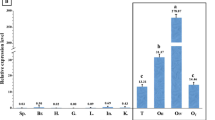
Abstract
As a member of the Sox gene family, Sox3 plays a vital role in gonadal development and gametogenesis. Nevertheless, the exact expression pattern of this gene in fish is still unknown. Here, we identified the Sox3 gene of Centropyge vrolikii, namely, Cv-Sox3. The Cv-Sox3 mRNA expression in the ovary and testis was detected by reverse transcription–polymerase chain reaction (RT-PCR) analysis, and the mRNA expression level of Cv-Sox3 in the ovary in the resting stage was significantly higher than that in other tissues. The phylogenetic tree and alignment of multiple sequences were constructed to analyze the evolutionary relationships of Cv-Sox3. Cv-Sox3 was relatively conserved in the evolution of teleost fish, indicating the importance and similarity of its function. The in situ hybridization results demonstrate that Cv-Sox3 was present in the follicle cells and cytoplasm of oocytes in the ovary of different stages, and the positive signals occurred in germ cells of the testis. After interfering with Cv-Sox3, the growth rate of ovarian cells in culture became slow, and the expression of ovary-bias-related genes Cyp19a and Foxl2 significantly increased. Meanwhile, the expression of testis-bias-related genes Dmrt1, Sox9, Cyp11a, Amh, and Sox8 significantly decreased. These results suggest that Cv-Sox3 gene might be expressed in the germ cells of male and female gonads during gonadal development. This study provides a precise expression pattern of Cv-Sox3 and demonstrates that Cv-Sox3 might play a significant role in the reproductive regulation of C. vrolikii.
Graphical abstract
In this study, Sox3 of C. vrolikii (Cv-Sox3) was cloned to understand the expression pattern in the gonadal development, which is expressed in germ cells, involved in the process of gonadal development. The results demonstrated that Cv-Sox3 may play a significant role in the reproductive regulation of C. vrolikii.









Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- C. vrolikii :
-
Centropyge vrolikii
- Cv- Sox3 :
-
Sox3 Gene of C.vrolikii
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- qRT-PCR:
-
Quantitative RT-PCR
- O-2:
-
Perinucleolus follicle
- O-4:
-
Vitellogenic follicle
- T:
-
Testis
- PFA:
-
Paraformaldehyde
- HD:
-
Homeodomain
- Mm:
-
Molecular mass
- pI:
-
Isoelectric point
- N-J method:
-
Neighbor-joining method
- ISH:
-
In situ Hybridization
- FBS:
-
Fetal bovine serum
- EGF:
-
Epidermal Growth Factor
- HGF:
-
Hepatocyte Growth Factor
- β-FGF:
-
Fibroblast Growth Factor
- CMC:
-
Carboxy Methyl Cellulose
- N-AG:
-
N-acetyl-D-( +)-glucosamine
- 2-Me:
-
2-Mercaptoethanol
- OC-2:
-
Oocytes at stage II
- OC-4:
-
Oocytes at stage IV
References
Bergstrom DE, Young M, Albrecht KH, Eicher EM (2000) Related function of mouse SOX3, SOX9, and SRY HMG domains assayed by male sex determination. Genesis 28:111–124
Brunelli S, Silva Casey E, Bell D, Harland R, Lovell-Badge R (2003) Expression of Sox3 throughout the developing central nervous system is dependent on the combined action of discrete, evolutionarily conserved regulatory elements. Genesis 36:12–24
Buston P (2003) Forcible eviction and prevention of recruitment in the clown anemonefish. Behav Ecol 14:576–582
Bylund M, Andersson E, Novitch BG, Muhr J (2003) Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci 6:1162–1168
Caetano LC, Gennaro FG, Coelho K, Araújo FM, Vila RA, Araújo A, de Melo Bernardo A, Marcondes CR, de Sousa C, Lopes SM, Ramos ES (2014) Differential expression of the MHM region and of sex-determining-related genes during gonadal development in chicken embryos. Genet Mol Res : GMR 13:838–849
Cheah PS, Thomas PQ (2015) SOX3 expression in the glial system of the developing and adult mouse cerebellum. Springerplus 4:400
Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122:509–520
Dee CT, Hirst CS, Shih YH, Tripathi VB, Patient RK, Scotting PJ (2008) Sox3 regulates both neural fate and differentiation in the zebrafish ectoderm. Dev Biol 320:289–301
Degani G (2014) Expression of SOX3 and SOX9 genes in gonads of blue gourami. Adv Biol Chem 4:322–330
Gao J, Li P, Zhang W, Wang Z, Wang X, Zhang Q (2015) Molecular cloning, promoter analysis and expression profiles of the sox3 gene in Japanese FLOunder, Paralichthysolivaceus. Int J Mol Sci 16:27931–27944
Green, E. (2003). International trade in marine aquarium species: using the Global Marine Aquarium Database. In Marine Ornamental Species, pp. 29–48.
