Abstract
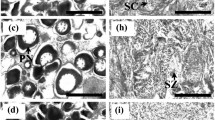
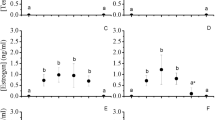
The histological process of gonadal differentiation, together with the endocrine changes of sex steroid hormones and some of their precursors, was studied in hatchery-produced greater amberjack Seriola dumerili from 101 until 408 days post-hatching (dph), with samplings conducted every 50 days. Histological processing showed that sex differentiation began at 101 dph with the formation of the ovarian cavity in females, while the presumptive males did not yet contain any germ cells in their gonad. At 150 dph, we observed the first germ cells in the developing testes. Sex differentiation in almost all sampled individuals was complete at 408 dph. No size dimorphism was observed between the sexes, and the sex ratio was 1:1, suggesting that there was no influence of early rearing in captivity on sex differentiation. Plasma concentrations of adrenosterone (Ad), androstenedione (Δ4), 11-ketotestosterone (11ΚΤ), testosterone (Τ), estradiol (Ε2), progesterone (P4) and 17,20β-dihydroxy-4-pregnen-3-one (17,20βP) were measured in males and females with the use of liquid chromatography tandem mass spectrometry (LC–MS/MS) to examine their role in the sex differentiation process. From the seven hormones, the only one that exhibited differences between the sexes was 11-KT and the plasma 11-KT concentration was found to be a useful indication of greater amberjack sex. Variations were observed in the mean values of Ad, Δ4, 11-KT, T, P4 and 17,20βP over time in one or both sexes, indicating their involvement in the sex differentiation process.






Similar content being viewed by others
Data availability
The original data of the study are available on request.
Code availability
Not applicable
References
Abbink W, Blanco Garcia A, Roques JAC, Partridge GJ, Kloet K, Schneider O (2012) The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 330–333:130–135. https://doi.org/10.1016/j.aquaculture.2011.11.043
Akhavan SR, Falahatkar B, Ward JM, Lokman PM (2019) 11-Ketotestosterone induces oocyte growth, but does not affect oocyte cytology in pre-vitellogenic captive beluga, Huso huso L. Comp Biochem Physiol b: Biochem Mol Biol 232:51–59. https://doi.org/10.1016/j.cbpb.2019.02.009
Anastasiadi D, Vandeputte M, Sánchez-Baizán N, Allal F, Piferrer F (2018) Dynamic epimarks in sex-related genes predict gonad phenotype in the European sea bass, a fish with mixed genetic and environmental sex determination. Epigenetics 13:988–1011. https://doi.org/10.1080/15592294.2018.1529504
Anonymous (1998) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 55:251–257
Aoki R, Chuda H, Washio Y, Masuma S, Kato K (2019) Sex discrimination of cultured greater amberjack Seriola dumerili using steroid hormones. Fisheries Sci. https://doi.org/10.1007/s12562-019-01379-z
Banh QQ, Domingos JA, Zenger KR, Jerry DR (2017) Morphological changes and regulation of the genes dmrt1 and cyp11b during the sex differentiation of barramundi (Lates calcarifer Bloch). Aquaculture 479:75–84. https://doi.org/10.1016/j.aquaculture.2017.05.022
Baroiller J-F, Guiguen Y, Fostier A (1999) Endocrine and environmental aspects of sex differentiation in fish. Cell Mol Life Sci 55:910–931
Bennett HS, Wyrick AD, Lee SW, McNeil JH (1976) Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol 51:71–97
Bertho S et al (2018) The unusual rainbow trout sex determination gene hijacked the canonical vertebrate gonadal differentiation pathway. Proc Natl Acad Sci U S A 115:12781–12786. https://doi.org/10.1073/pnas.1803826115
Blázquez M, Zanuy S, Carrillo M, Piferrer F (1998) Structural and functional effects of early exposure to estradiol-17ß and 17-ethynylestradiol on the gonads of the gonochoristic teleost Dicentrarchus labrax. Fish Physiol Biochem 18:37–47
Borg B (1994) Androgens in teleost fishes. Comp Biochem Physiol 109C:219–245
Bowyer JN, Booth MA, Qin JG, D’Antignana T, Thomson MJS, Stone DAJ (2014) Temperature and dissolved oxygen influence growth and digestive enzyme activities of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac Res 45:2010–2020. https://doi.org/10.1111/are.12146
Budd MA, Banh QQ, Domingos AJ, Jerry RD (2015) Sex control in fish: approaches, challenges and opportunities for aquaculture. Journal of Marine Science and Engineering 3 https://doi.org/10.3390/jmse3020329.
