Abstract
To combat the loss of species due to emerging infectious diseases, scientists must incorporate ecological parameters, such as temperature and humidity, to understand how the environment affects host–pathogen interactions. The fungal disease chytridiomycosis is a compelling case study to investigate the role of both temperature and humidity on infectious disease, as both the fungal pathogen (Batrachochytrium dendrobatidis, Bd) and the host (amphibians) are heavily influenced by these abiotic factors. We performed two experiments to investigate the importance of relative humidity and temperature on frog immunity (production of antimicrobial skin secretions) and disease development in captive golden frogs (Atelopus zeteki) of Panama. We found that the quantity of skin secretions significantly decreased over time in frogs moved from low to medium and high relative humidity treatments. Following Bd exposure, frogs in high temperature (26–27 °C) and high relative humidity (80–90%) had lower pathogen loads and survived significantly longer than frogs kept in all other treatment conditions, including high temperature and low relative humidity. These results suggest that high relative humidity may be an important, although less understood, mediator of Bd infection and the survival of golden frogs. Because the environment can drastically alter disease dynamics, understanding how temperature and humidity influence chytridiomycosis outcomes in golden frogs may be essential for the success of the reintroduction of captive frogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emerging infectious diseases (EIDs) are diseases that are newly recognized or have recently increased in incidence or geographic range (McArthur 2019). In recent decades, EIDs have profoundly impacted both human and wildlife populations (Morens et al. 2004; Tompkins et al. 2015; Schmeller et al. 2020). To fully recognize the scope and devastation of EIDs in both humans and wildlife, scientists have adopted relatively new approaches that apply an ecological perspective to investigate the role of the environment in shaping the outcome of host–pathogen interactions (Hawley and Altizer 2011). This concept has been classically illustrated with the heuristic of the disease triad, which demonstrates how pathogens, hosts, and their shared environment are interconnected (Hueffer et al. 2011; James et al. 2015). The disease triad framework has become integral to understanding disease dynamics in wildlife because it strategically encompasses the environment and how it can impact the ecology and evolution of both pathogens and hosts (James et al. 2015).
Of all three components of the disease triad, the environment is often the most difficult to define, and the most complex, as it is influenced by multiple, oscillating abiotic factors, such as temperature and humidity (Engering et al. 2013). Despite its complexity, many studies examining how abiotic factors influence disease have taken a relatively simplified approach, frequently focusing solely on the effect of temperature on pathogens and their hosts (Webb et al. 2010; Bailey et al. 2017; Price et al. 2019; Mordecai et al. 2019; Cohen et al. 2020). Temperature experiments are pervasive across many disease systems, including the pathogens of humans (Mordecai et al. 2019), amphibians (Sonn et al. 2017; Price et al. 2019), corals (Ward et al. 2007), fish (Karvonen et al. 2010; Bailey et al. 2017), insects (Thomas and Blanford 2003), and plants (Webb et al. 2010). However, understanding the impact of both temperature and humidity on disease systems has become increasingly important, especially in the context of global climate change, where compounding abiotic stressors are likely to alter infection dynamics across disease systems (Lafferty and Mordecai 2016).
One ideal study system to investigate the importance of environmental components on disease outcomes is the chytridiomycosis panzootic in amphibians (Puschendorf et al. 2009; Turner et al. 2021). Chytridiomycosis, a disease caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd), infects amphibian epidermis and has led to the decline of hundreds of amphibian species globally (Scheele et al. 2019). Given that both the pathogen and the hosts are ectothermic, understanding the role of the environment is critical in determining the mechanisms underpinning chytridiomycosis outcomes (Turner et al. 2021). Bd is generally thought to be a cold-adapted pathogen, growing optimally between 17 and 25 °C (Piotrowski et al. 2004; Woodhams et al. 2008; Sheets et al. 2021). While Bd can maintain relatively high fitness across a range of low temperatures (Woodhams et al. 2008; Voyles et al. 2012), temperature can change the rate that Bd completes its life cycle, as well as the number of infectious zoospores released, which is thought to alter Bd pathogenicity (Richards-Zawacki 2010; Voyles et al. 2012; Langhammer et al. 2013; Rowley and Alford 2013). Additionally, temperature may be a key determinant of differential pathogenicity among closely related Bd strains (Russell et al. 2019).
As well as affecting Bd, temperature also plays important roles in altering the amphibian immune response (reviewed in Rollins-Smith 2017). Amphibians are generally thought to have increased immune responses at high temperatures with diminished effectiveness against pathogens in low temperatures (Rollins-Smith and Woodhams 2012). One key component of amphibian innate immunity are skin secretions, which can inhibit Bd in numerous species (Woodhams et al. 2006, 2007). Skin secretion production can be reduced in cold temperatures (Robak et al. 2019) and varies seasonally (Le Sage et al. 2021; Rosa et al. 2022).
Since the environment influences both the pathogen and hosts, disease outcomes are often heterogenous across spatio-temporal gradients (Puschendorf et al. 2011; McMillan et al. 2020). Spatial heterogeneity may create disease refugia (locations where amphibian hosts persist despite infection and without severe disease-induced impacts) due to environmental conditions that favor the host and/or disfavor the pathogen (Puschendorf et al. 2011; Heard et al. 2015). Additionally, several studies have shown increased Bd prevalence at high-elevation sites compared to low-elevation sites in tropical regions (e.g., in Australia, McDonald et al. 2005; Woodhams and Alford 2005; Puschendorf et al. 2011; and Central America, Puschendorf et al. 2009; Zumbado-Ulate et al. 2014). Disease dynamics also shift over time where Bd prevalence fluctuates across temperate (spring, summer, autumn, winter) and tropical (wet, dry) seasons (Berger et al. 2004; McDonald et al. 2005; Woodhams and Alford 2005; Kinney et al. 2011; Ruggeri et al. 2020; Rosa et al. 2022; Wilber et al. 2022). These studies support environmental factors as critical components of chytridiomycosis, with different outcomes given the ‘right’ environmental conditions for the host or pathogen. While the role of temperature has been well documented, relative humidity has been comparatively understudied in research on chytridiomycosis (Murray et al. 2013; Bienentreu and Lesbarreres 2020; Schmeller et al. 2022).