Guiguen Y, Fostier A, Piferrer F, Chang CF (2010) Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol 165:352–366
Hargrave M, Karunaratne A, Cox L, Wood S, Koopman P, Yamada T (2000) The HMG box transcription factor gene Sox14 marks a novel subset of ventral interneurons and is regulated by sonic hedgehog. Dev Biol 219:142–153
Hong Q, Li C, Ying R, Lin H, Li J, Zhao Y, Cheng H, Zhou R (2019) Loss-of-function of sox3 causes follicle development retardation and reduces fecundity in zebrafish. Protein Cell 10:347–364
Hughes JF, Page DC (2015) The biology and evolution of mammalian Y chromosomes. Annu Rev Genet 49:507–527
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Jeng SR, Wu GC, Yueh WS, Kuo SF, Dufour S, Chang CF (2018) Gonadal development and expression of sex-specific genes during sex differentiation in the Japanese eel. Gen Comp Endocrinol 257:74–85
Jiang YH, Han KH, Wang SH, Chen Y, Wang YL, Zhang ZP (2018) Identification and expression of transcription factor sox2 in large yellow croaker Larimichthyscrocea. Theriogenology 120:123–137
Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H (1998) Involvement of Sox 1, 2 and 3 in the early and subsequent molecular events of lens induction. Development (Cambridge, England) 125:2521–2532
Kamachi Y, Uchikawa M, Kondoh H (2000) Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet : TIG 16:182–187
Katoh Y, Katoh M (2005) Comparative genomics on SOX2 orthologs. Oncol Rep 14:797–800
Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P (2002) Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev Dyn Off Publ Am Assoc Anatomists 225:384–391
Koyano S, Ito M, Takamatsu N, Takiguchi S, Shiba T (1997) The Xenopus Sox3 gene expressed in oocytes of early stages. Gene 188:101–107
Laronda MM, Jameson JL (2011) Sox3 functions in a cell-autonomous manner to regulate spermatogonial differentiation in mice. Endocrinology 152:1606–1615
Laudet V, Stehelin D, Clevers H (1993) Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res 21:2493–2501
Laumonnier F, Ronce N, Hamel BC, Thomas P, Lespinasse J, Raynaud M, Paringaux C, Van Bokhoven H, Kalscheuer V, Fryns JP et al (2002) Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet 71:1450–1455
Liman M, Wenji W, Renjie S, Quanqi Z, Jie Q, Zhigang W (2019) Characterization of Sox3 gene in an ovoviviparous teleost, black rockfish (Sebastes schlegelii). J Ocean Univ China 18:431–440
McAninch D, Thomas P (2014) Identification of highly conserved putative developmental enhancers bound by SOX3 in neural progenitors using ChIP-Seq. PLoS One 9:e113361
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Dev Growth Differ 50(Suppl 1):S195-219
Nakamura M (2009) Sex determination in amphibians. Semin Cell Dev Biol 20:271–282
Okuda Y, Yoda H, Uchikawa M, Furutani-Seiki M, Takeda H, Kondoh H, Kamachi Y (2006) Comparative genomic and expression analysis of group B1 sox genes in zebrafish indicates their diversification during vertebrate evolution. Dev Dyn Off Publ Am Assoc Anatomists 235:811–825
Oshima Y, Naruse K, Nakamura Y, Nakamura M (2009) SOX3: a transcription factor for Cyp19 expression in the frog Rana rugosa. Gene 445:38–48
Pasquier J, Cabau C, Nguyen T, Jouanno E, Severac D, Braasch I, Journot L, Pontarotti P, Klopp C, Postlethwait JH et al (2016) Gene evolution and gene expression after whole genome duplication in fish: the PhyloFish database. BMC Genomics 17:368
Pevny LH, Lovell-Badge R (1997) Sox genes find their feet. Curr Opin Genet Dev 7:338–344
Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R (1998) A role for SOX1 in neural determination. Development (cambridge, England) 125:1967–1978
Raverot G, Weiss J, Park SY, Hurley L, Jameson JL (2005) Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev Biol 283:215–225
Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ (1997a) Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn Off Publ Am Assoc Anatomists 209:323–332
Rex M, Uwanogho DA, Orme A, Scotting PJ, Sharpe PT (1997b) cSox21 exhibits a complex and dynamic pattern of transcription during embryonic development of the chick central nervous system. Mech Dev 66:39–53
Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R (2004) SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet 36:247–255
Rousseau F, Vincent A, Rivella S, Heitz D, Triboli C, Maestrini E, Warren ST, Suthers GK, Goodfellow P, Mandel JL et al (1991) Four chromosomal breakpoints and four new probes mark out a 10-cM region encompassing the fragile-X locus (FRAXA). Am J Hum Genet 48:108–116
Schepers GE, Teasdale RD, Koopman P (2002) Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell 3:167–170
Shin HS, An KW, Park MS, Jeong MH, Choi CY (2009) Quantitative mRNA expression of Sox3 and DMRT1 during sex reversal, and expression profiles after GnRHa administration in black porgy, Acanthopagrus schlegelii. Comparative biochemistry and physiology. Part b, Biochem Mol Biol 154:150–156
Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD et al (1994) Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15:342–355
Stevanović M, Lovell-Badge R, Collignon J, Goodfellow PN (1993) SOX3 is an X-linked gene related to SRY. Hum Mol Genet 2:2013–2018