Budzinski H, Devier MH, Labadie P, Togola A (2006) Analysis of hormonal steroids in fish plasma and bile by coupling solid-phase extraction to GC/MS. Anal Bioanal Chem 386:1429–1439. https://doi.org/10.1007/s00216-006-0686-9
Chang C-F, Hung C-Y, Chiang M-C, Lan S-C (1999) The concentrations of plasma sex steroids and gonadal aromatase during controlled sex diffrentiation in grey mullet, Mugil cephalus. Aquaculture 177:37–45
Chen J, Fan Z, Tan D, Jiang D, Wang D (2018) A review of genetic advances related to sex control and manipulation in tilapia. J World Aquacult Soc 49:n/a-n/a https://doi.org/10.1111/jwas.12479
Chen SX, Bogerd J, Garcia-Lopez A, de Jonge H, de Waal PP, Hong WS, Schulz RW (2010) Molecular cloning and functional characterization of a zebrafish nuclear progesterone receptor. Biol Reprod 82:171–181. https://doi.org/10.1095/biolreprod.109.077644
Corriero A, Wylie MJ, Nyuji M, Zupa P, Mylonas CC (2021) Reproduction of greater amberjack (Seriola dumerili) and other members of the family Carangidae. Rev Aquacult online:1–35 https://doi.org/10.1111/raq.12544.
Crespo S, Grau A, Padrós F (1994) The intensive culture of 0-group amberjack in the western Mediterranean is compromised by disease problems. Aquacult Int 2:262–265
Depeche J, Sire O (1982) In vitro metabolism of progesterone and 17α-hydroxyprogesterone in the testis of the rainbow trout, Salmo gairdneru Rich., at different stages of spermatogenesis. Reprod Nutr Dev 22:427–438
EU (2010) Directive 2010/63/EU of the European parliament and the council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L 276:33–79
Fakriadis I, Miccoli A, Karapanagiotis S, Tsele N, Mylonas CC (2020a) Optimization of a GnRHa treatment for spawning commercially reared greater amberjack Seriola dumerili: Dose response and extent of the reproductive season. Aquaculture 521:735011. https://doi.org/10.1016/j.aquaculture.2020.735011
Fakriadis I, Sigelaki I, Papadaki M, Papandroulakis N, Raftopoulos A, Tsakoniti K, Mylonas CC (2020b) Control of reproduction of greater amberjack Seriola dumerili reared in aquaculture facilities. Aquaculture 519:734880. https://doi.org/10.1016/j.aquaculture.2019.734880
Feist G, Schreck CB, Fitzpatrick MS, Redding JM (1990) Sex steroid profiles of coho salmon (Onchorhynchus kisutch) during early development and sexual differentiation. Gen Comp Endocrinol 80:299–313
Fernández-Montero A et al (2017) Effect of temperature on growth performance of greater amberjack Seriola dumerili (Risso 1810) Juveniles. Aquac Res 49:908–918. https://doi.org/10.1111/are.13537
Gonzalez A, Fernandino JI, Somoza GM (2015) Effects of 5alpha-dihydrotestosterone on expression of genes related to steroidogenesis and spermatogenesis during the sex determination and differentiation periods of the pejerrey, Odontesthes bonariensis. Comp Biochem Physiol A Mol Integr Physiol 182:1–7. https://doi.org/10.1016/j.cbpa.2014.12.003
Guiguen Y, Fostier A, Piferrer F, Chang C-F (2010) Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol 165:352–366
Jiang YX, Shi WJ, Ma DD, Zhang JN, Ying GG, Zhang H, Ong CN (2019) Male-biased zebrafish sex differentiation and metabolomics profile changes caused by dydrogesterone. Aquat Toxicol 214:105242. https://doi.org/10.1016/j.aquatox.2019.105242
Kawabe K, Kato K, Kimura J, Okamura Y, Ando K, Saito M, Yoshida K (1996) Rearing of broodstock fish and egg-taking from amberjack Seriola dumerili in Chichijima, Ogasawara Islands, Southern Japan. Aquac Sci 44:151–157. https://doi.org/10.11233/aquaculturesci1953.44.151
Kawase J, Aoki JY, Hamada K, Ozaki A, Araki K (2018) Identification of sex-associated SNPs of greater amberjack (Seriola dumerili). J Genomics 6:53–62. https://doi.org/10.7150/jgen.24788
Kobayashi Y, Nagahama Y, Nakamura M (2013) Diversity and plasticity of sex determination and differentiation in fishes. Sex Dev 7:115–125. https://doi.org/10.1159/000342009
