Abstract
Group living results in various benefits and costs, which often depend on group size and ontogenetic state of the individual. Therefore, certain group sizes are favourable over others, often depending on individual age or intrinsic state. Under natural conditions many amphibians can be found in aggregations, especially during the larval stage. Yet, whether these aggregations are the result of active social preferences or are driven by environmental factors has only been tested in a limited number of taxa. This study explores social preferences and group size discrimination in golden mantella (Mantella aurantiaca) tadpoles. We gave tadpoles the choice between different numbers of conspecifics using a two-choice design. To test for general social tendency, tadpoles could either join a group of three or remain solitary (3 vs. 0). To test for group size preferences, we tested tadpoles in two different ratios: 2 vs. 1 and 4 vs. 2 conspecifics. We repeated the trials weekly until metamorphosis to determine potential shifts in preference through ontogeny. Tadpoles preferred being with a group over being alone, and the strength of this preference declined with increasing age. Furthermore, tadpoles preferred to be close to the larger of two groups. This preference was stronger in the 2 vs. 1 treatment. Mantella aurantiaca tadpoles, therefore, show clear social tendencies and possess the ability to spontaneously discriminate between group sizes. The lower preference at higher group sizes might indicate cognitive limitations or lower benefits of choosing the larger of two groups when overall group size is high. These findings are one of the few showing social preferences depending on group size in tadpoles and contribute to our understanding of social behaviour in amphibians in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group living has evolved independently in many animal species as a result of a range of selection pressures (Ward and Webster 2016). It offers a variety of benefits, such as increased vigilance and predator detection (Roberts 1996; Michelena and Deneubourg 2011), dilution of predation risk (Wrona and Dixon 1991; Lehtonen and Jaatinen 2016) and increased foraging efficiency (Kurvers et al. 2010). However, there are also associated costs, such as intra-group competition (Krause and Ruxton 2002; Shrader et al. 2007; Sheppard et al. 2018), increased conspicuousness (Botham and Krause 2005; Ioannou et al. 2009), and an increase in pathogen susceptibility (Côté and Poulin 1995; but see Ezenwa et al. 2016 for an argument how group living may lead to increased pathogen resistance). These benefits and costs are often dependant on group size (Ward and Webster 2016). They might furthermore change through ontogeny, especially when they are linked to body size, leading to ontogenetic shifts in social preferences (Ward and Webster 2016). For example, some fish species group when they are young, but live solitary as they age and increase in size (Ryer and Olla 1991; Macpherson 1998). This may be due to an increase in intraspecific competition at larger sizes (Despland and Hamzeh 2004). Alternatively, the opposite has also been observed, where initially solitary animals become gregarious as they grow to reduce predation risk (Fobert et al. 2020).
Social aggregations have been described in several amphibians, especially during the larval stage. Here, environmental factors like food distribution (Sontag et al. 2006), time of day (Beiswenger 1977; Branch 1983) or weather (Espinoza and Quinteros 2008), and intrinsic factors like body size (Leu et al. 2013; Hase and Kutsukake 2019) or kinship (Blaustein and O’Hara 1986; Blaustein and Waldman 1992; Pizzatto et al. 2016) have been shown to mediate group formation. Thus far, these studies focus on a limited range of taxa, with a strong focus on Ranidae (e.g., Blaustein and O’Hara 1987; Balestrieri et al. 2019) and Bufonidae (e.g., Beiswenger 1977; Breden et al. 1982; Balestrieri et al. 2019).
With a multitude of factors affecting group size and social preference, individuals need to be able to assess the costs and benefits of joining or dispersing from a group. The ability to discriminate between group sizes can be invaluable to this. Several amphibian species possess the ability to discriminate between different numerosity, especially when foraging. Green-and-black poison dart frogs (Dendrobates auratus), European fire-bellied toads (Bombina bombina) and red-backed salamanders (Plethodon cinereus), for example, prefer the larger of two food quantities, with some species-dependent differences in how derived these preferences are (Uller et al. 2003; Stancher et al. 2015; Khatiwada and Burmeister 2022). Studies testing for numerosity discrimination in amphibians in a social context are scarce so far (but see Balestrieri et al. 2019).
Ontogenetic shifts have been described for various tadpole behaviours including anti-predator responses (Gazzola et al. 2017) and activity. While spiny toad (Bufo spinosus), American toad (Bufo americanus), and clawed frog (Xenopus leavis) tadpoles show an increase in activity with increasing age (Golden et al. 2000; Smith et al. 2008; Cheron et al. 2021), activity decreases with age in green frog (Rana clamitans) and common toad (Bufo bufo) tadpoles (Smith et al. 2008; Kurali et al. 2018). These differences in direction might be explained, for example, as outcomes of different anti-predator strategies. Comparable ontogenetic changes of social preferences have been described for only a few amphibians, with Japanese mountain brown frogs (Rana ornativentris) tadpoles preferring to group with smaller individuals with increasing age (Hase and Kutsukake 2019). Other amphibian larvae preferentially associate with individuals of similar size, including Southern leopard frogs (Rana utricularia; Alford and Crump 1982), Woodhouse’s toads (Anaxyrus woodhousii; Breden et al. 1982), and white-lined leaf frogs (Phyllomedusa vaillanti; Branch 1983). This has been suggested to be a result of size-dependent habitat preference and swimming capabilities.