Sutton E, Hughes J, White S, Sekido R, Tan J, Arboleda V, Rogers N, Knower K, Rowley L, Eyre H et al (2011) Identification of Sox3 as an XX male sex reversal gene in mice and humans. J Clin Investig 121:328–341
Takehana Y, Matsuda M, Myosho T, Suster ML, Kawakami K, Shin IT, Kohara Y, Kuroki Y, Toyoda A, Fujiyama A et al (2014) Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat Commun 5:4157
Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H (2004) Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol 24:8834–8846
Uchikawa M, Kamachi Y, Kondoh H (1999) Two distinct subgroups of group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev 84:103–120
Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT (1995) Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev 49:23–36
Watanabe M, Kawasaki K, Kawasaki M, Portaveetus T, Oommen S, Blackburn J, Nagai T, Kitamura A, Nishikawa A, Kodama Y et al (2016) Spatio-temporal expression of Sox genes in murine palatogenesis. Gene Expr Patterns : GEP 21:111–118
Wegner M (1999) From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27:1409–1420
Weiss J, Meeks JJ, Hurley L, Raverot G, Frassetto A, Jameson JL (2003) Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol 23:8084–8091
Wiebe MS, Nowling TK, Rizzino A (2003) Identification of novel domains within Sox-2 and Sox-11 involved in autoinhibition of DNA binding and partnership specificity. J Biol Chem 278:17901–17911
Woo K, Fraser SE (1995) Order and coherence in the fate map of the zebrafish nervous system. Development (cambridge, England) 121:2595–2609
Xia X, Huo W, Wan R, Zhang L, Xia X, Chang Z (2017) Molecular cloning and expression analysis of Sox3 during gonad and embryonic development in Misgurnusanguillicaudatus. Int J Dev Biol 61:565–570
Xu Y, Zhong Z, Zhang Z, Feng Y, Zhao L, Jiang Y, Wang Y (2022) Establishment and characterization of the gonadal cell lines derived from large yellow croaker (Larimichthyscrocea) for gene expression studies. Aquaculture 546:737300
Yao B, Zhou L, Wang Y, Xia W, Gui JF (2007) Differential expression and dynamic changes of SOX3 during gametogenesis and sex reversal in protogynous hermaphroditic fish. Journal of experimental zoology. Part a, Ecol Genet Physiol 307:207–219
Yu H, Xinxin D, Li X, Qu J, Zhu H, Wang X (2018) Genome-wide identification and transcriptome-based expression analysis of sox gene family in the Japanese flounder Paralichthysolivaceus. J Oceanol Limnol 36:1731–1745
Zhong Z, Ao L, Wang Y, Wang S, Zhao L, Ma S, Jiang Y (2021) Comparison of differential expression genes in ovaries and testes of Pearlscale angelfish Centropygevrolikii based on RNA-Seq analysis. Fish Physiol Biochem 47:1565–1583
Acknowledgements
Thanks to Prof. Shu-hong Wang, Fisheries College, Jimei University (Xiamen), for providing the fish for this study. Thanks to Prof. Jian-guang Qin, Flinders University (Australia), for polishing and revising the English of this manuscript.
Funding
This research was supported by the Natural Science Foundation of Fujian Province (2021J01824) and the Ornamental Aquarium Engineering Research Centre in University of Fujian Province; the Fujian Provincial Department of Education (JAT190350); and the Innovation Training program for College students of Fujian Province (202110390039; 202110390043).
Author information
Authors and Affiliations
Contributions
Yan Feng and Zhao-wei Zhong are in charge of sampling, research proposal, experiment execution, bioinformatics analysis, and article preparation. Yan Xu, Ze-yu Zhang, Lu-lu Ao, and Zhen Yang are in responsibility of the sampling, cell interference experiment, and quantitative analysis. Yong-hua Jiang and Yi-lei Wang are in responsibility of conceptualization, formal analysis, writing — review and editing, and funding acquisition. The final manuscript was reviewed and approved by all writers.
Corresponding authors
Ethics declarations
Ethics approval
The sample collection and experimental protocols were approved by the Animal Care and Use Committee of the Fisheries College of Jimei University (Animal Ethics No. 1067). All animal handling and methods were performed according to the relevant guidelines.
Consent to participate
All authors have discussed the study procedures and have been satisfied with the relevant questions, and all have agreed to participate in the study.
Consent for publication
All authors read and approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The functional domains of Cv-Sox3 were highly conserved among teleosts.
• The Cv-Sox3 was significantly expressed in the gonads and located in the germ cells.
• The expression level of Cv-Sox3 in the ovary was significantly higher than that in the testis.
• The expression of sex-related genes was regulated in different degrees after interfering with Cv-Sox3.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Zhong, Zw., Xu, Y. et al. Characterization of the transcription factor Sox3 regulating the gonadal development of pearlscale angelfish (Centropyge vrolikii). Fish Physiol Biochem 48, 1193–1207 (2022). https://doi.org/10.1007/s10695-022-01110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01110-3