Abstract
This study examined whether the aerobic swimming capacity of zebrafish juveniles is affected by the exposure of the yolk-sac larvae to sublethal concentration of Microcystis aeruginosa extract (200 mg dw L−1). Critical swimming speed significantly decreased in the pre-exposed fish (9.2 ± 1.0 vs 11.3 ± 1.4 TL s−1 in the control group). Exposure did not have any significant effects on the shape of the heart ventricle, rate of skeletal abnormalities, and growth or survival rates. Decreased swimming performance due to the early and short exposure to M. aeruginosa could have negative impacts on fish in the wild.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial harmful algal blooms (CyanoHABs) constitute a growing threat for ecosystems and public health (Paerl 2014). Microcystis aeruginosa is one of the predominant species in CyanoHABs that produces the endotoxin microcystins (MCs), together with a variety of other biologically active compounds (e.g., retinoids and oestrogenic compounds, Pipal et al. 2020; b-cyclocitral and b-Ionone, Li et al. 2021). M. aeruginosa (MA) extracts have high acute toxicity to the early life stages (ELS) of fish, mainly in the form of reduced survival and elevated malformation rates (Ghazali et al. 2009; Jonas et al. 2015; Saraf et al. 2018). At sublethal exposure levels, MA extracts may have less severe but persistent effects on developing fish. Sergi et al. (2022) demonstrated that exposure of zebrafish embryos (up to hatching, 48 h post-fertilization, hpf) to MA extracts resulted in decreased swimming performance, rounder heart ventricles, and elevated rates of vertebral abnormalities at later developmental stages.
Following our recent work on the prolonged effects of MA on zebrafish (Sergi et al. 2022), here, we hypothesized that exposure to sublethal levels of MA during the yolk-sac larval stage (from hatching to swimbladder inflation, 54–96 hpf) could also induce long-lasting effects in the swimming performance and anatomy (ventricular shape, skeleton abnormalities) of zebrafish juveniles.
Materials and methods
Zebrafish culture and exposure to M. aeruginosa extracts
Yolk-sac larvae were exposed by immersion to MA extract (200 mg biomass dw L−1), in glass beakers containing 150 larvae in 200 mL medium volume. Clean water was used for the control group. All trials were performed in two independent replicates. Following a 42-h exposure period, first-feeding larvae were reared up to the metamorphosis stage (22 dpf, ca 12–13 mm total length) in clean water, following the methodology of Sergi et al. (2022). In brief, larvae were reared into cubic net pens of 4.5 L volume each (0.1 mm mesh size), at 28.0 °C (± 0.5 °C), 86–95% oxygen saturation, 14/10 h light–dark photoperiod, 520–620 μS cm−1 conductivity, and 7.1–7.6 pH. To ensure common abiotic conditions for all experimental groups, pens were positioned into one common aquarium of 40 L volume, equipped with a biological filter. Larvae were fed five times daily with Artemia nauplii (Artemia AF, INVE, Determonde, Belgium) and commercial dry microdiets (Zebrafeed, Sparos Lda, Olhao, Portugal).
The selection of the tested extract concentration was based on the results of previous studies showing that 200 mg dw L−1 is the higher level that does not affect the survival rate of zebrafish embryos (Sergi et al. 2022). To test whether the selected level was not lethal for the early larval stage too, preliminary duplicated trials were performed by subjecting zebrafish yolk-sac larvae (54–96 hpf) to five extract concentrations (0, 50, 100, 200, 400 mg dw L−1). Fifty newly hatched yolk-sac larvae were used for each replicate and condition.
In all the trials, during the exposure period, oxygen saturation levels were controlled at normal levels by a gentle aeration of the medium, through pipette tips. The exposure medium was renewed twice daily. Before every medium renewal, dead embryos were removed, and oxygen levels, water temperature, and pH were measured. The effect of MA extract concentration on the measured abiotic conditions and fish survival rate was tested by means of the Kruskal–Wallis and Mann–Whitney U tests.
Extract preparation
Cell-free crude MA extracts of lyophilized mass of M. aeruginosa (PCC 7806) culture were prepared as described in Sergi et al. (2022). Lyophilised MA mass was diluted in nanopure water and submitted to ultrasonic treatment on ice. The suspensions of broken cells were centrifuged, and the supernatants were stored at − 20 °C. Crude MA extracts contained 1.15 mg MCs L−1 at 200 mg biomass dw L−1 (Sergi et al. 2022).
Swimming performance assay and heart morphology
Relative critical swimming speed (RUcrit) was estimated by conducting incremental swimming tests, in a swimming apparatus composed of a swimming tunnel (70 cm length, 10 cm depth, 5 cm width) and two holding tanks (Koumoundouros et al. 2009). Different flow regimes were obtained using external magnetic pumps with adjustable valves. An electromagnetic flow meter (Valeport, Model 801) was used to calibrate water speed in the tunnel. After a 5-min acclimation period in static water, fish were exposed to an increasing water velocity (2 TL s−1 raise every 15 min), at 28 °C, until each individual was fatigued and unable to swim. After the swimming tests, fish were anesthetized (MS222), measured for TL, and fixed in buffered formalin. From each experimental group and replicate, 10–12 (22–24 in total) fish were tested for RUcrit.
