Abstract
To avoid overheating of the batteries, which could lead to a fire, Lithium-ion batteries are provided with a thermal management system using refrigeration liquids. Since some of the commercial dielectric liquids used as refrigeration liquids contain halogens, their presence will contribute to a formation of hazardous emissions such as hydrofluoric acid during a potential fire. In this study, a simulation of a high temperature accident has been performed for lithium-ion batteries cooled with the direct immersion cooling systems using single-phase dielectric liquids to define their contribution to HF formation. Four commercial refrigeration liquids based on perfluoropolyethers, hydrofluoroether and polyalphaolefin were investigated in this work. By simulation of a fire, it was observed that the refrigeration liquids delayed the smoke formation by a factor of 2 to 2.5 in comparison to the case when the battery was burned without the cooling liquid. By analysis of the fluoride concentration in the washing system, it was determined that without the refrigeration liquid approximately 46.8 mmol/l of [F] was captured after the fire. When refrigeration liquids based on two perfluoropolyethers and hydrofluoroether were applied, the fluoride concentration in the washing system was 259 mmol/l, 173 mmol/l and 145 mmol/l, respectively. This work also proposed the reaction mechanisms of the refrigeration liquid´s decomposition during a fire. It was concluded that the refrigeration liquid based on polyalphaolefin does not contribute to the additional formation of hydrofluoric acid due to the chemical stability and low content of fluoride and can be considered as a more sustainable alternative for a direct cooling system for Lithium-ion batteries.
Similar content being viewed by others
1 Introduction
Lithium-ion batteries (LiBs) are now the most employed power source for portable electronic devices and fully electric and hybrid engines [1,2,3,4,5,6] since they can provide high energy and power per unit of the battery weight, as they are lighter and smaller than other rechargeable batteries [7, 8]. During the charge and discharge cycles, a significant quantity of heat is created inside of LiBs, due to the exothermic chemical reactions [9]. This generated heat negatively influences the performance, life span, and safety of LiBs [10,11,12]. Furthermore, the electric vehicles are increasingly required to use higher energy density batteries than ever before and to accumulate more cells in the pack, in order to increase the mileage that can be covered before the next charging. This trend further increases the internal heat generation and accumulation that might be expected during normal operations [13]. Further, the exothermic reactions can be triggered in the case of abuse of LiBs, such as mechanical abuse, overcharging or high temperature operation [14]. Battery failure can lead to the release of a combustible gas mixture (hydrogen, methane, organic carbonates, or propane) and under certain conditions thermal runaway can occur [15]. Also, the heat released by the exothermic reactions can cause a thermal runaway, manifested as an uncontrollable rise in the reaction rate. The mechanism of thermal runaway can be described as a chain of chemical reactions [16] that starts in a single battery cell, usually with the decomposition of the solid electrolyte interphase (SEI) layer followed by a reaction between the anode and the electrolyte, the decomposition of electrolyte, and the melting of the separator made from polyethylene (PE) or polypropylene (PP) [17]. This can result in the ejection of a large amount of dark smoke and hot sparks. The main risk is that when this process happens within the individual cells, it can propagate throughout the entire battery and it can cause an explosion [15, 18]. During the burning of LiBs, the generated toxic smoke can contain chemical components such as carbon monoxide (CO) and hydrogen fluoride (HF) [11, 19,20,21]. The main source of the flammable substances in the battery is the electrolyte. Generally, the electrolyte is based on halogens and organic solvents such as diethyl carbonate, polypropylene carbonate, and ethylene carbonate and salts. The most common salt is lithium hexafluorophosphate (LiPF6) but also other Li-salts (LiBF4, LiClO4 or LiSO3CF3) can be used. When overheated, the electrolyte will decompose and be released from the battery cells. The gases do not need to be ignited instantly. At higher temperature hydrogen fluoride (HF), phosphorus pentafluoride (PF5) and phosphoryl fluoride (POF3) can be formed as a consequence of the electrolyte and binder—polyvinylidene fluoride (PVDF) decomposition. Compounds with fluorine content can also be applied as flame retardant materials for the components such as electrolyte or separator. They can even be used as the additives for cathode and anode materials, usually in a form of e.g. fluorophosphates. The decomposition of LiPF6 salt is promoted by the presence of water/humidity according to the following reactions [21]:
