Abstract
Directional sexual selection drives the evolution of traits that are most closely linked to reproductive success, giving rise to trait exaggeration and sexual dimorphism. Exaggerated structures are often costly and, therefore, thought to be expressed in a condition-dependent manner. Sexual selection theory thus predicts a direct link between directional sexual selection, sexual dimorphism, and sex-specific condition dependence. However, only a handful of studies investigate the relationship between sexual dimorphism and condition dependence. Using 21 genetic lines of Drosophila prolongata, we here compared the degree of sexual dimorphism and sex-specific condition dependence, measured as allometric slopes, in sexually selected and non-sexual traits. Our data revealed male-biased sexual dimorphism in all traits examined, most prominently in the sexually selected forelegs. However, there was no relationship between the degree of sex-specific condition dependence and sexual dimorphism across traits and genetic lines. Our results contradict theoretical predictions and highlight the importance of understanding the role of exaggerated traits in the context of both sexual and natural selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection is predicted to favor the exaggeration of pre- and post-mating traits whenever the extent of their elaboration increases reproductive success through male-male competition or female choice (Darwin 1871; West-Eberhard 1983; Andersson 1994; Emlen 2008; Lüpold et al. 2016). Since sexual selection is stronger on males than on females in most species (Bateman 1948; Trivers 1972; Clutton-Brock and Parker 1992), traits acting as ornaments or armaments often evolve absolutely or relatively greater expression in males relative to females (i.e. male-biased sexual dimorphism). However, trait elaboration is typically costly in terms of energy and fitness (e.g., Somjee et al. 2018; Rometsch et al. 2021), and a male’s resource allocation to sexual traits —and thereby the net fitness benefits gained— depends on his somatic, genetic, or epigenetic condition. Condition dependence is a form of developmental plasticity where an individual’s available metabolic resources determine the extent of trait expression by optimizing the relative resource allocation between somatic maintenance and reproduction (Nur and Hasson 1984; Andersson 1986; Rowe and Houle 1996; Hill 2011). Although any trait has the potential to vary with condition, sexually selected traits are predicted to be particularly sensitive to condition due to their diversion of resources from somatic maintenance and survival (Rowe and Houle 1996; Cotton et al. 2004).
With stronger sexual selection on males than on females, resulting in the evolution of male-biased sexual dimorphism in condition-dependent traits, it follows that condition dependence itself should be sexually dimorphic. In other words, variation among males in the expression of sexually selected traits should be more tightly linked to their bearers’ underlying condition than that among females. Consequently, traits that differ more between the sexes should also show greater divergence in sex-specific condition dependence than less dimorphic ones, mediated by sex-specific resource diversion from somatic maintenance and differential viability costs (Rowe and Houle 1996). Even though theory predicts such links between sexual selection, sexual dimorphism and condition-dependent trait expression, however, only few empirical studies have integrated some of these predictions (Bonduriansky 2007a, b; Oudin et al. 2015; Miller et al. 2016; Rohner and Blanckenhorn 2018).
In addition to the paucity of studies exploring condition dependence of traits in the context of sexual dimorphism, other limitations in interpreting the link between sexual selection and condition dependence are also prevalent. Specifically, many studies provide correlational rather than experimental evidence of condition dependence, and they are often limited to a focal trait without appropriate control traits or accounting for variation in body size (reviewed in Cotton et al. 2004; but see, Rohner and Blanckenhorn 2018; Fox et al. 2019; Cattelan et al. 2020, for some recent examples addressing these issues). Stronger and less biased evidence can come from comparisons across multiple traits that inform about their relative condition dependence within the same set of individuals, thereby placing the trait of interest in the context of general trait variation (Arnqvist and Thornhill 1998; Bonduriansky and Rowe 2005; Fairbairn 2005; Rohner and Blanckenhorn 2018). Additionally, since individuals can vary in their sensitivity to developmental stress, in their efficiency in turning acquired resources into growth and in their resource allocation strategy, studying condition dependence in a genetic context seems particularly important for sexually selected traits, in which trait elaboration is often assumed to signal genetic quality (Iwasa et al. 1991; Rowe and Houle 1996). Yet, the genetic contribution to condition dependence is rarely studied (but see, David et al. 2000; Kemp and Rutowski 2007; Hubbard et al. 2015).