After swimming tests, formalin-fixed specimens (4–5 per experimental replicate, 9–10 per treatment) were stained with phosphomolybdic acid and examined for cardiac ventricle shape, by micro-CT imaging (SkyScan 1172, 2.0–2.5 μm pixel size, 50 kV voltage, 199 μA, 650 ms exposure time, 0.4° rotation step, 180° total rotation). Obtained projection images were then reconstructed to cross-sections and imported in the Amira v.5.2 software (Visage Imaging, Burlington USA). Ventricle morphometry was performed on the sagittal plane, as it was defined by the 1st vertebra, the ventral tip of cleithra, and the posterior of bulbus arteriosus. The ventricle length-to-depth ratio (VL/VD) was used for the measurement of ventricle roundness (Sergi et al. 2022).
The effect of MA exposure on the RUcrit and ventricle roundness was tested using the Mann–Whitney U test.
Skeletal abnormalities and fish growth
Fish total length (TL) was measured at 22 dpf (days post-fertilization), on a random sample of 46–56 fish per replicate, individually anesthetized (2-phenoxyethanol, 0.2–0.3 mL L−1) and photographed. Fish samples were then stained for bone and cartilage (Walker and Kimmel 2007) and microscopically examined for the presence of skeletal abnormalities (e.g., deformations, missing elements, fusions). The effect of MA exposure on fish TL was tested using the Mann–Whitney U test. Differences in abnormality rates between the exposed and control groups were tested using G-test (Sokal and Rolhf 1981).
Results and discussion
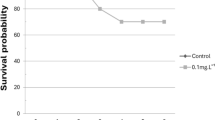
Within the tested range, results indicated that MA extract concentration did not significantly affect fish survival (p > 0.05, Fig. 1), water oxygen concentration, and pH (Table S1).
Consistent with our initial hypothesis, pre-exposure to MA extract had a significant effect on fish swimming performance at late metamorphosis (p < 0.001). Compared with controls, MA-exposed fish presented a 17.9% decrease of the mean RUcrit (9.2 ± 1.0 in the pre-exposed vs 11.3 ± 1.4 TL s−1 in the control group, mean ± SD of the pooled data) (Fig. 2A). No significant differences existed in fish TL between the control and the exposed fish which were tested for RUcrit (Table S2). The observed decrease in RUcrit (present study) is similar to that reported by Sergi et al. (2022, 14.3%) to result from zebrafish exposure to MA during the embryonic stage.
The effect of yolk-sac larval exposure to M. aeruginosa extract (200 mg dw L−.1) on the swimming performance and anatomy of zebrafish juveniles. A Critical swimming speed (RUcrit), separately for each experimental replicate (RepA, RepB), or pooled. B Mean frequency of vertebral defects (arrows in B'). C Ventricle shape (VL/VD). C' Oblique slice showing the distance measurements taken (ven, ventricle; ba, bulbus arteriosus). One, ventriculo-bulbar valve. Two, apex. Three and 4 define the maximum ventricle depth (VD), perpendicularly to ventricle length (VL). Error bars = 1 SD. Numbers in parentheses (A, C) give the size of the samples. ***p < 0.001
Our initial hypothesis was rejected in the case of abnormalities frequency (Fig. 2B, B') and ventricle shape (Fig. 2C, C'), which presented no significant differences between the pre-exposed and control fish (p > 0.05). Detected abnormalities appeared mainly in the form of abnormal haemal and neural processes of the vertebral column (Fig. 2B'). Contrarily to our results, Sergi et al. (2022) showed that MA exposure during the embryonic stage resulted to an increased (by 11 times) abnormality rate and ventricle roundness (by 13.8%) (Table 1). Similarly to findings by Sergi et al. (2022), at the end of metamorphosis, no significant differences in survival rate and fish size were observed between the pre-exposed and the control groups (p > 0.05, Fig. 3A, B).