In the present study we aimed at broadening our understanding of larval anuran social preferences by testing a member of the Mantella radiation, endemic to Madagascar. We used tadpoles of the endangered golden mantella (Mantella aurantiaca) as model organisms (IUCN SSC Amphibian Specialist Group; 2020). The golden mantella is a small-bodied terrestrial species that only occurs in fragmented, humid forests close to the town of Moramanga in east-central Madagascar (Randrianavelona et al. 2010). Adults can be found sheltering closely together during cool winter months (Edmonds et al. 2020), implying some kind of social preferences. Breeding occurs nearby seasonal, ephemeral ponds, with females laying egg-masses of on average 60–80 eggs on land (Edmonds et al. 2015). After approximately seven days the tadpoles hatch and are washed into the ponds, either due to their own movement, or aided by heavy rains. Tadpoles take approximately 6–8 weeks to metamorphose, at which time the froglets assume the terrestrial habits of the adults (Edmonds et al. 2015). This species is herbivorous in its larval stages and does not cannibalise, unlike some other amphibian larvae (e.g., Caldwell and de Araújo 1998; Summers 1999).
Through repeated testing at seven-day intervals we wanted to elucidate general social preferences as well as potential ontogenetic shifts in preference in M. aurantiaca. We first tested whether there is a general preference to be social over being alone (group size of 3 vs. 0, experiment one). Second, we assessed preference strengths in different group size combinations (2 vs. 1, 4 vs. 2, experiments two and three, respectively). As (larger) groups are thought of as being beneficial, we hypothesise that M. aurantiaca tadpoles prefer being with a group, and that they will discriminate between the two quantities at this low numerosity and ratio. Third, we tested for changes in social preferences during ontogeny. As larger size enhances individual conspicuousness and, consequently, detectability by visually oriented predators, we predict an increase in social preferences during ontogeny. Finally, we aimed at elucidating how activity of tadpoles changes during ontogeny. As there are examples for increases as well as decreases in activity in other anuran species, we predicted that activity would change with increasing size.
Materials and methods
Subjects
We kept adult golden mantellas in naturalistic set-ups at the amphibian facility of Manchester Metropolitan University (Newton-Youens et al. 2022), where we obtained two egg-masses spawned by different males and females. Tadpoles of the first egg-mass hatched on the 28th of March 2022 (n = 42 tadpoles), while tadpoles of the second egg-mass hatched on the 30th of March 2022 (n = 56 tadpoles). To keep track of the tadpoles’ pedigree, the two different egg-masses were housed separate from each other in two tanks (Exo-Terra mini faunariums, 230 × 155 × 170 mm) until 5 weeks of age, when they were moved to two separate larger tanks (Exo-Terra large faunariums, 370 × 220 × 250 mm). These holding tanks were filled with tannin-enriched water, (3.5 l in the smaller tanks, 5.0 l in the larger ones) made by soaking Indian almond leaves (Terminalia catappa) in aged tap water for a minimum of 24 hours. We provided Java moss (Taxiphyllum barbieri) and additional almond leaves for shelter. Tanks were kept in a climate-controlled unit, maintained on a 12:12 photo- and thermoperiod of 23°C during the day and 19°C at night. Lighting was provided by a standard ‘daylight’ lamp (30 W T8 ‘warm white’ 36” fluorescent tube, GE Lighting, Hungary). Tadpoles were fed the same diet for the duration of the experiment, which consisted of a 2:2:1 uniform ground mixture of fish flakes (Tetramin Tropical Food, Tetra, Germany), pellets (AmphibiGold, Arcadia Reptile, UK) and spirulina (Naturya, Netherlands). Ten grams of this dry mixture were added to 20 ml of water to create a thick paste before being frozen. Food was available ad libitum. It was replaced twice per week following routine cleaning, consisting of 20–30% water changes and the removal of excess food and waste material.
Experimental set-up
We started the experiments during the second week after hatching, with test animals being 14 and 11 days old, respectively. We repeated the trials every seven days for seven weeks, at which point the first tadpoles completed metamorphosis. Before each trial, we measured the used tadpoles to the nearest 0.5 mm on lined graph paper, which we placed underneath the tanks. Tadpoles measured on average 4 mm snout-vent length (SVL) at the beginning of the study and 8.6 mm SVL at metamorphosis stage.
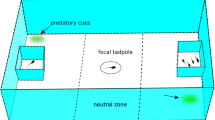
We used eight identical test tanks throughout the experiment (Fig. 1), which were visually isolated from each other using light green, opaque foam wrapped around the outside of each tank. Test tanks were clear plastic containers measuring 180 × 110 × 125 mm (l × w × h) for trials in weeks 2–5. We used larger tanks (370 × 220 × 250 mm) for weeks 6–8 to account for tadpoles’ larger size. Tanks were filled to a height of 50 mm with aged tap water treated with Indian almond leaves. Water was changed after every trial. Test tanks were maintained under the same light and temperature regime as the housing tanks. We divided each test tank into three compartments using clear, perforated plastic slides, allowing the exchange of visual and olfactory cues between the compartments (Fig. 1). The compartments on the left and right side served as stimulus compartments to hold the tadpole groups. For weeks 2–5 each of these stimulus compartments measured 40 mm in length. The compartment in the middle served as a test chamber that contained the respective test tadpole. For weeks 2–5 the test chamber measured 100 mm. The test chamber was visually divided into three zones, indicated on lined graph paper placed underneath the test tank. The zones in front of the left and right stimulus compartment measured 20 mm each and served as choice zones. The remaining 60 mm wide area between the choice zones served as a neutral zone (Fig. 1). As the test tanks were double the size during week 6–8, the dimensions of all compartments and zones were doubled as well. The eight test tanks were arranged in a 4 × 2 grid. A camera (Axis companion cube L, f = 2.0, fish-eye lens) was placed above every two tanks allowing video recordings of two trials simultaneously.