In insects, as in many other taxa, the relative size of sexual traits is strongly influenced by the resource availability during juvenile development (David et al. 2000). In cyclorrhaphan Diptera, adult structures grow mostly during late larval and pupal development. Because late third-instar larvae and pupae cannot acquire more energy by feeding, the developmental precursors underlying different adult tissues develop in an energetically closed system wherein they directly compete for resources (Nijhout and Emlen 1998; Heming 2018). In adults with juvenile development under different nutritional conditions, shifts in relative resource allocation to trait growth directly relate to the dependency of trait expression on resource availability, i.e. condition (Rohner and Blanckenhorn 2018; Shingleton and Frankino 2018). To the extent that condition covaries with body size due to the greater metabolic resource pool available to larger pupae (Blanckenhorn 2000), larger individuals may be able to allocate relatively more resources to a fitness-enhancing trait (e.g., sexual ornament) before experiencing a viability cost compared to smaller individuals (Bonduriansky and Day 2003). If so, that condition-dependent fitness trait would scale disproportionately (i.e., positively allometrically) with body size across individuals (Bonduriansky and Day 2003). Studying nutrition-dependent trait expression using static allometries (i.e., the degree to which trait size changes with overall body size) thus permits testing whether trait exaggeration and sexual dimorphism relate to (sex-specific) condition dependence, and how the expression of one trait depends on the investment in others.
An ideal system to study how sexual selection drives sex-specific condition dependence is the fruit fly Drosophila prolongata. As an exception within the Drosophilidae, D. prolongata males are much larger than females (Kudo et al. 2015; Rohner et al. 2018a, b) and develop dramatically enlarged forelegs with conspicuous black and white stripes (Setoguchi et al. 2014; Fig. 1). Males use these forelegs to strike their opponents in dyadic fights (Kudo et al. 2015), and to wave at, or occasionally stimulate the abdomen of, the female during courtship (Setoguchi et al. 2014, 2015; Perdigón Ferreira and Lüpold 2022). These functions, combined with the male-biased sexual dimorphism in foreleg expression and overall body size, suggest intense premating sexual selection on males, with forelegs as a primary target. For example, Perdigón Ferreira and Lüpold (2022) showed that males stimulating the female’s abdomen during courtship through “leg vibration” (Setoguchi et al. 2014, 2015) had significantly higher mating success compared to males that did not show this behavior.
Morphology of the foreleg, hindleg, and wing of male and female Drosophila prolongata. The lower panel illustrates the linear measurements, using the male foreleg and wing diagram as examples. FL: femur length; FW: femur width; TL: tibia length; TW: tibia width; TaL: tarsus1 length; WL: wing length; WW: wing width. Throughout this study, these abbreviations for leg components are preceded by the leg (fore- or hindleg; e.g., FFL for forefemur length, HTW for hindtibia width)
Here, using D. prolongata isofemale lines, we investigated the link between sexual dimorphism and sex-specific condition dependence (i.e., static allometries) in a genetic context. Overall, we tested the hypothesis that traits under sexual selection in males (e.g., exaggerated forelegs) show higher levels of male-biased sexual dimorphism and condition dependence than other traits (e.g., hindlegs not involved in mating behavior). Rather than focusing solely on total leg size, we also tested for differential patterns of condition dependence in different parts of the leg to infer a possible role of any such part in the unique male behaviors.
Materials and methods
Study organism
Drosophila prolongata is a member of the rhopaloa subgroup within the melanogaster species group, with a geographic distribution that includes southwestern China, northeastern India, Myanmar and Vietnam (Singh and Gupta 1977; Toda 1991; Setoguchi et al. 2014). For this study, we used flies from 42 isofemale lines that were originally collected in their natural habitat near Sa Pa (22°20’N, 103°52’E), Vietnam, in 2004 and 2015 by H. Takamori (Kudo et al. 2015), and in 2018 by J.P.F. (Perdigón-Ferreira and Lüpold 2022). In order to avoid variation across our genotypes that might result from differential inbreeding depression (e.g., due to deleterious recessive alleles; Wright et al. 2008), we crossed males of one isofemale line with virgin females of another in 21 independent pairwise combinations (i.e., using each isofemale line only once). Throughout our experiments, we used these heterozygous F1 genotypes (henceforth referred to as ‘lines’). We maintained all larvae and adult flies in a climate chamber on a 14:10 light:dark cycle at 18 °C and 60% humidity.