Kohn YY, Lokman PM, Kilimnik A, Symonds JE (2013) Sex identification in captive hapuku (Polyprion oxygeneios) using ultrasound imagery and plasma levels of vitellogenin and sex steroids. Aquaculture 384–387:87–93. https://doi.org/10.1016/j.aquaculture.2012.12.020
Koumoundouros G, Divanach P, Anezaki L, Kokkari C, Sterioti A, Divanach P, Kentouri M (2002) Temperature sex determination in the European sea bass, Dicentrarchus labrax (L., 1758) (Teleostei, Perciformes, Moronidae): critical sensitive ontogenetic phase. J Exp Zool 292:573–579
Kozul V, Skaaramuca B, Kraljevic M, Dulcic J, Glamuzina B (2001) Age, growth and mortality of the Mediterranean amberjack Seriola dumerili (Risso 1810) from the south-eastern Adriatic Sea. J Appl Ichthyol 17:134–141
Li M, Sun L, Wang D (2019) Roles of estrogens in fish sexual plasticity and sex differentiation. Gen Comp Endocrinol 277:9–16. https://doi.org/10.1016/j.ygcen.2018.11.015
Liang YQ et al (2015) Long-term exposure to environmentally relevant concentrations of progesterone and norgestrel affects sex differentiation in zebrafish (Danio rerio). Aquat Toxicol 160:172–179. https://doi.org/10.1016/j.aquatox.2015.01.006
Liu S et al (2000) Expression of cytochrome P45011b gene during gonadal sex differentiation and spermatogenesis in rainbow trout Oncorhynchus mykiss. J Steroid Biochem 75:291–298
Lokman PM, George KA, Divers SL, Algie M, Young G (2007) 11-Ketotestosterone and IGF-I increase the size of previtellogenic oocytes from shortfinned eel, Anguilla australis, in vitro. Reproduction 133:955–967. https://doi.org/10.1530/REP-06-0229
Marino G, Mandich A, Massari A, Andaloro F, Porrello S (1995a) Aspects of reproductive biology of the Mediterranean amberjack (Seriola dumerilii Risso) during the spawning period. J Appl Ichthyol 11:9–24
Marino G, Porrello S, Andarolo F, Massari A, Mannich A (1995b) Aspects of reproductive biology of Mediterranean amberjack (Seriola dumerilii Risso. 1810): Gonadal development. Cahiers Options Méditerranéenes 16:115–124
McDowell EM, Trump BF (1976) Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 100:405–414
Metcalfe JD, Craig JF (2011) Ethical justification for the use and treatment of fishes in research: an update. J Fish Biol 78:393–394. https://doi.org/10.1111/j.1095-8649.2010.02900.x
Micale V, Genovese L, Greco S (1998) Gonadal development in cultured amberjack Seriola dumerili (Risso, 1810). Anim Biol 7:125–130
Micale V, Maricchiolo G, Genovese L (1997) Hormonal stimulation and induced maturation in Seriola dumerili (Risso, 1810). Biol Mar Mediterr 4:327–329
Micale V, Maricchiolo G, Genovese L (1999) The reproductive biology of the amberjack, Seriola dumerilii (Risso 1810). I. Oocyte development in captivity. Aquac Res 30:349–355. https://doi.org/10.1046/j.1365-2109.1999.00336.x
Mylonas CC et al (2005) Influence of rearing temperature during the larval and nursery periods on growth and sex differentiation in two Mediterranean strains of Dicentrarchus labrax. J Fish Biol 67:652–668. https://doi.org/10.1111/j.0022-1112.2005.00766.x
Mylonas CC, Papandroulakis N, Smboukis A, Papadaki M, Divanach P (2004) Induction of spawning of cultured greater amberjack (Seriola dumerili) using GnRHa implants. Aquaculture 237:141–154. https://doi.org/10.1016/J.Aquaculture.2004.04.015
Mylonas CC, Zohar Y, Pankhurst NW, Kagawa H (2011) Reproduction and broodstock management. In: Pavlidis M, Mylonas CC (eds) Sparidae: Biology and Aquaculture of Gilthead Seabream and Related Species. Blackwell Science Publishers, London, pp 95–131
Nagahama Y (1994) Endocrine regulation of gametogenesis in fish. Int J Dev Biol 38:217–229
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Dev Growth Differ 50:S195–S219. https://doi.org/10.1111/j.1440-169X.2008.01019.x
Nakamura M (1984) Effects of Estradiol-1ß on gonadal sex differentiation in two species of salmonids, the masu salmon, Oncorhynchus masou and the chum salmon, Oncohynchus keta. Aquaculture 43: 83–90