Top view of the test tank, here showing a 3 vs. 0 trial. Dashed lines represent the clear, perforated barriers used to create stimulus compartments; grey dash-dotted lines represent the markings on the bottom of the tank indicating the division of the middle compartment into a neutral zone and two choice zones
Experimental procedure
We chose tadpoles haphazardly for each trial. All individuals in a given trial were within 0.5 mm SVL, to allow for minimal impact of size differences on test tadpoles’ preferences. At the start of the trial the stimulus tadpoles were carefully placed in the outer compartments and a test tadpole was placed in a small, cylindrical vial made of clear plastic (35 mm diameter) placed in the centre of the neutral zone for a five-minute acclimation period (Dadda et al. 2003). After the acclimation period, we removed the plastic vial, and remotely started the video recordings, using the Axis companion app on the researcher’s laptop to avoid interfering with the study. Video recordings lasted for a total of 20 min per trial. Afterwards, tadpoles were returned to their original tanks.
The first aim of the study was to test for overall social tendencies of the tadpoles. To that aim, we inserted a stimulus group of three tadpoles into one stimulus compartment, while the other stimulus compartment remained empty (experiment one). The second aim of the study was to test for group size preferences. To that aim we ran trials with two different group size combinations. Stimulus groups either consisted of one and two tadpoles respectively (absolute difference = 1, ratio = 0.5, experiment two), or of two and four tadpoles, respectively (absolute difference = 2, ratio = 0.5, experiment three). All tadpoles within a given trial were familiar full sibs in order to exclude potential influences of different levels of familiarity, relatedness or age on individuals’ choice (Blaustein and O’Hara 1987; Blaustein and Waldman 1992). Within each run, four of the tanks had the larger stimulus group on the left-hand side and four had the larger group on the right-hand side to control for potential side biases.
All three experiments were repeated weekly for seven weeks. We ran 8 trials per egg-mass for each of the three separate experiments every week, resulting in 16 trials per experiment in each week and 112 trials in total for each of the experiments. All test tadpoles were only used once per week; stimulus tadpoles were used more than once, but never in the same group combination during a given week. Hence, each test tadpole could have been involved in a minimum of 0 and a maximum of 7 trials in total (that is if it was chosen every single week). However, given that we chose the tadpoles haphazardly it is unlikely that such extreme values occurred frequently. Furthermore, each experiment was repeated 112 times (that is 16 trials x 7 weeks), reducing the impact of an individual test animal, even if it would have been tested the maximum of 7 times. Details on the repeats of the different experiments in each week can be found in Table S1.
Data analysis
We analysed all video recordings using the Axis companion app video software. We recorded the time in seconds the subject tadpole spent in each choice zone. As a measure of general activity, we counted how often tadpoles crossed the lines indicating both choice zones (that is entering and leaving the choice zone). We considered a test tadpole to be in a given zone if at least its head crossed the lines indicated on the bottom of the test chamber (Fig. 1). Individual differences in activity might lead to less active tadpoles spending generally less time in the choice zones close to both stimulus groups. Hence, differences in time spent close to the larger of two shoals might indicate differences in preference strength or differences in general activity. To allow a meaningful comparison of preferences between different treatments and different weeks, we therefore calculated a preference index by dividing the time (s) the tadpole spent in the choice zone close to the larger shoal by the time it spent in both choice zones combined (cf. Raveh et al. 2019; Durrer et al. 2020):
An index larger than 0.5 thus indicates a preference for the larger stimulus group and an index below 0.5 indicates a preference for the smaller stimulus group, with values closer to 1 or 0 indicating a stronger preference or avoidance, respectively. As the intercept in our models on the preference index shows if the estimates are different from zero, we subtracted 0.5 from the index and used this variable for further analyses. Note that Fig. 2 shows the index before subtracting 0.5.
Preference indices of the tadpoles at different ontogenetic stages in the three different experiments. In experiment one, tadpoles preferred to be with the group of 3 over being alone, and this preference declined with increasing age. In experiment two and three, tadpoles preferred the larger of the two groups. This preference was more pronounced in experiment two. Bold lines indicate medians, boxes interquartile ranges, whiskers 95% CI and circles outliers. The dashed line indicates a preference index of 0.5, values above the line indicate a preference for the larger shoal
To analyse the overall social tendencies of the tadpoles (experiment one) a generalised linear model (GLM) was fitted, with preference index as the dependent variable and age in weeks as a fixed effect, testing for ontogenetic shifts in social preference.
To test for a difference in preference strength between different group sizes (experiments two and three), we fitted a generalised linear mixed effects model (GLMM), with preference index as the dependent variable, experiment as a fixed effect and age in weeks as a random effect to account for using the same individuals in different weeks. As this GLMM revealed a difference in preference indices between the two experiments, we continued by analysing the data of experiment two and three separately. A GLM was fitted for each experiment, with preference index as the dependent variable and week as a fixed effect to test for an ontogenetic shift in social preference. As this was not the case (see results), we changed the week term to become a random effect to account for the repeated use of individuals between weeks and ran GLMMs. Here, a significant intercept would indicate a general preference for one of the stimulus group sizes.