Manipulation of condition through changes in larval diet
To evaluate the condition dependence of trait expression, we manipulated the amount of food available to each developing larva. To this end, we first allowed adult flies of each pair of parental lines to feed and oviposit on standard fly food medium (replaced daily). Across 7 consecutive days, we then collected up to 600 first-instar larvae from each line and transferred them in groups of 50 to culturing vials with 3 different nutrient dilutions (each across 4 replicate vials per line and diet). The high-condition diet (“high condition” or “H”) contained 13 g of standard fly food (consisting of 55 g corn, 80 g agar, 100 g flour, 75 g glucose, 100 g fresh yeast, 10ml Nipagin antimicrobial agent per liter of food medium). We then diluted this standard food medium with water and agar to the same consistency but containing either half (“medium condition” or “M” diet) or one fifth (“low condition” or “L” diet) of the original nutrients in the same volume of medium. Towards the end of immature development, we checked all vials and tubes daily, and collected, counted, and froze all newly emerged individuals for later measurements.
Morphometric measurements
For each male and female fly, we carefully removed with forceps the left foreleg, hindleg, and wing, and mounted them in Euparal between a glass microscope slide and a coverslip. We measured thorax length (distance between the tip of the scutellum and the base of the head, lateral view) of all individuals to the nearest 25 μm using a Leica MS5 stereomicroscope with an ocular reticle. This measurement was used as an estimate of body size, because it scales nearly isometrically with body weight and is a widely used proxy of body size in the Drosophila literature (e.g., Rohner et al. 2018a). We then captured photos of all appendages using a Leica M205 C stereoscope with an ORCA-Flash4.0 LT + Digital CMOS camera C11440 attached to it. Based on landmarks digitized using tpsDig2 version 2.32 (Rohlf 2016), we measured the lengths of the femur, tibia, and first tarsal segment of the fore- and hindleg, respectively (for measurements and abbreviations see Fig. 1). We further measured the maximal widths of the femur and tibia of each leg, as well as the length and width of the wing (Fig. 1).
Statistical analyses
To test for the diet treatment effect (hereafter referred to as “treatment”) on the larval survival rate, we used a generalized linear mixed-effects model (GLMM) with a binomial error distribution, using vial nested within line as random effect (hereafter, “line/vial” random effect) to control for the non-independence of flies within lines and vials during development. We further used linear mixed-effects models (LMEs) to test for an effect of treatment and sex, as well as their interaction, on both egg-to-adult development time and adult body size, again using line/vial as a random effect. The analysis on body size was done using log-transformed thorax length as the response variable. We also tested for the effects of our treatments on the relative size of all focal body parts by performing separate LMEs including thorax length (our estimate of body size) as a covariate and the line/vial random effect. To estimate the contribution of the random effect to the total variance, we compared models with and without the line/vial random effect in likelihood ratio tests (LRTs). Throughout, we focused only on random intercepts due to much poorer performance of random-slope analyses in model comparisons.
To estimate sex-specific condition dependence, we followed the approach described in Rohner and Blanckenhorn (2018). Instead of analyzing the effect of the three treatments as discrete categories, we considered their effect on body and trait size as continuous and estimated sex-specific static allometries. We restricted these analyses to the H and L individuals to capture the full range of body sizes. In addition, this allowed us to keep the M individuals for estimates of sexual dimorphism (see below) to avoid spurious correlations between condition dependence and sexual dimorphism by using partially overlapping individuals between sexes and treatments (Oudin et al. 2015). In brief, we log-transformed all trait values and then calculated sex-specific allometric slopes of all traits against body size. This means that we obtained 42 slopes for each trait (i.e., one slope for each sex and line). To test for deviations from isometry (β = 1) in the sex-specific relationship between trait size and body size, we performed ordinary least squares (OLS) regressions using the sma() function of the R package smatr version 3.4.8 (Warton et al. 2012), which includes the robust option that controls for the possible effect of outliers on the slope estimation inference. Even though allometric relationships are often calculated by standardized (or reduced) major-axis regressions, OLS regressions have recently been shown not to underestimate slopes as previously thought, and to be less sensitive to extreme values (Al-Wathiqui and Rodríguez 2011; Kilmer and Rodríguez 2017). We then tested whether the male and female trait-specific allometries were correlated across lines by performing an LME with trait and line as random effects. Noteworthy, the sma() function does not allow the inclusion of random effect impeding us from controlling for possible vial effects. We again tested for a possible contribution of line to the total variance using likelihood ratio tests. Further, we used the logarithm of the ratios of male over female allometric slopes [i.e., log(male slope) – log(female slope)] to estimate the trait-specific sex differences in condition dependence, resulting in 21 indices of condition dependence for each trait.