Nakamura M, Nagahama Y (1993) Ultrastructural study on the differentiation and development of steroid-producing cells during ovarian differentiation in the amago salmon, Oncorhynchus rhodurus. Aquaculture 112:237–251
Navarro-Martín L, Blázquez M, Viñas J, Joly S, Piferrer F (2009) Balancing the effects of rearing at low temperature during early development on sex ratios, growth and maturation in the European sea bass (Dicentrarchus labrax).: limitations and opportunities for the production of highly female-biased stocks. Aquaculture 296:347–358. https://doi.org/10.1016/j.aquaculture.2009.07.022
Nouri MZ, Kroll KJ, Webb M, Denslow ND (2020) Quantification of steroid hormones in low volume plasma and tissue homogenates of fish using LC-MS/MS. Gen Comp Endocrinol 296:113543. https://doi.org/10.1016/j.ygcen.2020.113543
Ospina-Álvarez N, Piferrer F (2008) Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 3(7):e2837. https://doi.org/10.1371/journal.pone.0002837
Ozaki Y, Higuchi M, Miura C, Yamaguchi S, Tozawa Y, Miura T (2006) Roles of 11beta-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology 147:5139–5146. https://doi.org/10.1210/en.2006-0391
Papadaki M, Piferrer F, Zanuy S, Maingot E, Divanach P, Mylonas CC (2005) Growth, sex differentiation and gonad and plasma levels of sex steroids in male- and female-dominant populations of Dicentrarchus labrax L. obtained through repeated size grading. J Fish Biol 66:938–956. https://doi.org/10.1111/j.0022-1112.2005.00639.x
Pastor E, Grau A, Riera F, Pou S, Massuti E, Grau AM (2000) Experiences in the culture of new species in the “Estacion de Acuicultura” of the Balearic Government (1980–1998). In: Basurco B (ed) Cahiers Options Méditerranéennes, vol 47. Mediterranean Marine Aquaculture Finfish Species Diversification. C.I.H.E.A.M, Zaragoza, Spain, pp 371–379
Pavlidis M, Koumoundouros G, Sterioti A, Somarakis S, Divanach P, Kentouri M (2000) Evidence of temperature-dependent sex determination in the European sea bass (Dicentrarchus labrax L.). J Exp Zool 287:225–232
Pérez JA et al (2020) The ontogeny of greater amberjack digestive and antioxidant defence systems under different rearing conditions: a histological and enzymatic approach. Aquacult Nutr 26:1908–1925. https://doi.org/10.1111/anu.13128
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Piferrer F, Anastasiadi D, Valdivieso A, Sanchez-Baizan N, Moraleda-Prados J, Ribas L (2019) The model of the conserved epigenetic regulation of sex. Front Genet 10:857. https://doi.org/10.3389/fgene.2019.00857
Rothbard S, Moav B, Yaron Z (1987) Changes in steroid concentrations during sexual ontogenesis in tilapia. Aquaculture 83:153–166
Rougeot C, Krim A, Mandiki SN, Kestemont P, Melard C (2007) Sex steroid dynamics during embryogenesis and sexual differentiation in Eurasian perch, Perca fluviatilis. Theriogenology 67:1046–1052. https://doi.org/10.1016/j.theriogenology.2006.12.006
Saillant E, Fostier A, Menu B, Haffray P, Chatain B (2001) Sexual growth dimorphism in sea bass Dicentrarchus labrax. Aquaculture 202:371–387
Samaras A, Pavlidis M, Lika K, Theodoridi A, Papandroulakis N (2017) Scale matters: performance of European sea bass, Dicentrarchus labrax, L. (1758), reared in cages of different volumes. Aquac Res 48:990–1005. https://doi.org/10.1111/are.12942
Sarropoulou E et al (2017) Full genome survey and dynamics of gene expression in the greater amberjack Seriola dumerili. Gigascience 6:1–13. https://doi.org/10.1093/gigascience/gix108
Sarter K, Papadaki M, Zanuy S, Mylonas CC (2006) Permanent sex inversion in 1-year-old juveniles of the protogynous dusky grouper (Epinephelus marginatus) using controlled-release 17α-methyltestosterone implants. Aquaculture 256:443–456. https://doi.org/10.1016/j.aquaculture.2006.01.034