Populations of the Panamanian golden frogs (Atelopus zeteki) were once abundant along montane streams but proved to be highly susceptible to Bd (Bustamante et al. 2010; Becker et al. 2011; Ellison et al. 2015; Gass and Voyles 2022). The decline of golden frogs was well noted because the frogs are highly conspicuous, active along streams during the day, and exhibit bright coloration (Lötters 1996). Although a Bd epizootic reduced golden frogs to near extinction (Lips et al. 2006; McCaffery et al. 2015), some populations are persisting in Panama, even though Bd is still present and highly pathogenic (Perez et al. 2014; Voyles et al. 2018; Byrne et al. 2021; Rosa et al. 2022). The historical sites where golden frogs are distributed have different ecological characteristics. For example, golden frogs persist at similar elevations (between 500 and 800 m) but in sites situated on either aspect of the Cordillera Central (the south-facing slopes towards the Pacific Ocean and the North facing slopes towards the Caribbean Sea; Fig. 1a). Accordingly, these two sites have dramatically different microhabitat conditions (Fig. 1b, c), which may play an important role in the disease dynamics and evolutionary trajectories in these golden frog populations. Understanding the role of temperature and humidity on the differential recovery of golden frog populations is likely to be crucial for the success of reintroduction strategies for this species. For example, this information can offer direction on how managers could proceed with translocations and/or the directed release of animals from captivity into environments that would best promote survival and mediate the risk of mortality due to severe chytridiomycosis.
Two sites in Panama where golden frogs have persisted following outbreaks of the lethal amphibian disease chytridiomycosis (a). The Caribbean site (black) and the Pacific site (gray) are at similar elevations (between 500 and 800 m) but situated on either aspect of the Cordillera Central (the south-facing slopes towards the Pacific Ocean and the North facing slopes towards the Caribbean Sea). These two sites have different relative humidity (b) and temperature (c) profiles (data from temperature and humidity recording devices (Onset HOBO Pro v2, temp/PH) placed in amphibian microhabitats in 2014)
To disentangle the role of humidity and temperature in chytridiomycosis, we conducted two experiments using environmental chambers to control temperature and relative humidity. In Experiment 1, hereafter referred to as the “Humidity Experiment”, we tested if host immunity (production of skin secretions) would be altered under three different levels of relative humidity (RH), followed by a Bd inoculation experiment. We predicted that humidity would alter the quantity of skin secretions produced by frogs, with more skin secretions produced in drier conditions. In Experiment 2, hereafter referred to as the “Humidity and Temperature Experiment”, we investigated two levels (high and low) of both temperature and relative humidity to see if variations of these conditions altered susceptibility to disease. We predicted that frogs would be the most successful in the high temperature and low relative humidity treatments since these conditions mirror putative disease refugia sites for amphibians in tropical regions (Woodhams and Alford 2005; Puschendorf et al. 2009, 2011; Zumbado-Ulate et al. 2014).
Methods
Experimental setup
In the Humidity Experiment, we tested to see if hosts that experienced a shift in relative humidity conditions would alter the production of skin secretions. We collected baseline samples from captive Panamanian golden frogs (Atelopus zeteki), that were acclimated to 21 °C and low (50%) relative humidity (RH; more details provided in the supplementary material). We extracted skin secretions from half of the frogs and subsequently randomly assigned them to one of three environmental chambers set to maintain the same temperature (21 °C) but at three different levels of relative humidity: high (80%), medium (65%), and low (55%) RH (Supplemental Fig. 1). We allowed frogs to replenish their skin secretions and collected samples a second time after 30 days (enough time for the skin secretions to naturally replenish; Rollins-Smith 2005; Ramsey et al. 2010). We allowed an additional 30 days for frogs to replenish their secretions, and we exposed frogs (N = 10) to Bd or a control, sham solution (N = 10) in each chamber (Supplemental Fig. 1).
For the Humidity and Temperature Experiment, we investigated the importance of humidity and temperature on A. zeteki survival after Bd exposure. We chose temperature and humidity combinations based on two field sites in Panama where wild populations of golden frogs are persisting despite Bd infection (Fig. 1). We placed the first treatment group (N = 20) into a high RH (80–90%) and high temperature (26–27 °C) environmental chamber. We placed the second treatment group (N = 20) into a high RH (80–90%) and a low temperature (20–21 °C) environmental chamber. We placed the third treatment group (N = 20) into a low RH (50–60%) and a high temperature (26–27 °C) environmental chamber. We placed the final treatment group (N = 20) in a low RH (50–60%) and a low temperature (20–21 °C) room. We exposed N = 10 frogs from each treatment group to the Bd isolate Rio Maria, while we exposed N = 10 frogs to a sham exposure solution. We exposed frogs to Bd or the sham solution for 24 h while in their respective temperature and humidity conditions, after which the frogs were returned to their original containers with fresh water. Frogs subsequently remained in their respective temperature and humidity treatments for the duration of the experiment.