As a measure of overall tadpole activity, we used the number of movements in and out of the preference zones as a proxy. A separate linear model (LM) was fitted to test for differences in tadpole total activity in each experiment with total number of movements between choice zones as the dependent variable and age in weeks as fixed effect. All data were tested for collinearity and multiple collinearity (Zuur et al. 2010) and no violations were detected. Gaussian error distributions and identity link functions were adopted for the models. Week was considered as a continuous predictor as it follows a set pattern. All statistical tests were run using R (version 4.1.2).
Results
In experiment one (3 vs. 0) tadpoles significantly preferred to be with the group over being alone (GLM: β = 0.056 ± 0.012 (SD), t = 4.99, df = 111, P < 0.001, Fig. 2). The strength of this preference declined with increasing age (β = -0.012 ± 0.006, t = -2.07, df = 111, P = 0.04, Fig. 2).
Preference indices of experiment two (2 vs. 1) and three (4 vs. 2) differed significantly (GLMM, β = -0.058 ± 0.017, t = -3.32, df = 207, P = 0.001), with tadpoles in experiment two showing stronger preferences for the larger group than in experiment three (Fig. 2). Analysing both experiments separated revealed a preference for the larger shoal in both experiments (GLM, 2 vs. 1: β = 0.095 ± 0.014, t = 6.871, df = 107, P < 0.001; 4 vs. 2: β = 0.037 ± 0.011, t = 1.086, df = 107, P < 0.001). In both experiments age at testing had no significant effect on preferences: Experiment two: (β = 0.009 ± 0.007, t = 1.33, df = 107, P = 0.19), experiment three: (β = 0.006 ± 0.006, t = 1.09, df = 107, P = 0.28; Fig. 2).
Test animals in all three experiments showed a considerable amount of activity, measured as the total number of movements in and out of each of the choice zones (Fig. 3). Activity decreased with increasing age in all three experiments (experiment one: LM, β = -1.75 ± 0.595, t = -2.95, df = 110, P = 0.004; experiment two: β = -0.358 ± 0.66, t = -2.06, df = 106, P = 0.04), though this trend failed statistical significance in experiment three (β = -1.033 ± 0.587, t = -1.76, df = 106, P = 0.08, Fig. 3).
Activity of the tadpoles at different ontogenetic stages, measured as the total number of movements in and out of each prescribed choice zone. In experiment one and two, activity significantly declined with increasing age, and there was a similar trend also in experiment three. Bold lines indicate medians, boxes interquartile ranges, whiskers 95% CI and circles outliers
Discussion
Amphibian species are often considered to be solitary-living animals. Still, while this often holds true for post-metamorphic individuals (but see Edmonds et al. (2020) for an exception in adult golden mantellas), larvae are frequently found in dense aggregations, and the mechanisms shaping these groups often remain elusive. Living in groups comes with various costs and benefits, which often depend on group size. The ability to discriminate between group sizes can therefore improve an individual’s fitness as it will allow evaluating the costs and benefits of joining a particular group. Here, we show that Mantella aurantiaca tadpoles prefer being in a group over being alone (experiment one). Furthermore, tadpoles in our study discriminated between group sizes and preferred being with the larger of two groups, especially in experiment two, where stimulus group sizes were smaller.
Tadpoles in this study showed a preference for the larger shoals, conforming to previous studies on amphibians (Leu et al. 2013; Balestrieri et al. 2019) and fishes (e.g., Gómez-Laplaza and Gerlai 2011; Mehlis et al. 2015; Durrer et al. 2020). The preference for a larger group indicates the potential ability of M. aurantiaca to discriminate between low numerical quantities, however it is unclear what their capabilities at larger numerosity or larger shoal ratios would be. For example, tadpoles of green toads (Bufotes balearicus) and edible frogs (Pelophylax esculentus) differed in acuity when discriminating between small numbers, with B. balearicus only discerning between groups of a ratio of 4 vs. 1 while P. esculentus also differentiated 4 vs. 3 (Balestrieri et al. 2019). Two distinct mechanisms for discriminating numerosity have been shown to occur in animals. The object tracking system is used for tracking small precise quantities, normally up to the value of four (Trick and Pylyshyn 1994). The approximate number system is less precise and useful for tracking larger quantities (Gallistel and Gelman 2000). In our study, the maximum stimulus group size was four, and hence fell within the boundaries of the object tracking system. Still, preferences were more pronounced in experiment two, where the total difference in group size was smaller, but at the same time also the total number of stimulus individuals was low. Whether these results are based on limited numerical capacities or variation in motivation to join a certain group cannot be discerned in our study. For example, it is plausible to assume that the benefits of joining a larger group is diminished by its costs, e.g., due to increased competition. Hence, tadpoles’ motivation to join a group might be mediated by weighing the costs against the benefits, and this balance might be different in the 2 vs. 1 scenario than in the 4 vs. 2 scenario. Furthermore, it remains to be determined whether our result is evidence of true number discrimination capabilities, as seen in other amphibians (Uller et al. 2003; Balestrieri et al. 2019; Khatiwada and Burmeister 2022) and some fishes (Stancher et al. 2013), or if it may be attributed to physical variables, such as the increased space use or higher individual density in larger shoals (Frommen et al. 2009; Stancher et al. 2015; Xiong et al. 2018).