To calculate sexual dimorphism, we used the flies originating from treatment “M”. We first removed any trait variation due to overall body size by calculating the residual trait sizes derived from LMEs of the log-transformed focal trait size against thorax length across both sexes combined, with line/vial as a random effect. We then z-transformed these residuals across sexes before averaging them within each sex. The trait-specific mean difference between sexes (\(\stackrel{-}{x}\)males − \(\stackrel{-}{x}\)females) represents the index of relative trait size dimorphism. Here too, we obtained one index of sexual dimorphism per line (i.e., 21 values per trait). We then tested if these values were associated with the trait-specific sex differences in condition dependence (above) in an LME with trait as random effect. Again, we tested for the significance of the line random effect using an LRT. We conducted all statistical analyses in R v.4.0.4 (R Core Team 2021).
In summary, we tested for the degree of sexual dimorphism, condition dependence, and their relationship across 12 morphological traits. Based on observations of male courtship and fighting behavior (Perdigón Ferreira and Lüpold 2022), we predicted male forelegs to be the most sexually dimorphic trait, with wings and hindlegs showing intermediate and low levels of dimorphism, respectively. In addition, we expected male forelegs, a trait putatively under directional sexual selection (Setoguchi et al. 2014, 2015), to show the steepest allometric slopes when compared to all other traits (see Bonduriansky and Day 2003). Finally, we predicted a positive correlation between the degrees of condition dependence and sexual dimorphism across traits (Bonduriansky 2007a).
Results
Effect of larval food limitation on survival, development, and body size
We obtained 2061 adult males (1000 H, 741 M, and 320 L) and 1,685 adult females (797 H, 548 M, and 340 L). In a binomial GLMM, larvae reared under the H treatment survived better than those subjected to food limitation (M and L treatments) (H: mean (95% CI) = 46.5% (45.0–47.9); M: 34.3% (32.6–36.1); L: 16.9% (15.6–18.2); treatment effect; Wald χ22 = 202.66, P < 0.001). The line/vial random effect was highly significant (LRT: χ22 = 480.89, P < 0.001).
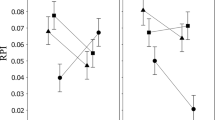
An LME further revealed that the mean development time of the H treatment was 29% and 36% shorter compared to M and L ones, respectively (F2,199.96 = 226.78, P < 0.001; Fig. 2a). Females also developed faster than males in all treatments (F1,204.15 = 634.85, P < 0.001; females, H: mean = 16.7 (15.8–17.6); M: 22.1 (21.3–23.0); L: 24.5 (23.6–25.4) days; males, H: mean = 17.7 (16.8–18.6); M: 24.6 (23.8–25.5); L: 27.6 (26.7–28.5) days), and the response to the treatments was stronger in males than in females (treatment × sex interaction: F2,204.02 = 45.66, P < 0.001). Again, we found a significant line/vial random effect (LRT: χ22 = 379.03, P < 0.001).
A Development time (from day of egg hatching to day of adult emergence) for males and females between high, medium and low nutrient concentration in the larval diet. B Body size (thorax length) for males and females across the same three treatments. Circles and triangles indicate the least-squares means, error bars their 95% confidence intervals. Means sharing a letter are not significantly different (P > 0.05; Sidak–adjusted comparisons)
Finally, treatment had a significant effect on body size (Fig. 2b), with flies of the H treatment being larger than those of the M and L treatments (F2,191.2 = 176.47, P < 0.001). In addition, males were larger than females (F1,1931.9 = 1604.30, P < 0.001), and the treatment effect was stronger on males than on females (treatment × sex interaction: F2,1924 = 3.38, P = 0.034). Line/vial identity contributed significantly to the total variance in body size (LRT: χ22 = 459.36, P < 0.001). We found similar treatment × sex interactions for the relative size of most leg and wing traits (Table 1).