Collection of skin secretions
We used the hormone norepinephrine (NE; 40 nmol per gram body mass, Gass and Voyles 2022) to stimulate the frog’s sympathetic nervous system, causing them to release stored skin secretions from their cutaneous granular glands following standard methods that have been described (Rollins-Smith 2005; Gass and Voyles 2022, and see supplemental information). Since NE is eliminated from the body within minutes (Smith and Maani 2019), we could be confident that this reduction of skin secretions has minimal long-term effects on the immune system. After NE injection, skin secretions remain depleted from 21–30 days, after which they are naturally replenished (Rollins-Smith 2005; Ramsey et al. 2010; Pask et al. 2013). We controlled for any potential effects of NE by including N = 10 frogs that received injections of sterile amphibian phosphate-buffered saline [aPBS; 6.6 g/L sodium chloride, 1.15 g/L sodium phosphate, and 0.2 g/L potassium phosphate in 1 L of distilled water, filter sterilized using a sterile nylon mesh filter (Millipore Express)].
Bd challenge assays in vitro
To determine the inhibitory effectiveness of the skin secretions taken from the frogs that received NE injections, we conducted an in vitro Bd challenge assay. We cultured the Bd isolate “Rio Maria” (Voyles et al. 2018) using a nutrient rich tryptone-gelatin hydrolysate-lactose (TGhL) media in 25 cm2 tissue culture flasks at 21 °C. We observed Bd flasks daily until the day of peak zoospore activity when we filtered the culture to remove any sporangia (Voyles 2011) and counted Bd zoospores using a hemocytometer. We diluted the zoospores to a concentration of approximately 5 × 105 zoospores per ml−1 in 96 well plates. We prepared three replicate wells for each skin secretion sample containing 40 µl of skin secretions, 50 µl of TGhL media, and 10 µl of live Bd. We included three wells per sample as positive controls for Bd growth, containing 40 µl of HPLC water, 50 µl of TGhL media, and 10 µl of live Bd. We also included three negative control wells per sample containing 40 µl skin secretions, 50 µl TGhl media, and 10 µl of heat-killed Bd. We monitored the plates daily for zoospore activity and used a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay and measured OD at 570 nm using a microplate reader on the day of peak zoospore release (five days after passaging; Lindauer et al. 2019). We subtracted the OD reading from the negative control wells from our sample and positive control wells to normalize the OD data.
Bd exposures in vivo
The Bd exposures for both experiments were set up identically. First, we weighed each frog to the nearest 0.1 g and measured snout-vent length (SVL) to the nearest 0.1 mm to calculate body condition (mass/SVL; Dodd 2010; Whiteman et al. 2012). We measured body condition as a gauge of frog health, as both Bd infection and altered humidity and temperature regimes have the potential to impact frog body condition (Supplemental Fig. 3; Retallick and Miera 2007). We swabbed each frog with a Medical Wire swab (MWE REF100, Wiltshire, England, UK) 10 times on the ventral side, 10 times on each thigh, and 5 times on each hand and foot (Hyatt et al. 2007). We analyzed these swabs using qPCR to determine that frogs were negative for Bd before beginning the experiment. We took measurements of frog mass, SVL, and collected diagnostic swab samples at two-week intervals until the end of the experiments.
We cultured the Bd isolate Rio Maria, originally from Panama and highly pathogenic in Atelopus frogs (Voyles et al. 2018; Gass and Voyles 2022), using the methods described above. We counted Bd zoospores using a hemocytometer and diluted them using TGhL media until they reached a concentration of 6.5 X 104 zoospores per ml−1. We then placed frogs into the exposure containers (7.6 cm diameter, 3.5 cm height) containing 11 ml of 20% Holtfretter’s solution [250 ml, (in mMol) 6.0 NaCl, 0.06 KCL, 0.09, CaCl2, 0.24, NaCO3, pH 7.0; Wright and Whitaker 2001]. We added 1 ml of live Bd culture to the exposure container for the Bd exposed frogs. For the sham-exposed frogs, we added 1 ml TGhL media. The frogs remained in the exposure containers for 24 h, after which we placed them back into their housing containers with 100 ml of 20% Holtfretter’s solution. We monitored frogs twice daily until we observed clinical signs of severe disease (Voyles et al. 2009) when we collected one final diagnostic Bd swab and euthanized via shallow immersion in a bath of 0.1% tricaine methanesulfonate (MS-222; Fisher Scientific, WA, USA).
DNA extraction and qPCR amplification
We used a DNeasy Blood and Tissue DNA Extraction Kit following the manufacturer’s directions (Qiagen, Valencia, CA, USA; animal tissue protocol) to extract Bd DNA from the diagnostic swabs. We followed this with a real-time quantitative polymerase chain reaction (qPCR) run on a QuantStudio 3 Real-Time PCR instrument (Life Technologies, Singapore) to quantify the Bd load for each swab (Boyle et al. 2004). We analyzed samples in singlicate with an internal positive control (IPC, Garland et al. 2010) and a dilution set of plasmid standards (Pisces Molecular, Boulder, CO) to quantify Bd load. To convert plasmid copy numbers to genomic equivalents, we used the formula Bd load = Quantity * 40. If one of the samples appeared positive, we checked the Cycle Threshold (Ct) value to determine if a low-level infection was likely and we verified that qPCR was working properly by confirming IPC amplification. We re-ran any samples that produced uncertain results.
Statistical analysis
To determine if there were differences in the amount of skin secretions released at the beginning of the humidity experiment among the three treatment groups, we used a non-parametric Kruskal–Wallis test because our data were not normally distributed despite transformations. To compare the amount of skin secretions released over time (i.e., by each group at the beginning of the experiment and after a month), we used a paired Wilcoxon sign rank test because the data were not normally distributed.
To compare how Bd load changed over time among the Bd exposed groups for both experiments, we ran a GLMM with a Poisson distribution with Bd load as the dependent variable, treatment group and time as interacting fixed effects, and individual frogs as the random effect (package: “lme4,” function: “glmer,” Bates et al. 2014). To compare mean survival time as well as Bd load, presented as genomic equivalents, on the date of death for the Bd exposed frogs we used an ANOVA followed by Tukey post hoc tests or a Kruskal–Wallis test if data were not normally distributed. We generated a Kaplan–Meier survival curve and analyzed survival data using a log-rank test using the R package “survival” (Therneau and Gramsch 2000). We performed all statistical analyses using R version 4.1.3 (R Core Team 2022).