As the clear partitions between the stimulus and test compartment were perforated, test tadpoles had visual and chemical contact to the stimulus groups. While quantity discrimination is usually tested based on visual cues, chemical information of conspecifics has been repeatedly shown to be involved in social decisions of aquatic animals (e.g., Thünken et al. 2010; Ward and Currie 2013; Raveh et al. 2019). Indeed, some fish species use chemical cues to differentiate between groups containing different numbers of conspecifics (Ward et al. 2002; Santacà et al. 2021). Using our setup, we cannot differentiate between the cues tadpoles use when choosing between groups.
The ontogenetic stage has the potential to play a considerable role in group association in amphibians. This can be due to increases in cognitive capabilities changing kin-recognition abilities (Gramapurohit et al. 2006), changes in diet (Hase and Kutsukake 2019) or increased swimming capabilities and therefore an increased ability to avoid predators (Brown and Taylor 1995; Balestrieri et al. 2019), all of which will play a role in the benefits associated with group living. For example, a switch from an herbivorous to an omnivorous diet in Japanese mountain brown frogs (Rana ornativentris) can result in increased competition and thus reduced group association (Hase and Kutsukake 2019). Number discrimination capabilities have also been shown to change during ontogeny (Stancher et al. 2013; Vallortigara 2017; Balestrieri et al. 2019). In the present study tadpoles show some ontogenetic shift in general social tendencies (experiment one, 3 vs. 0), with a decline in preference for the group during the later stages of development. The propensity to socialise less at later developmental stages may be a result of increased conspecific competition or a change in predator avoidance strategies at larger sizes (Brown and Taylor 1995; Relyea 2003). However, even though preferences decreased in experiment one as the larvae aged, they remained above the 0.5 threshold throughout the study. Additionally, experiments two and three showed no clear relationship between age and preference strength, suggesting that ontogenetic shifts may not be a general driver of tadpole sociality but might be situation dependent.
In our study, tadpoles showed decreased levels of activity with increasing size, and this decrease was comparable in all three experiments. These decreased activity levels might be caused by an ontogenetic shift in antipredator behaviour from fleeing or shoaling to hiding (Smith et al. 2008), or by an increase in foraging efficiency with increasing size (Kurali et al. 2018). Such changes in activity have the potential to influence the outcome of social preference tests, as a reduced activity might decrease sample rates, lowering the chance to detect existing preferences (cf. Fischer and Frommen 2013). Whether the shown reduction in preference in our study can be explained by reduced activity would be an interesting topic for future research.
In all three experiments, tadpoles were allowed to choose between groups composed of related individuals only. Earlier studies in anuran larvae have shown that tadpoles reared in kin groups showed more even growth rates (Pakkasmaa and Aikio 2003), lower aggressiveness (Fouilloux et al. 2022) and higher activity (Hokit and Blaustein 1995), and that tadpoles prefer to shoal with kin over non-kin (e.g., Blaustein and O’Hara 1986; Blaustein and Waldman 1992; Hase and Kutsukake 2019). However, when competition is high, related individuals in some species show lower mass at metamorphosis (Hokit and Blaustein 1994) and increased amounts of kin-directed cannibalism (Pfennig et al. 1993; Gray et al. 2009). In such scenarios, individuals might prefer to group with unrelated individuals or with smaller groups (cf. Krause 1993; Frommen et al. 2007, for comparable results in fishes). How such potentially detrimental effects of relatedness interact with preferences for certain group sizes will be a worthwhile question for future studies.
In conclusion, by preference testing for different shoal sizes we show that golden mantella tadpoles actively form social aggregations. They furthermore possess the ability to discern between different group sizes, although the mechanisms underpinning this need further exploration. These findings provide valuable insight into mechanisms driving social aggregations in amphibians and deepen our understanding of the ecology of the endangered golden mantella and other members of the Mantellidae. Finally, our results can inform husbandry guidelines for this commonly kept, endangered species, supporting the idea that group housing may be preferable at the tadpole stage.
Data Availability
Data will be made available upon acceptance of the manuscript.
Code Availability
Not applicable.