To avoid thermal runaway and keep the temperature in a range that does not negatively influence the performance of the battery, the LiBs are provided with a battery thermal management system [18, 20, 22,23,24]. Generally, the surface of the battery cells in electric vehicles have been cooled with forced directed ambient or cooled air. An alternative method consists of an indirect liquid-cooled system in which a cooled water–glycol mixture is pumped through pipes and so-called cold plates close to the cells [20, 23]. A third and more effective thermal management system consist of the direct immersion in a cooling single-phase dielectric liquid [20]. These liquids are forced to circulate with a pump to ensure a constant flow to make continuous contact with all the battery modules [20, 25]. Dielectric coolants have higher thermal conductivity, density, and specific heat capacity than air and therefore perform more effectively as cooling media than air. Many of the commercial dielectric liquids used as refrigeration liquids (RL) contain halogens due to their performances in avoiding or significantly delaying thermal runaway. Cooling liquids are expected to have chemical stability at higher temperatures. However, there is limited knowledge about the formation of hydrofluoric acid in the case of high temperature accidents involving LiBs using a cooling system based on refrigeration liquids containing halogens. It is unknown if the use of these RLs could increase the quantity of toxic gas released. Such data can contribute to a more sustainable design of future batteries and knowledge about the risks associated with accidents with LiBs thermal runaway. This study aimed to determine the formation of HF in the case of a high temperature accident (at 700 ºC) involving the Li-ion batteries using the cooling system based on four commercial refrigeration liquids containing fluorine. The main goal of the work was to determine quantitively how much the selected liquids contribute to the formation of HF during the fire of LiBs.
2 Materials and Methods
2.1 Materials and Thermal Treatment
LiBs cells Samsung INR21700-50E with a weight of 69 g were cut in half to obtain representative samples with a weight of ~ 34.5 g. The cell chemistry was based on graphite/LiNi0.8Co0.15Al0.05O2. Each sample was partially immersed in 5 ml of refrigeration liquid (RL) to simulate a real operational ratio. Four commercial refrigeration liquids were tested: PFPE1 and PFPE2 (perfluoropolyethers), HFE (hydrofluoroether) and PAO (polyalphaolefin). Due to the confidentiality reasons, more exact composition of refrigeration liquids cannot be disclosed and is not exposed in any part of the article. To determine how much HF can be generated by a refrigeration liquid in a high temperature event, thermal treatment at 700°C was performed, since 700°C is the average surface temperature of the battery cells during thermal runaway [25]. The aim was to trigger the decomposition of the organic material and refrigeration liquid.
3 Equipment for the Thermal Treatment
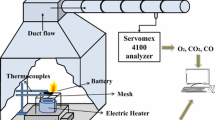
The thermal treatment was performed in a tubular furnace (Nabertherm GmbH Universal Tube Furnace RT 50-250/13). Each sample was placed in an alumina sample holder and inserted into a high-purity 65 cm alumina tube (Al2O3, 99.7%, Degussit AL23, Aliaxis). Custom-made stainless-steel connectors were added to both ends of the tube [26, 27]. When the furnace reached the selected temperature, the alumina tube was then inserted into the tubular furnace. A constant flow of approximately 340 ml/min of air was pumped through the tube, with a flowmeter used to regulate the gas flow at the system inlet. The exhaust gas was bubbled through three plastic cylinders filled with 150 ml of MilliQ water (ultrapure water with a resistivity of 18.2 MΩ cm (at 25°C) and a TOC value below 5 ppb), as shown in Figure 1. Using a thermocouple, it was controlled that the samples were not subjected to a temperature higher than 80ºC in this part of the tube. The MilliQ water from the plastic wash bottles was collected and analyzed. The MilliQ water from the gas-washing bottle directly connected with the alumina tube is referred to as B1. Consequently, the MilliQ water from the other gas-washing bottles is referred to as B2 and B3, respectively. Plastic bottles were selected to prevent HF loss via the reaction with glass vessels. All experiments and measurements were carried out in triplicates.