Condition dependence and sexual dimorphism
Independent of the sex, individuals of the 21 different genotypes showed similar allometric patterns within traits (Supplementary Table S1, Fig. S1). Out of 504 allometric slopes calculated, 252 were not significantly different from one (i.e., isometry), 194 shallower than one (i.e., hypoallometry), 23 were steeper than one (i.e., hyperallometry), and 35 were not significantly different from zero (Suppl. Table S1 and Fig. S1). Most traits that showed hyperallometric scaling in some lines (FFW, FTW, FTaL, HFW, and HTaL; for definitions see Fig. 1) did so in males but not in females (Suppl. Table S1). Overall, allometric slopes were not significantly correlated between the sexes (LME using trait and line identity as random effects: F1,230.92 = 2.87, P = 0.092), despite most lines showing a positive, albeit not always significant, relationship between male and female allometric slopes (Table 2). Among all traits, hindfemur width was the only one with a hyperallometric slope in all lines, at least for males, whilst all other traits were either hypo- or isometric in both sexes (Fig. 3). The line random effect explained a significant portion of the total variance (LRT: χ21 = 71.15, P < 0.001).
Relationship between static allometric slopes of males and females. Rectangles (foreleg traits) and dots (hindleg and wing traits) reflect means across the 21 lines with 95% confidence intervals. Horizontal and vertical dashed lines indicate isometry. Values above the horizontal and to the right of the vertical dashed lines indicate hyperallometry, and those below or to the left indicate hypoallometry. Traits labeled as in Fig. 1 except for being preceded by an F = foreleg or an H = hindleg
The relative sexual dimorphism of all traits considered was male-biased (Fig. 4). All foreleg parts measured showed the highest degree of sexual dimorphism whereas wing length and width, together with hindtibia width, were the least dimorphic. However, even though most traits were more condition-dependent in males, forefemur, foretibia and foretarsus lengths were significantly more condition-dependent in females compared to males (Fig. 4). In addition, the degree of relative sexual dimorphism was not correlated with the sex difference in condition dependence (linear mixed model using trait and line identity as random effects: F175.46 = 0.292, P = 0.591; Fig. 4). The significant line effect (LRT: χ21 = 35.13, P < 0.001) suggests genetic variation in the strength of the relationship between the sex difference in condition dependence and sexual dimorphism. The lack of a relationship between the sex-specific trait expression and condition dependence was not the result of contrasting associations between lines that canceled one another but rather of no significant correlation in all but one line (Table 2).
Relationship between sex-specific condition dependence (log(slopemales) – log(slopefemales)) and relative sexual trait size dimorphism (\(\stackrel{-}{x}\)males − \(\stackrel{-}{x}\)females). Rectangles (foreleg traits) and dots (hindleg and wing traits) are the trait-specific means across all 21 lines. Horizontal dashed lines indicate no difference in condition dependence between males and females and values above and below indicate male- and female-biased condition dependence, respectively. Note that all values on the x-axis are larger than zero, indicating male-biased sexual dimorphism. Error bars represent 95% confidence intervals. Traits labeled as in Fig. 1 except for being preceded by an F = foreleg or an H = hindleg
Discussion
Sexual selection theory predicts that traits under directional selection should be more sexually dimorphic and more condition-dependent than traits that are mostly under natural selection that acts similarly on both sexes (Rowe and Houle 1996; Cotton et al. 2004). Our results do not support the prediction that sex-specific trait exaggeration goes hand in hand with a sex-specific increase in condition dependence (Bonduriansky 2007b). Although the foreleg traits showed a high degree of sexual dimorphism in the predicted direction (i.e., male-biased sexual dimorphism), they were not more condition-dependent than other traits. That is, some foreleg parts that were more exaggerated in males were more condition-dependent in females, conflicting with theoretical predictions (Andersson 1986; Pomiankowski 1987; Rowe and Houle 1996). Below we discuss the evidence for directional sexual selection in male morphology, the relationship with sexual dimorphism and sex-specific condition dependence, and how it shapes trait covariation.
Evidence of sexual selection acting on male foreleg and wing morphology
As in many drosophilids (e.g., Spieth 1974), wings are used in several courtship elements in D. prolongata, such as “unilateral wing vibration”, “bilateral wing vibration”, and “wing waving” (Setoguchi et al. 2014). Wing shape has been shown to affect mating success in male D. melanogaster (Menezes et al. 2013), and similar effects could also apply to D. prolongata (but see Perdigón Ferreira and Lüpold 2022). In addition to wings, male D. prolongata use their forelegs for repeated waving movements when courting females. This behavior is not limited to D. prolongata, but it is about 30 times more frequently observed than in closely related species (Setoguchi et al. 2014). In addition, male D. prolongata use their forelegs to stimulate the female abdomen with drumming movements after protracted courtship, possibly to increase the receptivity of reluctant females. Unlike all other drosophilids studied so far (e.g., Vedenina et al. 2013), however, male D. prolongata do not approach and court females from behind, but rather face the female and reach around her (Setoguchi et al. 2014; Perdigón Ferreira and Lüpold 2022). Consequently, this unusual way of stimulating the female abdomen could have played a pivotal role in the evolution of male foreleg exaggeration in this species (Setoguchi et al. 2014; Perdigón Ferreira and Lüpold 2022) either via directional (e.g. favoring males with larger legs) or stabilizing (e.g. favoring an average leg length) selection.