References
Alford RA, Crump ML (1982) Habitat partitioning among size classes of larval southern leopard frogs, Rana utricularia. Copeia 1982(2):367–373. https://doi.org/10.2307/1444617
Balestrieri A, Gazzola A, Pellitteri-Rosa D, Vallortigara G (2019) Discrimination of group numerousness under predation risk in anuran tadpoles. Anim Cogn 22(2):223–230. https://doi.org/10.1007/s10071-019-01238-5
Beiswenger RE (1977) Diel patterns of aggregative behavior in tadpoles of Bufo americanus, in relation to light and temperature. Ecology 58(1):98–108. https://doi.org/10.2307/1935111
Blaustein AR, O’Hara RK (1986) An investigation of kin recognition in Red-legged frog (Rana aurora) tadpoles. J Zool 209(3):347–353. https://doi.org/10.1111/j.1469-7998.1986.tb03598.x
Blaustein AR, O’Hara RK (1987) Aggregation behaviour in Rana cascadae tadpoles: association preferences among wild aggregations and responses to non-kin. Anim Behav 35(5):1549–1555. https://doi.org/10.1016/S0003-3472(87)80026-X
Blaustein AR, Waldman B (1992) Kin recognition in anuran amphibians. Anim Behav 44(2):207–221. https://doi.org/10.1016/0003-3472(92)90027-7
Botham MS, Krause J (2005) Shoals receive more attacks from the wolf-fish (Hoplias malabaricus Bloch, 1794). Ethology 111(10):881–890. https://doi.org/10.1111/j.1439-0310.2005.01122.x
Branch L (1983) Social behavior of the tadpoles of Phyllomedusa vaillanti. Copeia 1983(2):420–428. https://doi.org/10.2307/1444385
Breden F, Lum A, Wassersug R (1982) Body size and orientation in aggregates of toad tadpoles Bufo woodhousei. Copeia 1982(3):672–680. https://doi.org/10.2307/1444669
Brown RM, Taylor DH (1995) Compensatory escape mode trade-offs between swimming performance and maneuvering behavior through larval ontogeny of the wood frog, Rana sylvatica. Copeia 1995(1):1–7. https://doi.org/10.2307/1446794
Caldwell JP, de Araújo MC (1998) Cannibalistic interactions resulting from indiscriminate predatory behavior in tadpoles of poison frogs (Anura: Dendrobatidae). Biotropica 30(1):92–103. https://doi.org/10.1111/j.1744-7429.1998.tb00372.x
Cheron M, Raoelison L, Kato A, Ropert-Coudert Y, Meyer X, MacIntosh AJ, Brischoux F (2021) Ontogenetic changes in activity, locomotion and behavioural complexity in tadpoles. Biol J Linn Soc 134(1):165–176. https://doi.org/10.1093/biolinnean/blab077
Côté IM, Poulin R (1995) Parasitism and group size in social animals: a meta-analysis. Behav Ecol 6(2):159–165. https://doi.org/10.1093/beheco/6.2.159
Dadda M, Sovrano VA, Bisazza A (2003) Temporal pattern of social aggregation in tadpoles and its influence on the measurement of lateralised response to social stimuli. Physiol Behav 78(2):337–341. https://doi.org/10.1016/S0031-9384(02)01001-6
Despland E, Hamzeh S (2004) Ontogenetic changes in social behaviour in the forest tent caterpillar, Malacosoma disstria. Behav Ecol Sociobiol 56:177–184. https://doi.org/10.1007/s00265-004-0767-8
Durrer L, Taborsky M, Frommen JG (2020) Group-size preferences in a shoaling cichlid. Behaviour 157(5):415–431. https://doi.org/10.1163/1568539X-bja10007
Edmonds D, Rakotoarisoa JC, Rasoanantenaina S, Sam SS, Soamiarimampionona J, Tsimialomanana E, Dolch R, Rabemananjara F, Youssouf, Rabibisoa N, Robsomanitrandrasana E (2015) Captive husbandry, reproduction, and fecundity of the golden mantella (Mantella aurantiaca) at the Mitsinjo breeding facility in Madagascar. Salamandra 51(4):315–325
Edmonds D, Adamovicz L, Rakotoarisoa JC, Soamiarimampionona J, Harris R (2020) Seasonal activity patterns of golden mantellas (Mantella aurantiaca). J Herpetol 54(3):325–330. https://doi.org/10.1670/18-050
Espinoza RE, Quinteros S (2008) A hot knot of toads: aggregation provides thermal benefits to metamorphic Andean toads. J Therm Biol 33(2):67–75. https://doi.org/10.1016/j.jtherbio.2007.10.004
Ezenwa VO, Ghai RR, McKay AF, Williams AE (2016) Group living and pathogen infection revisited. Curr Opin Behav Sci 12:66–72. https://doi.org/10.1016/j.cobeha.2016.09.006
Fischer S, Frommen JG (2013) Eutrophication alters social preferences in three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol 67(2):293–299. https://doi.org/10.1007/s00265-012-1449-6
Fobert EK, Reeves SE, Swearer SE (2020) Ontogenetic shifts in social aggregation and habitat use in a temperate reef fish. Ecosphere 11(12):e03300. https://doi.org/10.1002/ecs2.3300
Fouilloux CA, Fromhage L, Valkonen JK, Rojas B (2022) Size-dependent aggression towards kin in a cannibalistic species. Behav Ecol 33(3):582–591. https://doi.org/10.1093/beheco/arac020
Frommen JG, Luz C, Bakker TCM (2007) Nutritional state influences shoaling preference for familiars. Zoology 110(5):369–376. https://doi.org/10.1016/j.zool.2007.06.002
Frommen JG, Hiermes M, Bakker TCM (2009) Disentangling the effects of group size and density on shoaling decisions of three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol 63:1141–1148. https://doi.org/10.1007/s00265-009-0767-9
Gallistel CR, Gelman R (2000) Non-verbal numerical cognition: from reals to integers. Trends Cogn Sci 4(2):59–65. https://doi.org/10.1016/S1364-6613(99)01424-2