4 Ion Chromatography for Fluoride Analysis
Washing solutions B1-3 were analyzed using a Dionex DX100 Ion chromatograph to measure the concentration of fluorine as fluoride ions. The column used was a Dionex IonPacTM AS4A-SC RFICTM 4 × 250 mm Analytical. The eluent was a solution of 1.7 mM NaHCO3 and 1.8 mM Na2CO3.
5 Measurement of the pH in the Washing Solutions
The pH of the washing water was also measured. The pH meter was calibrated to an accuracy of ± 0.02 pH units at 25°C (Radiometer Analytical SAS) using three buffer solutions at pH 1, 4, and 7.
6 Determination of the Fluorine Content in the Refrigeration Liquids
The fluorine content of the samples was analyzed by using combustion ion chromatography, Metrohm, Switzerland. The samples were first subject to hydropyrolysis in quartz glass tube with a supply of oxygen and argon at 1050°C. Under this condition, the organic fluorine was converted into inorganic fluoride, and the carrier gas brought the formed HF to the absorption unit where the fluoride anions were formed. After that these fluoride ions were transferred to an ion exchange column for separation and the quantity was measured using conductivity detector. Details of the method can be obtained elsewhere [28]. This pyrolysis had been shown to be sufficient to convert fluoro-organics to fluoride [28,29,30]. The solvent used for dilution was also analyzed and a very low detectable level of fluorine was found (16 ng F/ml).
7 Analysis of Carbon, Hydrogen, and Fluorine
The oil by-product of the thermal treatment and washing solutions B1-3 were analyzed for carbon, hydrogen and fluorine content. The analysis of carbon and hydrogen was carried out by combustion analysis. The sample was completely and instantaneously oxidized by the combustion, which converts all the organic fluorinated hydrocarbons from the organic condensate and diluted hydrofluoric acid from the washing solution into a gas phase. The resulting combustion gases passed through a reduction furnace and were swept into the chromatographic column by the He carrier gas. Here they were separated and eluted as carbon dioxide and water and detected by a thermal conductivity detector (TCD), which gives an output signal proportional to the concentration of the individual components of the mixture. The instrument was calibrated with the analysis of known standard compounds. The fluorine was analyzed by means of combustion, followed by titration or ion chromatography.
8 Results and Discussion
8.1 The Analysis of Fluoride Content in the Refrigeration Liquids
The concentration of fluorine in the refrigeration liquids as determined through a combustion ion chromatographic analysis is shown in Table 1. PFPE1 and PFPE2 had the highest fluorine concentration. There was a very low concentration of fluorine detected in PAO.
9 Suggested Mechanism of Refrigeration Liquids Decomposition During the Fire
A combination of 1,1-difluoroethene and hexafluoropropene can be used to form a fluoroelastomer. When heated in a quartz tube to between 700°C or 900°C, smaller molecules are produced [31]. These can include the monomer which is reformed by thermally cracking the polymer. While normal alkenes are well known to undergo attack of electrophiles (electron seeking species) on the electron rich pi system of the alkene. For the highly fluorinated alkenes such as tetrafluoroethylene and hexafluoroisobutylene it is possible that they could cause similar lung injuries to those caused by phosgene. This is due to the fact that they are able to act as electrophiles on nucleophiles such as alkoxides. During the pyrolysis of the fluorinated polymer, it is likely that nucleophilic species such as water will be present. For example, if tetrafluoroethylene is combined with sodium ethoxide then it forms 1,1,2-trifluoro-2-ethoxyethene at room temperature [32]. It can be reasoned at the higher temperature that a neutral alcohol or water will be able to act as a nucleophile on the trifluoroalkene. This reaction will form both protons and fluoride anions as the side product. Thus, it will be able to form hydrogen fluoride. A possible mechanism of the reaction is proposed in Figure 2, which shows that the nucleophile attacks at the less electron poor carbon and ultimately forms pentafluoroacetone.