The effect of sexual selection on phenotypic traits can vary across ecological and social conditions (Miller and Svensson 2014; Evans and García-González 2016). Specifically, factors such as population density (Rittschof 2010; McCullough et al. 2018), sex ratio (Jann et al. 2000; Punzalan et al. 2010), or resource quality (Gillespie et al., 2014) can all change the strength and direction of sexual selection. Such effects might also play a role in D. prolongata. For example, in competitive mating trials using male duos, an outbred population, and fly food medium as the substrate, we found a mating advantage for the males that had developed under superior dietary conditions (and consequently were also larger in most cases; Perdigón Ferreira and Lüpold 2022). However, it is important to measure sexual selection in different contexts to better capture the naturally occurring fluctuations in sexual selection (Miller and Svensson 2014).
If fluctuations in the ecological and social context affect the strength and direction of sexual selection in D. prolongata males, this could explain the maintenance of phenotypic variation in body and trait size, despite the possible advantage of relatively larger males when fighting (Amino and Matsuo 2020) or courting (Perdigón Ferreira and Lüpold 2022). In addition, such fluctuations could have favored the evolution of interception (i.e., ‘stealing’ a female from a courting male) as an alternative reproductive tactic among relatively smaller males (Perdigón Ferreira and Lüpold 2022). In summary, assessing sexual selection under different and relevant ecological conditions can help to understand the circumstances that could have favored the evolution of the unique morphology and behavior of males and to better predict the type of allometric scaling (i.e., the level of condition dependence) shown by these sexually selected traits (Eberhard et al. 2018; McCullough and O’Brien 2022).
Allometric scaling and sexual selection
Based on the striking sexual dimorphism in forelegs and on their role, together with the wings, in courtship, we predicted that these traits should show particularly marked differences in condition dependence between males and females. However, we found no clear evidence of a relationship between the trait-specific extent of sexual dimorphism and the sex difference in relative condition dependence (see Fig. 4).
Hyperallometric scaling, that is, a disproportionate increase in trait size relative to organismal body size that ultimately reflects condition-dependent trait expression, is often expected to be driven by strong directional selection (Bonduriansky and Day 2003). Here, most traits, even those predicted to be under directional sexual selection, were instead isometric or hypoallometric. Despite these apparent contradictions, our results are by no means exceptional, in that secondary sexual traits have previously been shown to scale hypoallometrically (Eberhard et al. 2018). In fact, it has been emphasized that the mode and strength of sexual selection, as well as different forms of natural selection, and possible genetic correlations between the sexes, must be considered when making predictions (e.g., Simmons and Tomkins 1996; Eberhard 2002; Fairbairn 2005; Eberhard et al. 2018; Kelly 2022; McCullough and O’Brien 2022; Palaoro et al. 2022). The forelegs of D. prolongata, for example, are also used for locomotion and might thus be more constrained or functionally integrated with variation in other traits (e.g., mid- and hindlegs) than traits in other species that are used in a sexual context only (e.g., Kelly 2014, 2022).
Like D. prolongata, males of the drosophilid Chymomyza mycopelates use their forelegs to rapidly flick and touch the female abdomen during courtship, and to either display at, or slam, their opponents in contests (Eberhard 2002). Despite clear indication that male forelegs function as both signals and weapons, the allometric slopes of all foreleg parts considered here were hypoallometric and comparable to those of the hindlegs that are not directly involved in courtship or fighting (see Katsuki et al. 2014, for a similar example in a beetle species). More recently, Kelly (2022) showed that the allometric relationships of leg and wing traits in the Japanese beetle Popillia japonica were shallower than unity, independently of whether sexual selection was directional or stabilizing. The same study also highlighted the importance of considering other types of selection (e.g., viability) when predicting allometric patterns. Thus, the different sexual and non-sexual functions of male forelegs complicate predictions about their allometric scaling (Bonduriansky 2007a), highlighting the importance of considering the context of trait use when predicting or comparing allometries, including different forms of use in a sexual context (functional weapon, coercion, intimidation, or courtship; Eberhard et al. 2018).