To conclude, yolk-sac larval exposure to MA induced similar long-lasting effects on zebrafish swimming performance as in Sergi et al. (2022), but did not affect the ventricle shape and vertebral formation (Table 1). Despite the protective role of chorion, the sensitivity of zebrafish embryos to MA extracts (Table 1) might be linked to alterations of critical developmental processes, that take place before hatching and define fish development in the following stages. Somitogenesis and notochord differentiation in zebrafish take place before hatching (9–22 hpf, Kimmel et al. 1995), and, when defective, they result in the development of vertebral abnormalities during the following larval and juvenile period (Fleming et al. 2004; Lleras Forero et al. 2018). Similarly, long-lasting changes in ventricle shape (Sergi et al. 2022) might be linked with MA-induced changes of prior-to-hatching cardiac development events (e.g., chamber emergence, valvulogenesis, Glickman and Yelon 2002; Kalogirou et al. 2014). In the current study, RUcrit decrease in the MA pre-exposed fish was not linked to any cardiac-shape alterations or vertebral defects. Future studies could benefit from examining whether this decrease in RUcrit is linked to alterations of other features that are known to control aerobic swimming speed in fish (muscle physiology and/or functionality, mitochondria number, gill’s structure).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Fleming A, Keynes R, Tannahill D (2004) A central role for the notochord in vertebral patterning. Development 131:873–880. https://doi.org/10.1242/dev.00952
Ghazali IE, Saqrane S, Carvalho AP, Ouahid Y, Oudra B, Del Campo FF, Vasconcelos V (2009) Compensatory growth induced in zebrafish larvae after pre-exposure to a Microcystis aeruginosa natural bloom extract containing microcystins. Int J Mol Sci 10:133–146. https://doi.org/10.3390/ijms10010133
Glickman NS, Yelon D (2002) Cardiac development in zebrafish: coordination of form and function. Semin Cell Dev Biol 13:507–513. https://doi.org/10.1016/s1084952102001040
Jonas A, Scholz S, Fetter E, Sychrova E, Novakova K, Ortmann J, Benisek M, Adamovsky O, Giesy JP, Hilscherova K (2015) Endocrine, teratogenic and neurotoxic effects of cyanobacteria detected by cellular in vitro and zebrafish embryos assays. Chemosphere 120:321–327. https://doi.org/10.1016/j.chemosphere.2014.07.074
Kalogirou S, Malissovas N, Moro E, Argenton F, Stainier DYR, Beis D (2014) Intracardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardiovasc Res 104:49–60. https://doi.org/10.1093/cvr/cvu186
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Koumoundouros G, Ashton C, Xenikoudakis G, Giopanou I, Georgakopoulou E, Stickland N (2009) Ontogenetic differentiation of swimming performance in gilthead sea bream (Sparus aurata, Linnaeus 1758) during metamorphosis. J Exp Mar Biol Ecol 370:75–81. https://doi.org/10.1016/j.jembe.2008.12.001
Li H, Gu X, Chen H, Mao Z, Zeng Q, Yang H, Kan K (2021) Comparative toxicological effects of planktonic Microcystis and benthic Oscillatoria on zebrafish embryonic development: implications for cyanobacteria risk assessment. Environ Pollut 274:115852. https://doi.org/10.1016/j.envpol.2020.115852
Lleras Forero L, Narayanan R, Huitema LF, VanBergen M, Apschner A, Peterson-Maduro J, Logister I, Valentin G, Morelli LG, Oates AC, Schulte-Merker S (2018) Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife 7:e33843. https://doi.org/10.7554/eLife.33843.001
Paerl HW (2014) Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. eLife 4:988–1012. https://doi.org/10.3390/elife4040988
Pipal M, Priebojova J, Koci T, Blahova L, Smutna M, Hilscherova K (2020) Field cyanobacterial blooms producing retinoid compounds cause teratogenicity in zebrafish embryos. Chemosphere 241:125061. https://doi.org/10.1016/j.chemosphere.2019.125061
Saraf SR, Frenkel A, Harke MJ, Jankowiak JG, Gobler CJ, McElroy AE (2018) Effects of Microcystis on development of early life stage Japanese medaka (Oryzias latipes): comparative toxicity of natural blooms, cultured Microcystis and microcystin-LR. Aquat Toxicol 194:18–26. https://doi.org/10.1016/j.aquatox.2017.10.026
Sergi E, Orfanakis M, Dimitriadi A, Christou M, Zachopoulou A, Kourkouta Ch, Printzi A, Zervou S-K, Makridis P, Hiskia A, Koumoundouros G (2022) Sublethal exposure to Microcystis aeruginosa extracts during embryonic development reduces aerobic swimming capacity in juvenile zebrafish. Aquat Toxicol 243:106074. https://doi.org/10.1016/j.aquatox.2022.106074
Sokal R, Rohlf F (1981) Biometry. 2nd ed. WH Freeman, San Francisco, California, USA.
Walker MB, Kimmel CB (2007) A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem 82:23–28. https://doi.org/10.1080/10520290701333558
Acknowledgements
We express our thanks to Dr. Ch. Arvanitidis and Mrs. N. Keklikoglou for their contribution in ct-scanning.
Funding
Open access funding provided by HEAL-Link Greece. The study was partially funded by Greece and EU (European Social Fund-ESF, MIS-5033021, State Scholarships Foundation, IKΥ).
Author information
Authors and Affiliations
Contributions
AK: investigation, visualization, formal analysis, and writing—original draft. AD: investigation and supervision. GK: conceptualization, supervision, resources, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All the experiments were approved by the Animal Care Committee of the Biology Department, University of Crete (permit 187168/2022).
Consent to participate
Not applicable.
Consent for publication
All authors review and approve the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kekelou, A., Dimitriadi, A. & Koumoundouros, G. Sublethal exposure to Microcystis aeruginosa extracts during the yolk-sac larval stage reduces aerobic swimming speed in juvenile zebrafish. Fish Physiol Biochem 48, 1443–1447 (2022). https://doi.org/10.1007/s10695-022-01151-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01151-8