However, it is more likely that the nucleophile will attack at the other end of the alkene, this is due to the fact that the p orbital holding the negative charge in the carboanion shown top right will be only able to be stabilized by resonance with the trifluoromethyl group rather than the fluorine atom. This reaction will result in the formation of a carboxylic acid fluoride which will be able to react with water to form another equivalent of hydrogen fluoride and a carboxylic acid. The mechanism of described reaction is depicted in Figure 3.
While the carboxylic acid formed has a passing resemblance to fluoroacetic acid, it is unclear if the 2,3,3,3-tetrafluoropropinoic acid is toxic. It will be unable to form the fluorocitrate and thus it is likely to be far less toxic than fluoroacetate [28].
10 Ion Chromatographic Analysis of the Gas Product After Thermal Treatment
Dense white smoke was generated 2 min after the sample without RL was placed into the furnace. For the sample containing RL, the generation of the smoke started 5 min after the sample was introduced to the furnace. The amount of smoke slowly decreased but persisted until 30 min from the beginning of the heat treatment. At the end of the experiments, all RLs were decomposed to gas products, without leaving any condensed product in the sampler. The data in Table 2 show the amounts of HF released during the experiments. The use of PFPE1 contributes to the HF formation by a ratio of 5:1 ([F]RL/[F]LiB) when the weight ratio is 1:7 (wRL/wLiB) and produces the highest quantity of HF. For the PFPE1, PFPE2, and HFE only ~ 1% of the initial concentration of fluorine in the RL (Table 1) was detected by ion chromatographic analysis of the gas-washing water (Table 2). This indicates that only a small quantity of the fluoride is released as HF during the decomposition of the RLs. There is not a significant difference between the total concentration of fluorine released from thermal treatment of the LiB without refrigeration liquids and the concentration of fluorine released using PAO. Therefore, PAO did not contribute to any HF formation, whereas PFPE1, PFPE2 and HFE increased the HF formation. In common with Feldmann’s method for the collection of volatile methylated selenium species in nitric acid for the determination of the total selenium content of air [33] we choose to use multiple Dreschel bottles in series to enable an estimate of the total amount of volatile inorganic fluorine to be made. To a first approximation the amount of fluoride found in the bottles decreased exponentially as the stage number increased, taking the assumption that the amount of fluorine decreased exponentially estimates were made of the total amount of fluorine compounds which entered B1.
The difference between the estimated and captured fluorine concentration can be explained by additional fluorine coming from the battery electrolyte. The loss of fluorine is due to its reaction with metal compounds such as lithium and aluminum, which are both possible from the thermodynamic point of view as shown in the reaction mechanism summarized in Equations (4) and (5). Some loss of fluorine can also occur due to the formation of polycyclic aromatic hydrocarbons, which can be formed during the fire [34] and can be present as fluorinated.
As it is shown in Figures 4, 5 and 6, the majority of HF is captured in the first washing bottle of the washing system. The third bottle contained only residual concentration of HF.
Figure 7 shows the cumulative change of fluoride concentration in the washing bottles after the thermal treatment of all four refrigeration liquids.
11 Change of pH in the Washing System Due to HF Formation
The pH of the Milli Q water used to wash the off-gas varied depending on which RL was used (Table 3). This indicates the presence of hydrogen ions, most probably from HF. The pH increased from B1 to B3 for PFPE1, PFPE2, and HFE, corresponding to a decrease in F concentration in the washing solutions as shown in Table 2. The pH measured for PAO and for the LiBs thermal treated without refrigeration liquids has similar values in B1. In B2 and B3, RL 4 has a higher pH than LiBs without RL. Since PAO contains a negligible amount of F, this decrease is explained by the formation of other substances than HF.