Finally, it is worth noting that our results differ from another study comparing the allometric slope of foreleg length between the sexes in the same species. In a reduced major-axis regression, Luecke and Kopp (2019) reported leg length to be isometric in both sexes, but males had higher mean trait values (i.e., shift in elevation). However, like Luecke and Kopp’s (2019) results, we found comparable allometric patterns between males and females, suggesting that the developmental constraints on the final foreleg size are not completely removed.
Conclusion
Taken together, our results suggest that sexual dimorphism did not correlate with the degree of sex-specific condition dependence. Rather, our results illustrate that the relative importance of sexual selection in generating such patterns is likely to vary between species (e.g., Cotton et al. 2004) and to depend on the function of the trait (Eberhard et al. 2018). Moreover, even when theoretical models predict that these patterns should be common in secondary sexual traits, and empirical studies often find support thereof, there is nonetheless a notable proportion of traits that do not follow these patterns (e.g., Johnstone et al. 2009; Voje 2016). To better predict the relationship between exaggerated secondary sexual traits and other body parts among individuals, we need to better understand the role of such traits in the context of natural as much as sexual selection (e.g., Bro-Jørgensen et al. 2007). Only by investigating the costs and benefits of a multitude of fitness components can we better predict the outcome of selection acting on exaggerated secondary sexual traits.
Data availability
Analyses reported in this article can be reproduced using the data provided in Perdigón Ferreira et al. (2022).
Code availability
The code use for the analysis of the data will be deposited on the Dryad data repository upon acceptance of the paper.
References
Al-Wathiqui N, Rodríguez RL (2011) Allometric slopes not underestimated by ordinary least squares regression: a case study with Enchenopa treehoppers (Hemiptera: Membracidae). Ann Entomol Soc Am 104:562–566
Amino K, Matsuo T (2020) Intra- versus inter-sexual selection on sexually dimorphic traits in Drosophila prolongata. Zool Sci 37:1–7
Andersson M (1986) Evolution of condition-dependent sex ornaments and mating preferences-sexual selection based on viability differences. Evolution 40:804–816
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Arnqvist G, Thornhill R (1998) Evolution of animal genitalia: patterns of phenotypic and genotypic variation and condition dependence of genital and non-genital morphology in water strider (Heteroptera: Gerridae: Insecta). Genet Res 71:193–212
Bateman AJ (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–407
Bonduriansky R (2007a) Sexual selection and allometry: a critical reappraisal of the evidence and ideas. Evolution 61:838–849
Bonduriansky R (2007b) The evolution of condition-dependent sexual dimorphism. Am Nat 169:9–19
Bonduriansky R, Day T (2003) The evolution of allometry in sexually selected traits. Evolution 57:2450–2458
Bonduriansky R, Rowe L (2005) Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthostoma (Piophilidae). Evolution 59:138–151
Bro-Jørgensen J, Johnstone RA, Evans MR (2007) Uninformative exaggeration of male sexual ornaments in barn swallows. Curr Biol 17:850–855
Catellan S, Evans JP, García-Gonzalez F, Morbiato E, Pilastro A (2020) Dietary stress increases the total opportunity for sexual selection and modifies selection on condition-dependent traits. Ecol Lett 23:447–456
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Cotton S, Fowler K, Pomiankowski A (2004) Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc R Soc B 271:771–783
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
David P, Bjorksten T, Fowler K, Pomiankowski A (2000) Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature 406:186–188
Eberhard WG (2002) Natural history and behavior of Chymomyza mycopelates and C. exophthalma (Diptera: Drosophilidae), and allometry of structures used as signals, weapons, and spore collectors. Can Entomol 134:667–687
Eberhard WG, Rodríguez RL, Huber BA, Speck B, Miller H, Buzatto BA, Machado G (2018) Sexual selection and static allometry: the importance of function. Q Rev Biol 93:207–250
Emlen DJ (2008) The evolution of animal weapons. Annu Rev Ecol Evol Syst 39:387–413