Results
Experiment 1: Humidity experiment
There were no significant differences in the amount of skin secretions released among the RH treatment groups at the start of the experiment (χ2 = 4.2068, df = 2, p = 0.122) or after 30 days (χ2 = 2.434, df = 2, p = 0.2961; Fig. 2). However, the frogs in the high and medium RH groups had a significant decrease in the amount of skin secretions they produced after spending 30 days in the RH treatments (high RH conditions: Wilcoxon; Z = − 2.465, p = 0.014; medium RH conditions: Wilcoxon; Z = − 2.418, p = 0.016; Fig. 2). Conversely, the low RH group had no significant change in the amount of skin secretions released after 30 days (Wilcoxon; Z = − 0.602, p = 0.546; Fig. 2).
Mean concentrations (μg/gram body mass) of skin peptide samples collected from golden frogs (Atelopus zeteki) following injections of norepinephrine (40 nmol/gram body weight) in three treatment groups that were moved from low relative humidity (RH) to high, medium, and low RH. Time 1 refers to mean skin secretions collected from frogs at the beginning of the experiment (solid boxes). Time 2 refers to mean skin secretions collected from frogs after 30 days at each humidity treatment (open boxes). Frogs moved into the high RH and medium RH treatment groups produced significantly fewer skin secretions after 30 days in the new RH conditions
All Bd-exposed frogs became infected with Bd, developed clinical signs of chytridiomycosis, and tested positive for Bd at every sample collection time point. Although there was a trend suggesting slightly higher Bd loads for the frogs in the low and medium RH conditions, the differences were not significant over the course of the experiment (GLMM with a Poisson distribution, χ2 = 0.7229, df = 2, p = 0.7) or on the date of death (ANOVA: F = 0.565, p = 0.575). None of the control frogs showed clinical signs of infection or died of chytridiomycosis. While every control frog except one tested slightly Bd positive (2–3 Log GE + 1) on week 6, all control frogs were Bd negative with subsequent testing and never showed any signs of chytridiomycosis, suggesting that these qPCR results were likely false positives. In contrast, all the Bd-exposed frogs showed clinical signs of severe chytridiomycosis and died within 54 days of exposure. We observed a trend suggesting that frogs in the low and medium RH conditions succumbed to chytridiomycosis more rapidly than the frogs in the high RH conditions, although there were no significant differences in survival (mean number of days survived post inoculation: ANOVA: F = 2.75, p = 0.0825; Fig. 3a, survival: Kaplan–Meier log-rank test: χ2 = 5.8, p = 0.06; Fig. 3b).
Survival among groups of golden frogs (Atelopus zeteki) from the Humidity Experiment that were moved from low relative humidity (RH) to one of three treatment conditions, high (magenta), medium (dark orange), or low (light orange) RH, and subsequently inoculated with the lethal fungal pathogen Batrachochytrium dendrobatidis (Bd). There were no significant differences among treatment groups in overall survival (a) or mean days of survival (b)
Experiment 2: Humidity and temperature experiment
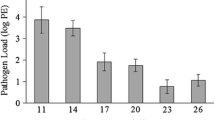
The Bd isolate Rio Maria was highly pathogenic in all groups of exposed frogs except for the high temperature and high RH group. Whereas every Bd-exposed frog in the other three treatment conditions tested Bd positive at every time point and died within 77 days of exposure, we found that three frogs in the high humidity and high temperature treatment group cleared Bd infection, and eight survived until the end of the experiment. We also found that there was a significant effect of the treatment group and time on the pathogen load among the Bd exposed groups (GLMM with a Poisson distribution, χ2 = 32.130, df = 3, p < 0.001; Fig. 4). Additionally, frogs in the high humidity and high temperature group survived significantly longer than all other groups (Kaplan Meier log-rank test: χ2 = 28, df = 3, p < 0.001, ANOVA: F = 68.42, df = 3, p < 0.001; Fig. 5).
Change in mean infection intensity (± SE) of all four Bd-exposed treatment groups from the Humidity and Temperature experiment: high relative humidity (RH) and high temperature (purple), high RH and low temperature (blue), low RH and high temperature (teal), and low RH and low temperature (mint). Infection intensity was calculated as the log (genomic equivalents + 1) following qPCR analysis
Survival among groups of golden frogs (Atelopus zeteki) in the Humidity and Temperature Experiment that were moved from low relative humidity (RH) to one of four treatment conditions: high relative humidity (RH) and high temperature (purple), high RH and low temperature (blue), low RH and high temperature (teal), and low RH and low temperature (mint) and subsequently inoculated with the lethal fungal pathogen Batrachochytrium dendrobatidis (Bd) or exposed to sham control solution (grey). The high RH and high temperature group had significantly greater overall survival (a) and mean days of survival (b) compared to the other three Bd exposed groups
Discussion
The environment plays a critical role in determining epizootics, pathogen prevalence, and infection outcomes across many disease systems (Altizer et al. 2006). While the importance of temperature on both pathogens and hosts has been extensively studied (Thomas and Blanford 2003; Ward et al. 2007; Webb et al. 2010; Karvonen et al. 2010; Sonn et al. 2017; Bailey et al. 2017; Price et al. 2019; Mordecai et al. 2019), the role of humidity in driving disease dynamics is much less clear. We used the chytridiomycosis disease system to investigate the importance of temperature and humidity on disease outcomes in a highly endangered amphibian.