12 Conclusions
The organic components of the LiBs decomposed, releasing a gas composed mainly of CO2, CO, and H2O, as already described in two previous works [26, 27]. The electrolyte and PVDF decomposed to release HF and an organic by-product rich in fluorine. It was observed that in a case of the lithium-ion battery fire the smoke formation was delayed 2 to 2.5 times when refrigeration liquid was present in comparison to the fire without the liquid. However, the use of the refrigeration liquid with a fluorine-based chemical composition leads to a consistent increase in the quantity of HF released in the event of a high temperature accident. It was determined that, without the refrigeration liquid approximately 46.8 mmol/l of [F] was captured after the fire. When refrigeration liquids based on two perfluoropolyethers and hydrofluoroether were applied, the fluoride concentration in the washing system was 259 mmol/l, 173 mmol/l and 145 mmol/l, respectively. on the other hand, the fluoride released as HF represents only a small quantity of the fluoride concentration present in the perfluoropolyethers and hydrofluoroether. In general fluoride released from those liquids represents around 1% in their initial concentration at the tested temperature. The refrigeration liquid based on polyalphaolefin did not contribute to additional HF formation and can be considered as a more environmentally friendly alternative for a cooling system.
References
Zheng X et al (2018) A mini-review on metal recycling from spent lithium ion batteries. Engineering 4:361–370
Schipper F, Aurbach D (2016) A brief review: past, present and future of lithium ion batteries. Russ J Electrochem 52:1095–1121
Zeng X, Li J, Singh N (2014) Recycling of spent lithium-ion battery: a critical review. Crit Rev Environ Sci Technol 44:1129–1165
Schmuch R, Wagner R, Hörpel G, Placke T, Winter M (2018) Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy 3:267–278
Zhang W, Xu C, He W, Li G, Huang J (2018) A review on management of spent lithium ion batteries and strategy for resource recycling of all components from them. Waste Manage Res 36:99–112
Mossali E et al (2020) Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J Environ Manage 264:110500
Chagnes A (2015) Fundamentals in electrochemistry and hydrometallurgy. Lithium Process Chem. https://doi.org/10.1016/b978-0-12-801417-2.00002-5
Hanisch C, Diekmann J, Stieger A, Haselrieder W, Kwade A (2015) Recycling of lithium-ion batteries. Handb Clean Energy Syst. https://doi.org/10.1002/9781118991978.hces221
Nazari A, Farhad S (2017) Heat generation in lithium-ion batteries with different nominal capacities and chemistries. Appl Therm Eng 125:1501–1517
Saw LH, Ye Y, Tay AAO (2013) Electrochemical-thermal analysis of 18650 lithium iron phosphate cell. Energy Convers Manag 75:162–174
Wang Q et al (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224
Sundin DW, Sponholtz S (2020) Thermal management of Li-ion batteries with single-phase liquid immersion cooling. IEEE Open J Veh Technol 1:82–92
Ma S et al (2018) Temperature effect and thermal impact in lithium-ion batteries: a review. Prog Nat Sci Mater Int 28:653–666
Andersson P, Blomqvist P, Lorén A (2013) Investigation of fire emissions from Li-ion batteries. SP Technical Research Institute of Sweden, Boras
Sun P, Bisschop R, Niu H, Huang X (2020) A review of battery fires in electric vehicles. Fire Technol. https://doi.org/10.1007/s10694-019-00944-3
Feng X et al (2014) Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J Power Sources 255:294–301
Feng X et al (2018) Thermal runaway mechanism of lithium ion battery for electric vehicles: a review. Energy Storage Mater 10:246–267
