (2020) Role of extrinsic apoptotic signaling pathway during definitive erythropoiesis in normal patients and in patients with Thalassemia. Review Int J Mol Sci 21:3325. https://doi.org/10.3390/ijms21093325
Rajme-Manzur D, Gollas-Galvan T, Vargas-Albores F, Martínez-Porchas M, Hernández-Oñate M-Á, Hernández-López J (2021) Granulomatous bacterial diseases in fish: An overview of the host’s immune response. Comp Biochem Physiol A Mol Integr Physiol 261:111058. https://doi.org/10.1016/j.cbpa.2021.111058
Ravelo-Nieto E, Cifuentes J, Ruiz Puentes P, Rueda-Gensini L, Quezada V, Ostos C, Muñoz-Camargo C, Reyes LH, Duarte-Ruiz A, Cruz JC (2023) Unlocking cellular barriers: silica nanoparticles and fullerenol conjugated cell-penetrating agents for enhanced intracellular drug delivery. Front Bioeng Biotechnol 11:1184973. https://doi.org/10.3389/fbioe.2023.1184973
Reda RM, El-Murr A, Abd Elhakim Y, El-Shahat W (2021) Aeromonas veronii detection in Egyptian fish farms with summer tilapia mortality outbreaks and the role of formic acid in limiting its spread. Aquac Res 53:940–956. https://doi.org/10.1111/ARE.15635
Reyes-Becerril M, Angulo C, Ascencio F (2015) Humoral immune response and TLR9 gene expression in Pacific red snapper (Lutjanus peru) experimentally exposed to Aeromonas veronii. Fish Shellfish Immunol 42:289–296. https://doi.org/10.1016/j.fsi.2014.11.002
Said AA, Reda RM, Metwally MMM, Abd El-Hady HM (2023) Therapeutic efficacy of coriander (Coriandrum sativum) enriched diets in Oreochromis niloticus: effect on hepatic-renal functions, the antioxidant-immune response and resistance to Aeromonas veronii. Fish Physiol Biochem 49:687–709. https://doi.org/10.1007/s10695-023-01220-6
Singh G, Lakhi KS, Kim IY, Kim S, Srivastava P, Naidu R, Vinu A (2017) Highly efficient method for the synthesis of activated mesoporous biocarbons with extremely high surface area for high-pressure CO2 adsorption. ACS Appl Mater Interfaces 9:29782–29297
Soni M, Qureshi QA, Mishra M, Nishad CS, Chhaba B, Das SA (2021) Common Aeromonas infections in ornamental fishes: a review. Biol Forum 13:433–439
Srivastava N, Shelly A, Kumar M, Pant A, Das B, Majumdar T, Mazumder S (2017) Aeromonas hydrophila utilises TLR4 topology for synchronous activation of MyD88 and TRIF to orchestrate anti-inflammatory responses in zebrafish. Cell Death Discov 3:17067
Suvarna KS, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques, 8th edn. Churchill Livingstone, Elsevier, England
Tabriz PT, Maghsodi PH, Afarin AJ, Heydari H, Amiry F, Sazegar MR (2023) Antibacterial composite: polymeric mesoporous silica nanoparticles and combination of imipenem/meropenem. J Mater Chem B 11:1971–1977. https://doi.org/10.1039/D2TB02442J
Winkler HC, Suter M, Naegeli H (2016) Critical review of the safety assessment of nano-structured silica additives in food. J Nanobiotechnol 14:1–9
Wong RSY (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Yang B, Song H, An D, Zhang D, Raza SH, Wang G, Shan X, Qian A, Kang Y, Wang C (2020) Functional analysis of preA in Aeromonas veronii TH0426 reveals a key role in the regulation of virulence and resistance to oxidative stress. Int J Mol Sci 21. https://doi.org/10.3390/ijms21010098
Young IS (2001) Measurement of total antioxidant capacity. Clin Pathol 54:339. https://doi.org/10.1136/jcp.54.5.339
Youssef HA, Ayoub HF, Soror E-I, Matter AF (2023) Virulence genes contributing to Aeromonas veronii pathogenicity in Nile tilapia (Oreochromis niloticus): approaching the development of live and inactivated vaccines. Aquacul Inter 31:1253–1267. https://doi.org/10.1007/s10499-022-01023-1
Zhu X, Qian Q, Wu C, Zhu Y, Gao X, Jiang Q, Wang J, Liu G, Zhang X (2022) Pathogenicity of Aeromonas veronii causing mass mortality of largemouth bass (Micropterus salmoides) and its induced host immune response. Microorganisms 10. https://doi.org/10.3390/microorganisms10112198
Acknowledgements
The authors thank the Aquatic Animal Medicine Department, Faculty of Veterinary Medicine, Zagazig University, for their kind help during the experimental procedures.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Heba H. Mahboub, Wafaa M. Gad, Enas K. Aziz, Mona Abdelghany Nasr, Esraa Fahmy, Dina Mohamed Mansour, Nesma Rasheed, Hanaa S. Ali, Sameh H. Ismail, and Afaf N. Abdel Rahman: conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, and visualization. Heba H. Mahboub and Afaf N. Abdel Rahman: writing original draft and writing review and editing.
Corresponding authors
Ethics declarations
Ethics approval
The Institutional Animal Care and Use Committee of Zagazig University, Egypt, approved the experimental protocol (ZU-IACUC/2/F/309/2022), and all applicable institutional standards were followed when caring for and using animals in this study.
Consent to participate
All authors have participated in this work.
Consent for publication
All authors review and approve the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahboub, H.H., Gad, W.M., Aziz, E.K. et al. Silica nanoparticles alleviate the immunosuppression, oxidative stress, biochemical, behavioral, and histopathological alterations induced by Aeromonas veronii infection in African catfish (Clarias gariepinus). Fish Physiol Biochem 50, 767–783 (2024). https://doi.org/10.1007/s10695-023-01274-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01274-6