The golden frogs of Panama (Atelopus zeteki) are members of one of the most imperiled groups of amphibians in the world (La Marca et al. 2005). These species are highly endangered in the wild but exist in large populations in captivity with prospective plans for reintroduction (Lewis et al. 2019). As such, determining what environmental conditions may create disease refugia is considered a key factor for decision making in reintroduction planning (Lewis et al. 2019). Our prediction that frogs would have the greatest survival at high temperature and low relative humidity (RH) was partially supported. We found increasing frog survival in the high temperature conditions. However, the high temperature was only protective when paired with high RH, not low RH as we originally predicted. That frogs survived better at high RH was surprising, as many putative disease refugia sites are characterized as hot and dry (Puschendorf et al. 2009, 2011).
Additionally, frogs produced significantly fewer skin secretions over 30 days when kept at high RH and medium RH when compared to the low RH group. One possible explanation for this is that skin secretions are produced to help prevent desiccation in frogs (Lillywhite and Licht 1975) but doing so may be energetically costly (Blennerhassett et al. 2019). As such, we suggest that the groups maintained at high and medium RH may have decreased the production of skin secretions as they were at a lower risk of desiccation (Lillywhite and Licht 1975). Furthermore, frogs that significantly decreased the amount of skin secretions in the high and medium RH conditions survived longer than the frogs in the low RH conditions (where frogs had no changes in skin secretion production over time).
The Humidity and Temperature Experiment revealed that frogs survived the best at the high temperature and high RH treatment, with three frogs clearing Bd infection, and eight frogs surviving past the end of the experiment. The fact that some frogs cleared Bd infection was particularly intriguing given that several previous studies have shown no recovery or survival of golden frogs exposed to Bd under a wide variety of experimental conditions, including temperature treatments (Bustamante et al. 2010), applications of anti-Bd probiotic microbes (Becker et al. 2011), and exposure to different Bd lineages (Langhammer et al. 2013). Only one previous study has been able to show survival in small number (five) of A. zeteki frogs (Becker et al. 2015). For our study, the finding that high temperature and high RH can allow some frogs to survive, and even clear well-established Bd infections, underscores how critically important local and regional environmental conditions can be for disease dynamics and survival for some host species.
Our results suggest that high temperature coupled with high RH was more protective than the other three temperature and humidity combinations against disease development and mortality in an otherwise highly susceptible amphibian host species. We suggest that the specific temperature and relative humidity conditions experienced by amphibians may have mediated the production of amphibian skin secretions such that pathogen reproduction (Bd growth rates) and disease development were altered. In a previous study, researchers found that skin secretions of captive A. zeteki were not protective, but rather enhanced Bd growth in vitro and may have exacerbated disease development and mortality (Gass and Voyles 2022). Our results corroborate those findings supporting that the production of skin secretions is associated with disease development in A. zeteki. Therefore, a reduction of skin secretions in high RH conditions may provide a mechanistic explanation for the survival and persistence of some host species in warm and humid sites in Panama.
While the temperature constraints of Bd are relatively well established (Piotrowski et al. 2004; Voyles et al. 2017) the importance of humidity in chytridiomycosis outcomes has been more difficult to unravel (Brannelly et al. 2015). Given that Bd is sensitive to desiccation (Johnson and Speare 2003) and relies on water to move for part of its life cycle (Longcore et al. 1999), water availability is a critical determinant of Bd infection outcome (Rowley and Alford 2007). Previous work with A. zeteki showed that frogs that could behaviorally avoid water survived longer than frogs who remained in water (and completely saturated) during a Bd exposure experiment (Bustamante et al. 2010). Additionally, Atelopus zeteki frogs have been shown to behaviorally thermoregulate to limit their disease risk during a Bd epidemic (Richards-Zawacki 2010). While the frogs in this study were not given the opportunity to behaviorally thermoregulate (as they were held at constant temperatures), they may have behaviorally responded to the RH conditions. All the frogs in both of our experiments had 100 ml of 20% Holtfretter’s solution constantly at one end of their containers, so it is possible that frogs in the low RH groups spent a greater amount of time in the water compared to frogs in the high RH treatments, which could have led to high mortality. Another possible explanation for the enhanced survival of frogs kept at high RH is that the humidity levels altered the amount of skin secretions produced such that frogs were able to allocate more energy towards other elements of their immune systems to limit Bd infection. These possibilities remain to be investigated.
This study investigated the role of relative humidity in chytridiomycosis in tropical amphibian host species. Our findings support several previous studies that suggested that high temperature conditions improve frog survival and may limit Bd prevalence and intensity of infection (Berger et al. 2004; Woodhams and Alford 2005; Bustamante et al. 2010). While some studies have shown that dry areas are ideal disease refugia sites for amphibians against chytridiomycosis (Woodhams and Alford 2005; Puschendorf et al. 2009, 2011; Zumbado-Ulate et al. 2014), this study suggests that dry conditions (RH less than 60%) may not be necessarily more protective against Bd in all amphibian host species. Pinpointing which environmental conditions will best promote host survival while limiting pathogen growth is important for selecting successful reintroduction sites for golden frogs (Hettyey et al. 2019). More broadly, these findings reinforce the importance of incorporating all facets of the disease triad (hosts, pathogens, and the environment) to elucidate patterns of disease emergence and inform conservation decisions on wildlife threatened by emerging infectious diseases.
Data availability
Data will be available on Dryad upon the publication of the manuscript.
References
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol 9(4):467–484
Bailey C, Segner H, Casanova-Nakayama A, Wahli T (2017) Who needs the hotspot? The effect of temperature on the fish host immune response to Tetracapsuloides bryosalmonae the causative agent of proliferative kidney disease. Fish Shellfish Immunol 63:424–437
Bates D, Mächler M, Bolker B, and Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823.