Rao Z, Wang S (2011) A review of power battery thermal energy management. Renew Sustain Energy Rev 15:4554–4571
Ribière P et al (2012) Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ Sci 5:5271–5280
Kim J, Oh J, Lee H (2019) Review on battery thermal management system for electric vehicles. Appl Therm Eng 149:192–212
Larsson F, Andersson P, Blomqvist P, Mellander BE (2017) Toxic fluoride gas emissions from lithium-ion battery fires. Sci Rep 7:1–13
Jung W (2005) Thermal management. In: Data conversion handbook. Elsevier, Amsterdam, pp 823–832. https://doi.org/10.1016/B978-075067841-4/50045-2
Yin L, Björneklett A, Söderlund E, Brandell D (2021) Analyzing and mitigating battery ageing by self-heating through a coupled thermal-electrochemical model of cylindrical Li-ion cells. J Energy Storage 39:102648
Li Z, Huang J, Yann Liaw B, Metzler V, Zhang J (2014) A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J Power Sources 254:168–182
Bandhauer TM, Garimella S, Fuller TF (2014) Temperature-dependent electrochemical heat generation in a commercial lithium-ion battery. J Power Sources 247:618–628
Lombardo G, Ebin B, Foreman MRSJ, Steenari B-M, Petranikova M (2019) Chemical transformations in li-ion battery electrode materials by carbothermic reduction. ACS Sustain Chem Eng 7:13668–13679
Lombardo G, Ebin B, Mark MR, Steenari BM, Petranikova M (2020) Incineration of EV Lithium-ion batteries as a pretreatment for recycling—determination of the potential formation of hazardous by-products and effects on metal compounds. J Hazard Mater 393:122372
Aro R, Eriksson U, Kärrman A, Reber I, Yeung LWY (2021) Combustion ion chromatography for extractable organofluorine analysis. iScience 24(9):102968
Kärrman A, Yeung LWY, Spaan KM, Lange FT, Nguyen MA, Plassmann M, de Wit CA, Scheurer M, Awad R, Benskin JP (2021) Can determination of extractable organofluorine (EOF) be standardized? First interlaboratory comparisons of EOF and fluorine mass balance in sludge and water matrices. Environ Sci Process Impacts 23(10):1458–1465
Dubocq F, Wang T, Yeung LWY, Sjöberg V, Kärrman A (2020) Characterization of the chemical contents of fluorinated and fluorine-free firefighting foams using a novel workflow combining nontarget screening and total fluorine analysis. Environ Sci Technol 54(1):245–254
González-Pérez JA et al (2014) Appraisal of polycyclic aromatic hydrocarbons (PAHs) in environmental matrices by analytical pyrolysis (Py–GC/MS). J Anal Appl Pyrol 109:1–8
Keller DA, Roe DC, Lieder PH (1996) Fluoroacetate-mediated toxicity of fluorinated ethanes. Fundam Appl Toxicol 30(2):213–219
Kunio O, Hajime B, Rimpei K (1962) Preparation and properties of alkyl trifluorovinyl ethers and related compounds. Bull Chem Soc Jpn 35(4):532–535
Willstrand O, Bisschop R, Blomqvist P, Temple A, Anderson J (2020) Toxic gases from fire in electric vehicles. In: RISE Report 2020:90
Acknowledgements
An acknowledgment goes to the Swedish Energy Agency (Grant No. 2018-008060) for providing the funding of the research related to the cooling liquids treatment. The authors would like to acknowledge the support of APR Technologies AB, in providing the samples and valuable discussions.
Funding
Open access funding provided by Chalmers University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lombardo, G., Foreman, M.R.S.J., Ebin, B. et al. Determination of Hydrofluoric Acid Formation During Fire Accidents of Lithium-Ion Batteries with a Direct Cooling System Based on the Refrigeration Liquids. Fire Technol 59, 2375–2388 (2023). https://doi.org/10.1007/s10694-023-01425-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10694-023-01425-4