Gazzola A, Balestrieri A, Ghitti M, Paganelli D, Galeotti P (2017) Behavioural and life history responses to predation risk by common frog tadpoles exposed to two predators during ontogeny. Acta Ethol 20:235–241. https://doi.org/10.1007/s10211-017-0266-3
Golden DR, Smith GR, Rettig JE (2000) Effects of age and group size on habitat selection and activity level in Xenopus laevis tadpoles. Trans Neb Acad Sci 26:23–27
Gómez-Laplaza LM, Gerlai R (2011) Spontaneous discrimination of small quantities: shoaling preferences in angelfish (Pterophyllum scalare). Anim Cogn 14:565–574. https://doi.org/10.1007/s10071-011-0392-7
Gramapurohit NP, Veeranagoudar DK, Mulkeegoudra Sv, Shanbhag BA, Saidapur SK (2006) Kin recognition in Bufo scaber tadpoles: ontogenetic changes and mechanism. J Ethol 24:267–274. https://doi.org/10.1007/s10164-006-0194-6
Gray HM, Summers K, Ibáñez RD (2009) Kin discrimination in cannibalistic tadpoles of the green poison frog, Dendrobates auratus (Anura, Dendrobatidae). Phyllomedusa 8(1):41–50
Hase K, Kutsukake N (2019) Developmental effects on social preferences in frog tadpoles, Rana ornativentris. Anim Behav 154:7–16. https://doi.org/10.1016/j.anbehav.2019.06.001
Hokit DG, Blaustein AR (1994) The effects of kinship on growth and development in tadpoles of Rana cascadae. Evolution 48(4):1383–1388. https://doi.org/10.2307/2410395
Hokit DG, Blaustein AR (1995) Predator avoidance and alarm-response behaviour in kin‐discriminating tadpoles (Rana cascadae). Ethology 101(4):280–290. https://doi.org/10.1111/j.1439-0310.1995.tb00365.x
Ioannou CC, Morrell LJ, Ruxton GD, Krause J (2009) The effect of prey density on predators: conspicuousness and attack success are sensitive to spatial scale. Am Nat 173(4):499–506. https://doi.org/10.1086/597219
IUCN SSC Amphibian Specialist Group (2020) Mantella aurantiaca. The IUCN Red List of Threatened Species 2020:e.T12776A508612. https://doi.org/10.2305//IUCN.UK.2020-3.RLTS.T12776A508612.en
Khatiwada S, Burmeister SS (2022) Quantity discrimination in a spontaneous task in a poison frog. Anim Cogn 25(1):27–32. https://doi.org/10.1007/s10071-021-01528-x
Krause J (1993) The influence of hunger on shoal size choice by three-spined sticklebacks, Gasterosteus aculeatus. J Fish Biol 43(5):775–780
Krause J, Ruxton G (2002) Living in groups. Oxford University Press, Oxford
Kurali A, Pásztor K, Hettyey A, Tóth Z (2018) Resource-dependent temporal changes in antipredator behavior of common toad (Bufo bufo) tadpoles. Behav Ecol Sociobiol 72:91. https://doi.org/10.1007/s00265-018-2503-9
Kurvers RHJM, Prins HHT, van Wieren SE, van Oers K, Nolet BA, Ydenberg RC (2010) The effect of personality on social foraging: shy barnacle geese scrounge more. Proc R Soc Lond B: Biol Sci 277(1681):601–608. https://doi.org/10.1098/rspb.2009.1474
Lehtonen J, Jaatinen K (2016) Safety in numbers: the dilution effect and other drivers of group life in the face of danger. Behav Ecol Sociobiol 70:449–458. https://doi.org/10.1007/s00265-016-2075-5
Leu ST, Whiting MJ, Mahony MJ (2013) Making friends: social attraction in larval green and golden bell frogs, Litoria aurea. PLoS ONE 8(2):e56460. https://doi.org/10.1371/journal.pone.0056460
Macpherson E (1998) Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes. J Exp Mar Biol Ecol 220(1):127–150. https://doi.org/10.1016/S0022-0981(97)00086-5
Mehlis M, Thünken T, Bakker TCM, Frommen JG (2015) Quantification acuity in spontaneous shoaling decisions of three-spined sticklebacks. Anim Cogn 18:1125–1131. https://doi.org/10.1007/s10071-015-0884-y
Michelena P, Deneubourg JL (2011) How group size affects vigilance dynamics and time allocation patterns: the key role of imitation and tempo. PLoS ONE 6(4):e18631. https://doi.org/10.1371/journal.pone.0018631
Newton-Youens J, Michaels C, Preziosi R (2022) Keeping the golden mantella golden: the effect of dietary carotenoid supplementation and UV provision on the colouration and growth of Mantella aurantiaca. J Zoo Aqua Res 10(2):74–81. https://doi.org/10.19227/jzar.v10i2.598
Pakkasmaa S, Aikio S (2003) Relatedness and competitive asymmetry–the growth and development of common frog tadpoles. Oikos 100(1):55–64. https://doi.org/10.1034/j.1600-0706.2003.11815.x
Pfennig DW, Reeve HK, SHerman PW (1993) Kin recognition and cannibalism in spadefoot toad tadpoles. Anim Behav 46(1):87–94. https://doi.org/10.1006/anbe.1993.1164
Pizzatto L, Stockwell M, Clulow S, Clulow J, Mahoney M (2016) How to form a group: effects of heterospecifics, kinship and familiarity in the grouping preference of green and golden bell frog tadpoles. Herpetol J 26(2):157–164
Randrianavelona R, Rakotonoely H, Ratsimbazafy J, Jenkins RKB (2010) Conservation assessment of the critically endangered frog Mantella aurantiaca in Madagascar. Afr J Herpetol 59(1):65–78
Raveh S, Langen K, Bakker TCM, Josephs C, Frommen JG (2019) Oddity, predation risk and social decisions in aquatic invertebrates. Ethology 125(2):106–113. https://doi.org/10.1111/eth.12835
Relyea RA (2003) Predators come and predators go: the reversibility of predator-induced traits. Ecology 84(7):1840–1848. https://doi.org/10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2