Becker MH, Harris RN, Minbiole KPC, Schwantes CR, Rollins-Smith LA, Reinert LK, Brucker RM, Domangue RJ, Gratwicke B (2011) Towards a Better Understanding of the Use of Probiotics for Preventing Chytridiomycosis in Panamanian Golden Frogs. EcoHealth 8(4):501–506
Becker MH, Walke JB, Cikanek S, Savage AE, Mattheus N, Santiago CN, Minbiole KPC, Harris RN, Belden LK, Gratwicke B (2015) Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc R Soc B 282(1805):20142881
Bienentreu JF, Lesbarrères D (2020) Amphibian disease ecology: are we just scratching the surface? Herpetol 76(2):153–166
Berger L, Speare R, Hines H, Marantelli G, Hyatt A, McDonald K, Skerratt L, Olsen V, Clarke J, Gillespie G, Mahony M, Sheppard N, Williams C, Tyler M (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J 82(7):434–439
Blennerhassett RA, Bell-Anderson K, Shine R, Brown GP (2019) The cost of chemical defence: the impact of toxin depletion on growth and behaviour of cane toads (Rhinella marina). Proc R Soc b 286(1902):20190867
Boyle D, Boyle D, Olsen V, Morgan J, Hyatt A (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Brannelly LA, Berger L, Marrantelli G, Skerratt LF (2015) Low humidity is a failed treatment option for chytridiomycosis in the critically endangered southern corroboree frog. Wildl Res 42(1):44
Bustamante HM, Livo LJ, Carey C (2010) Effects of temperature and hydric environment on survival of the Panamanian Golden Frog infected with a pathogenic chytrid fungus. Integr Zool 5(2):143–153
Byrne AQ, Richards-Zawacki CL, Voyles J, Bi K, Ibáñez R, Rosenblum EB (2021) Whole exome sequencing identifies the potential for genetic rescue in iconic and critically endangered Panamanian harlequin frogs. Glob Chan Biol 27(1):50–70
Cohen JM, Sauer EL, Santiago O, Spencer S, Rohr JR (2020) Divergent impacts of warming weather on wildlife disease risk across climates. Science 370:6519
Dodd CK (2010) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, Oxford
Ellison AR, Tunstall T, DiRenzo GV, Hughey MC, Rebollar EA, Belden LK, Harris RN, Ibáñez R, Lips KR, Zamudio KR (2015) More than skin deep: functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biol Evol 7(1):286–298
Engering A, Hogerwerf L, Slingenbergh J (2013) Pathogen–host–environment interplay and disease emergence. Emerg Microbes Infect 2(1):1–7
Garland S, Baker A, Phillott A, Skerratt L (2010) BSA reduces inhibition in a TaqMan® assay for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org 92(3):113–116
Gass, J, and Voyles J (2022) When defenses fail: Atelopus zeteki Skin secretions increase growth of the pathogen Batrachochytrium dendrobatidis. Integr Comp Biol icac060
Hawley DM, Altizer SM (2011) Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol 25(1):48–60
Heard GW, Thomas CD, Hodgson JA, Scroggie MP, Ramsey DSL, Clemann N (2015) Refugia and connectivity sustain amphibian metapopulations afflicted by disease. Ecology 18(8):853–863
Hettyey A, Ujszegi J, Herczeg D, Holly D, Vörös J, Schmidt BR, Bosch J (2019) Mitigating disease impacts in amphibian populations: capitalizing on the thermal optimum mismatch between a pathogen and its host. Front Ecol Evol 7:254
Hueffer K, O’Hara TM, Follmann EH (2011) Adaptation of mammalian host-pathogen interactions in a changing arctic environment. Acta Vet Scand 53(1):17
Hyatt A, Boyle D, Olsen V, Boyle D, Berger L, Obendorf D, Dalton A, Kriger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Colling A (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org 73:175–192
James TY, Toledo LF, Rödder D, Silva Leite D, Belasen AM, Betancourt-Román CM, Jenkinson TS, Soto-Azat C, Lambertini C, Longo AV, Ruggeri J, Collins JP, Burrowes PA, Lips KR, Zamudio KR, Longcore JE (2015) Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: Lessons from the first 15 years of amphibian chytridiomycosis research. Ecol Evol 5(18):4079–4097
Johnson ML, Speare R (2003) Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerg Infect Dis 9(8):915–921
Karvonen A, Rintamäki P, Jokela J, Valtonen ET (2010) Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. Int J Parasitol 40(13):1483–1488
Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ (2011) Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg’s “10,000 Zoospore Rule.” PLoS ONE 6(3):e16708
La Marca E, Lips KR, Lotters S, Puschendorf R, Ibanez R, Rueda-Almonacid JV, Schulte R, Marty C, Castro F, Manzanilla-Puppo J, Garcia-Perez JE, Bolanos F, Chaves G, Pounds JA, Toral E, Young BE (2005) Catastrophic population declines and extinctions in Neotropical Harlequin Frogs (Bufonidae: Atelopus)1. Biotropica 37(2):190–201
Lafferty KD, Mordecai EA (2016) The rise and fall of infectious disease in a warmer world. F1000Research 5:2040
Langhammer PF, Lips KR, Burrowes PA, Tunstall T, Palmer CM, Collins JP (2013) A fungal pathogen of amphibians, Batrachochytrium dendrobatidis, attenuates in pathogenicity with in vitro passages. PLoS ONE 8(10):e77630
Le Sage EH, LaBumbard BC, Reinert LK, Miller BT, Richards-Zawacki CL, Woodhams DC, Rollins-Smith LA (2021) Preparatory immunity: Seasonality of mucosal skin defenses and Batrachochytrium infections in Southern leopard frogs. J Anim Ecol 90(2):542–554
Lewis CHR, Richards-Zawacki CL, Ibáñez R, Luedtke J, Voyles J, Houser P, Gratwicke B (2019) Conserving Panamanian harlequin frogs by integrating captive-breeding and research programs. Biol Conserv 236:180–187