Sfakianakis DG, Papadakis IE, Papadaki M, Sigelaki I, Mylonas CC (2013) Influence of rearing temperature during early life on sex differentiation, haemal lordosis and subsequent growth during the whole production cycle in European sea bass Dicentrarchus labrax. Aquaculture 412–413:179–185. https://doi.org/10.1016/j.aquaculture.2013.07.033
Shu T, Zhai G, Pradhan A, Olsson PE, Yin Z (2020) Zebrafish cyp17a1 knockout reveals that androgen-mediated signaling is important for male brain sex differentiation. Gen Comp Endocrinol 295:113490. https://doi.org/10.1016/j.ygcen.2020.113490
Skaramuca B, Kozul V, Teskeredžić Z, Bolotin J, Onofri V (2001) Growth rate of tank-reared Mediterranean amberjack, Seriola dumerili (Risso 1810) fed on three different diets. J Appl Ichthyol 17:130–133
Smith GH, Murie DJ, Parkyn DC (2014) Nonlethal sex determination of the greater amberjack, with direct application to sex ratio analysis of the Gulf of Mexico stock. Mar Coast Fish 6:200–210. https://doi.org/10.1080/19425120.2014.927403
Thompson BA, Beasly M, Wilson CA (1999) Age distribution and growth of greater amberjack Seriola dumerili, from the north-central Gulf of Mexico. Fish B NOAA 97:362–371
Van den Hurk R, Lambert JGD, Peute J (1982) Steroidogenesis in the gonads of rainbow trout fry (Salmo gairdneri) before and after the onset of gonadal sex differentiation. Reprod Nutr Dev 22:413–425
Vizziano D, Le Gac F, Fostier A (1995) Synthesis and regulation of 17a-hydroxy-20ß-dihydroprogesterone in immature males of Oncorhynchus mykiss. Fish Physiol Biochem 14:289–299
Wang W, Zhu H, Tian Z, Sun A, Dong Y, Dong T, Hu H (2020) Effects of 11-Ketotestosterone on development of the previtellogenic ovary in the sterlet, Acipenser Ruthenus. Front Endocrinol (lausanne) 11:115. https://doi.org/10.3389/fendo.2020.00115
Xia X, Wang P, Wan R, Chang Z, Du Q (2019) Progesterone affects sex differentiation and alters transcriptional of genes along circadian rhythm signaling and hypothalamic-pituitary-gonadal axes in juvenile Yellow River Carp (Cyprinus carpio var.). Environ Toxicol 34:1255–1262. https://doi.org/10.1002/tox.22826
Yamamoto T (1969) Sex differentiation in fish. In: Hoar WS, Randal DJ (eds) Fish physiology, vol. 3. Reproduction, vol 3. vol 117–175. Academic Press, New York.
Funding
The study was supported by the project NewTechAqua (European Union´s Programme H2020, GA 862658) awarded to CCM ylonas.
Author information
Authors and Affiliations
Contributions
MPapadaki and CCMylonas designed the experiment. The fish husbandry was carried out by MA and sample collection was performed by MPapadaki, MA, NP and PK. Hormonal analyses were carried out by MM and TIA. Histological evaluations were carried out by MPapadaki and MPouli. Data analysis was performed by MPapadaki, TIA and MM. The manuscript was written by MPapadaki, MM and CCM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained by the relevant Greek authorities (National Veterinary Services) under the license No 255332 (ΑΔΑ: ΩΨ2Κ7ΛΚ-Η7Ξ). All procedures involving animals were conducted in accordance to the “Guidelines for the treatment of animals in behavioral research and teaching” (Anonymous 1998), the Ethical justification for the use and treatment of fishes in research: an update (Metcalfe and Craig 2011) and the “Directive 2010/63/EU of the European parliament and the council of 22 September 2010 on the protection of animals used for scientific purposes” (EU 2010). All authors have agreed to participate in the manuscript.
Consent for publication
All authors have agreed to submit the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Papadaki, M., Mandalakis, M., Anastasiou, T.I. et al. Histological evaluation of sex differentiation and early sex identification in hatchery-produced greater amberjack (Seriola dumerili) reared in sea cages. Fish Physiol Biochem 47, 1777–1792 (2021). https://doi.org/10.1007/s10695-021-01007-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-01007-7