Roberts G (1996) Why individual vigilance declines as group size increases. Anim Behav 51(5):1077–1086. https://doi.org/10.1006/anbe.1996.0109
Ryer CH, Olla BL (1991) Agonistic behavior in a schooling fish: form, function and ontogeny. Environ Biol Fishes 31:355–363. https://doi.org/10.1007/BF00002360
Santacà M, Dadda M, Bisazza A (2021) The role of visual and olfactory cues in social decisions of guppies and zebrafish. Anim Behav 180:209–217. https://doi.org/10.1016/j.anbehav.2021.08.017
Sheppard CE, Inger R, McDonald RA, Barker S, Jackson AL, Thompson FJ, Vitikainen EIK, Cant MA, Marshall HH (2018) Intragroup competition predicts individual foraging specialisation in a group-living mammal. Ecol Lett 21(5):665–673. https://doi.org/10.1111/ele.12933
Shrader AM, Kerley GIH, Kotler BP, Brown JS (2007) Social information, social feeding, and competition in group-living goats (Capra hircus). Behav Ecol 18(1):103–107. https://doi.org/10.1093/beheco/arl057
Smith GR, Burgett AA, Temple KG, Sparks KA, Winter KE (2008) The ability of three species of tadpoles to differentiate among potential fish predators. Ethology 114(7):701–710. https://doi.org/10.1111/j.1439-0310.2008.01505.x
Sontag C, Wilson DS, Wilcox RS (2006) Social foraging in Bufo americanus tadpoles. Anim Behav 72(6):451–1456. https://doi.org/10.1016/j.anbehav.2006.05.006
Stancher G, Sovrano VA, Potrich D, Vallortigara G (2013) Discrimination of small quantities by fish (redtail splitfin, Xenotoca eiseni). Anim Cogn 16:307–312. https://doi.org/10.1007/s10071-012-0590-y
Stancher G, Rugani R, Regolin L, Vallortigara G (2015) Numerical discrimination by frogs (Bombina orientalis). Anim Cogn 18:219–229. https://doi.org/10.1007/s10071-014-0791-7
Summers K (1999) The effects of cannibalism on amazonian poison frog egg and tadpole deposition and survivorship in Heliconia axil pools. Oecologia 119:557–564. https://doi.org/10.1007/s004420050819
Thünken T, Baldauf SA, Bersau N, Bakker TCM, Kullmann H, Frommen JG (2010) Impact of olfactory non-host predator cues on aggregation behaviour and activity in Polymorphus minutus infected Gammarus pulex. Hydrobiologia 654:137–145. https://doi.org/10.1007/s10750-010-0377-6
Trick LM, Pylyshyn ZW (1994) Why are small and large numbers enumerated differently? A limited-capacity preattentive stage in vision. Psychol Rev 101(1):80–102. https://doi.org/10.1037/0033-295X.101.1.80
Uller C, Jaeger R, Guidry G, Martin C (2003) Salamanders (Plethodon cinereus) go for more: rudiments of number in an amphibian. Anim Cogn 6:105–112. https://doi.org/10.1007/s10071-003-0167-x
Vallortigara G (2017) An animal’s sense of number. In: Adams J, Barmby P, Mesoudi A (eds) The nature and development of mathematics: Cross disciplinary perspectives on cognition, learning and culture. Routledge, New York, pp 43–65
Ward AJW, Currie S (2013) Shoaling fish can size-assort by chemical cues alone. Behav Ecol Sociobiol 67:667–673. https://doi.org/10.1007/s00265-013-1486-9
Ward A, Webster M (2016) Sociality: the behaviour of group-living animals. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-319-28585-6
Ward AJW, Axford S, Krause J (2002) Mixed-species shoaling in fish: the sensory mechanisms and costs of shoal choice. Behav Ecol Sociobiol 52:182–187. https://doi.org/10.1007/s00265-002-0505-z
Wrona FJ, Dixon RJ (1991) Group size and predation risk: a field analysis of encounter and dilution effects. Am Nat 137(2):186–201. https://doi.org/10.1086/285153
Xiong W, Yi L-C, Tang Z, Zhao X, Fu SJ (2018) Quantity discrimination in fish species: fish use non-numerical continuous quantity traits to select shoals. Anim Cogn 21:813–820. https://doi.org/10.1007/s10071-018-1214-y
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
We are grateful to Manon Schweinfurth and Aurélie Jolivald for helpful discussions on data analyses and to Fernando Vargas-Salinas and two anonymous referees for very constructive and thoughtful comments on earlier versions of our manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
D.W., J.N.-Y. and J.G.F. conceived of the study; D.W., J.N.-Y. and J.G.F. planned the experiment; D.W. and J.N.-Y. bred and cared for the animals; J.G.F supervised the research; D.W., J.N.-Y. and J.G.F. established the set-up; D.W. ran the experiments and analysed the videos; D.W. conducted the statistical analyses and was supported by J.G.F.; D.W., J.N.-Y. and J.G.F. worked on the manuscript drafts; all authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The experiment followed the ASAB/ABS guidelines for the treatment of animals in behavioural research and teaching. The experiments were approved by Manchester Metropolitan University (Project ID 35889) and are in accordance with good scientific practice guidelines and national legislation.
Consent to participate
Not applicable.
Consent for publication
All authors agreed on publication of the final draft of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wright, D., Newton-Youens, J. & Frommen, J.G. Four’s a crowd: social preferences for larger groups in golden mantella (Mantella aurantiaca) tadpoles. Evol Ecol 38, 23–36 (2024). https://doi.org/10.1007/s10682-023-10276-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-023-10276-y