Lillywhite HB, Licht P (1975) A comparative study of integumentary mucous secretions in amphibians. Comp Biochem Physiol 51(4):937–941
Lindauer A, May T, Rios-Sotelo G, Sheets C, Voyles J (2019) Quantifying Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans Viability. EcoHealth 16(2):346–350
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. PNAS 103(9):3165–3170
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. Et. Sp. Nov., a chytrid pathogenic to amphibians. Mycologia 91(2):219–227
Lötters S (1996) The neotropical toad genus Atelopus: checklist, biology, distribution. M. Vences and F. Glaw Verlags, Köln
McArthur DB (2019) Emerging infectious diseases. Nurs Clin North Am 54(2):297–311
McCaffery R, Richards-Zawacki CL, Lips KR (2015) The demography of Atelopus decline: harlequin frog survival and abundance in central Panama prior to and during a disease outbreak. Glob Ecol Conserv 4:232–242
McDonald RK, Méndez D, Müller R, Freeman AB, Speare R (2005) Decline in the prevalence of chytridiomycosis in frog populations in North Queensland. Australia Pac Conserv Biol 11(2):114
McMillan KM, Lesbarrères D, Harrison XA, Garner TWJ (2020) Spatiotemporal heterogeneity decouples infection parameters of amphibian chytridiomycosis. J Anim Ecol 89(4):1109–1121
Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, Rohr JR, Ryan SJ, Savage V, Shocket MS, Sippy R, Stewart Ibarra AM, Thomas MB, Villena O (2019) Thermal biology of mosquito-borne disease. Ecology 22(10):1690–1708
Morens DM, Folkers GK, Fauci AS (2004) The challenge of emerging and re-emerging infectious diseases. Nature 430(6996):242–249
Murray KA, Skerratt LF, Garland S, Kriticos D, McCallum H (2013) Whether the weather drives patterns of endemic amphibian chytridiomycosis: a pathogen proliferation approach. PLoS ONE 8(4):e61061
Pask JD, Cary TL, and Rollins-Smith LA (2013) Skin peptides protect juvenile leopard frogs (Rana pipiens) against chytridiomycosis. J Exp Biol jeb.084145
Perez R, Richards-Zawacki CL, Krohn AR, Robak M, Griffith EJ, Ross H, Gratwicke B, Ibáñez R, Voyles J (2014) Field surveys in Western Panama indicate populations of Atelopus varius frogs are persisting in regions where Batrachochytrium dendrobatidis is now enzootic. Amphib Reptile Conserv 8(2):6
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96(1):9–15
Price SJ, Leung WTM, Owen CJ, Puschendorf R, Sergeant C, Cunningham AA, Balloux F, Garner TWJ, Nichols RA (2019) Effects of historic and projected climate change on the range and impacts of an emerging wildlife disease. Glob Change Biol 25(8):2648–2660
Puschendorf R, Carnaval AC, VanDerWal J, Zumbado-Ulate H, Chaves G, Bolaños F, Alford RA (2009) Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: Proposing climatic refuges as a conservation tool. Divers Distrib 15(3):401–408
Puschendorf R, Hoskin CJ, Cashins SD, McDonald K, Skerratt LF, Vanderwal J, Alford RA (2011) Environmental refuge from disease-driven amphibian extinction: environmental refuge from extinction. Conserv Biol 25(5):956–964
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African Clawed Frog, Xenopus laevis. Infect Immunol 78(9):3981–3992
Retallick RW, Miera V (2007) Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis Aquat Organ 75(3):201–207
Richards-Zawacki CL (2010) Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc R Soc b 277(1681):519–528
Robak MJ, Reinert LK, Rollins-Smith LA, Richards-Zawacki CL (2019) Out in the cold and sick: Low temperatures and fungal infections impair a frog’s skin defenses. J Exp Biol jeb.209445
Rollins-Smith LA (2005) Antimicrobial peptide defenses in amphibian skin. Integr Comp Biol 45(1):137–142
Rollins-Smith LA (2017) Amphibian immunity–stress, disease, and climate change. Dev Comp Immunol 66:111–119
Rollins-Smith LA, Woodhams DC (2012) Amphibian immunity. Oxford University Press, New York, pp 92–143
Rosa GM, Perez R, Richards LA, Richards‐Zawacki CL, Smilanich AM, Reinert LK, Rollins‐Smith LA, Wetzel DP, Voyles J (2022) Seasonality of host immunity in a tropical disease system. Ecosphere 13(7)
Rowley J, Alford R (2007) Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis Aquat Org 77:1–9
Rowley JJ, Alford RA (2013) Hot bodies protect amphibians against chytrid infection in nature. Sci Rep 3(1):1515
Ruggeri J, Martins AGDS, Domingos AHR, Santos I, Viroomal IB, Toledo LF (2020) Seasonal prevalence of the amphibian chytrid in a tropical pond-dwelling tadpole species. Dis Aquat Org 142:171–176
Russell ID, Larson JG, von May R, Holmes IA, James TY, Davis Rabosky AR (2019) Widespread chytrid infection across frogs in the Peruvian Amazon suggests critical role for low elevation in pathogen spread and persistence. PLoS ONE 14(10):e0222718
Scheele BC, Foster CN, Hunter DA, Lindenmayer DB, Schmidt BR, Heard GW (2019) Living with the enemy: facilitating amphibian coexistence with disease. Biol Conserv 236:52–59
Schmeller DS, Courchamp F, Killeen G (2020) Biodiversity loss, emerging pathogens and human health risks. Biodivers Conserv 29:3095–3102
Schmeller DS, Cheng T, Shelton J, Lin CF, Chan-Alvarado A, Bernardo-Cravo A, Zoccarato L, Ding TS, Lin YP, Swei A, Fisher MC (2022) Environment is associated with chytrid infection and skin microbiome richness on an amphibian rich island (Taiwan). Sci Rep 12(1):16456
Sheets CN, Schmidt DR, Hurtado PJ, Byrne AQ, Rosenblum EB, Richards-Zawacki CL, Voyles J (2021) Thermal performance curves of multiple isolates of Batrachochytrium dendrobatidis, a lethal pathogen of amphibians. Front Vet Sci 8:687084
Smith MD, Maani CV (2019) Administration. In: Norepinephrine. Treasure Island, Florida StatPearls Publishing
Sonn JM, Berman S, Richards-Zawacki CL (2017) The influence of temperature on chytridiomycosis in vivo. EcoHealth 14(4):762–770
Therneau TM, Grambsch PM (2000) The cox model. In: Modeling survival data: extending the cox model. Statistics for biology and health. Springer, New York
Thomas MB, Blanford S (2003) Thermal biology in insect-parasite interactions. Trends Ecol Evol 18(7):344–350
Tompkins DM, Carver S, Jones ME, Krkošek M, Skerratt LF (2015) Emerging infectious diseases of wildlife: a critical perspective. Trends Parasitol 31(4):149–159
Turner A, Wassens S, Heard G, Peters A (2021) Temperature as a driver of the pathogenicity and virulence of amphibian chytrid fungus Batrachochytrium dendrobatidis: a systematic review. J Wildl Dis 57(3):477–494
Voyles J (2011) Phenotypic profiling of Batrachochytrium dendrobatidis, a lethal fungal pathogen of amphibians. Fungal Ecol 4(3):196–200
Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF, Speare R (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326(5952):582–585
Voyles J, Johnson LR, Briggs CJ, Cashins SD, Alford RA, Berger L, Skerratt LF, Speare R, Rosenblum EB (2012) Temperature alters reproductive life history patterns in Batrachochytrium dendrobatidis, a lethal pathogen associated with the global loss of amphibians. Ecol Evol 2(9):2241–2249
Voyles J, Johnson LR, Rohr J, Kelly R, Barron C, Miller D, Minster J, Rosenblum EB (2017) Diversity in growth patterns among strains of the lethal fungal pathogen Batrachochytrium dendrobatidis across extended thermal optima. Oecologia 184(2):363–373
Voyles J, Woodhams DC, Saenz V, Byrne AQ, Perez R, Rios-Sotelo G, Ryan MJ, Bletz MC, Sobell FA, McLetchie S, Reinert L, Rosenblum EB, Rollins-Smith LA, Ibáñez R, Ray JM, Griffith EJ, Ross H, Richards-Zawacki CL (2018) Shifts in disease dynamics in a tropical amphibian assemblage are not due to pathogen attenuation. Science 359(6383):1517–1519
Ward J, Kim K, Harvell C (2007) Temperature affects coral disease resistance and pathogen growth. Mar Ecol Prog 329:115–121
Webb KM, Oña I, Bai J, Garrett KA, Mew T, Vera Cruz CM, Leach JE (2010) A benefit of high temperature: increased effectiveness of a rice bacterial blight disease resistance gene. New Phytol 185(2):568–576
Whiteman HH, Wissinger SA, Denoël M, Mecklin CJ, Gerlanc NM, Gutrich JJ (2012) Larval growth in polyphenic salamanders: Making the best of a bad lot. Oecologia 168:109–118
Wilber MQ, Ohmer ME, Altman KA, Brannelly LA, LaBumbard BC, Le Sage EH, McDonnell NB, Muñiz Torres AY, Nordheim CL, Pfab F, Richards-Zawacki CL (2022) Once a reservoir, always a reservoir? Seasonality affects the pathogen maintenance potential of amphibian hosts. Ecology 103(9):e3759
Woodhams DC, Alford RA (2005) Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical queensland. Conserv Biol 19(5):1449–1459
Woodhams DC, Voyles J, Lips KR, Carey C, Rollins-Smith LA (2006) Predicted diseases susceptibility in a Panamanian amphibian assemblage based on skin peptide defenses. J Wildl Dis 42(2):207–218
Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv 10(4):409–417
Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA (2008) Life-history trade-offs influence disease in changing climates: strategies of an amphibian pathogen. Ecology 89(6):1627–1639
Wright KM, Whitaker BR (2001) Amphibian medicine and captive husbandry. Krieger Publishing Company, Malabar
Zumbado-Ulate H, Bolaños F, Gutiérrez-Espeleta G, Puschendorf R (2014) Extremely low prevalence of Batrachochytrium dendrobatidis in frog populations from neotropical dry forest of costa rica supports the existence of a climatic refuge from disease. EcoHealth 11(4):593–602
Acknowledgements
We thank A. Reeves, D. Benson, V. Poole, K. Barrett, and the Omaha and Maryland Zoos and Project golden frog for providing captive-bred frogs. We thank M.D. Basanta, L. Carriere, and I. Burgos for their assistance with data collection and animal husbandry.
Funding
This research was supported by the National Science Foundation (2120084 and 1846403 to JV).
Author information
Authors and Affiliations
Contributions
JG and JV conceived the ideas and designed the methodology; JG, JV, AJM, CS, and ML collected the data. JG and JV analyzed the data and wrote the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest or competing interests to share.
Ethical approval
We performed all experiments with approval from, and in accordance with, the ethical standards of the UNR Institutional Animal Care and Use Committee under protocol #20–08–1063.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gass, J., Miller, A.J., Sheets, C. et al. High relative humidity and temperature limit disease development and mortality in golden frogs of Panama, Atelopus zeteki, infected with Batrachochytrium dendrobatidis. Evol Ecol 38, 141–156 (2024). https://doi.org/10.1007/s10682-023-10247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-023-10247-3