Evans JP, Garcia-Gonzalez F (2016) The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J Evol Biol 29:2338–2361
Fairbairn DJ (2005) Allometry for sexual size dimorphism: testing two hypotheses for Rensch’s rule in the water strider Aquarius remigis. Am Nat 166:S69–S84
Fox RJ, Fromhage L, Jennions MD (2019) Sexual selection, phenotypic plasticity and female reproductive output. Phil Trans R Soc B 374:20180184
Gillespie SR, Tudor MS, Moore AJ, Miller CW (2014) Sexual selection is influenced by both developmental and adult environments. Evolution 68:3421–3432
Heming BS (2018) Insect development and evolution. Cornell University Press, Ithaca, NY
Hill GE (2011) Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol Lett 14:625–634
Hubbard JK, Jenkins BR, Safran RJ (2015) Quantitative genetics of plumage color: lifetime effects of early nest environment on a colorful sexual signal. Ecol Evol 5:3436–3449
Iwasa Y, Pomiankowski A, Nee S (1991) The revolution of costly mate preferences II. The “handicap” principle. Evolution 45:1431–1442
Jann P, Blanckenhorn WU, Ward PI (2000) Temporal and microspatial variation in the intensities of natural and sexual selection in the yellow dung fly Scatophaga stercoraria. J Evol Biol 13:927–938
Johnstone RA, Rands SA, Evans MR (2009) Sexual selection and condition-dependence. J Evol Biol 22:2387–2394
Katsuki M, Yokoi T, Funakoshi K, Oota N (2014) Enlarged hind legs and sexual behavior with male-male interaction in Sagra femorata (Coleoptera: Chrysomelidae). Entomol News 124:211–220
Kelly CD (2014) Sexual selection, phenotypic variation, and allometry in genitalic and non-genitalic traits in the sexually size-dimorphic stick insect Micrarchus hystriculeus. Biol J Linn Soc 113:471–484
Kelly CD (2022) Can patterns of static allometry be inferred from regimes of sexual selection in the japanese beetle? Evol Biol In press
Kemp DJ, Rutowski RL (2007) Condition dependence, quantitative genetics, and the potential signal content of iridescent ultraviolet butterfly coloration. Evolution 61:168–183
Kilmer JT, Rodríguez RL (2017) Ordinary least squares regression is indicated for studies of allometry. J Evol Biol 30:4–12
Kudo A, Takamori H, Watabe H, Ishikawa Y, Matsuo T (2015) Variation in morphological and behavioral traits among isofemale strains of Drosophila prolongata (Diptera: Drosophilidae). Entomol Sci 18:221–229
Luecke DM, Kopp A (2019) Sex-specific evolution of relative leg size in Drosophila prolongata results from changes in the intersegmental coordination of tissue growth. Evolution 73:2281–2294
Lüpold S, Manier MK, Puniamoorthy N, Schoff C, Starmer WT, Buckely Luepold SH, Belote JM, Pitnick S (2016) How sexual selection can drive the evolution of costly sperm ornamentation. Nature 533:535–538
McCullough E, O’Brien DM (2022) Variation in allometry along the weapon-signal continuum. Evol Ecol 36:591–604
McCullough EL, Buzatto BA, Simmons LW (2018) Population density mediates the interaction between pre- and postmating sexual selection. Evolution 72:893–905
Menezes BF, Vigoder FM, Peixoto AA, Varaldi J, Bitner-Mathé BC (2013) The influence of male wing shape on mating success in Drosophila melanogaster. Anim Behav 85:1217–1223
Miller CW, Svensson EI (2014) Sexual selection in complex environments. Annu Rev Entomol 59:427–445
Miller CW, McDonald GC, Moore AJ (2016) The tale of the shrinking weapon: seasonal changes in nutrition affect weapon size and sexual dimorphism, but not contemporary evolution. J Evol Biol 29:2266–2275
Nijhout HF, Emlen DJ (1998) Competition among body parts in the development and evolution of insect morphology. Dev Biol 95:3685–3689
Nur N, Hasson O (1984) Phenotypic plasticity and the handicap principle. J Theor Biol 110:275–297
Oudin MJ, Bonduriansky R, Rundle HD (2015) Experimental evidence of condition-dependent sexual dimorphism in the weakly dimorphic antler fly Protopiophila litigata (Diptera: Piophilidae). Bio J Linn Soc 116:211–220
Palaoro AV, García-Hernández S, Buzatto BA, Machado G (2022) Function predicts the allometry of contest-related traits, but not sexual or male dimorphism in the amazonian tusked harvestman. Evol Ecol 36:605–630
Perdigón Ferreira J, Lüpold S (2022) Condition- and context-dependent alternative reproductive tactic in Drosophila prolongata. Behav Ecol 33:213–221
Perdigón Ferreira J, Rohner PT, Lüpold S et al (2022) Condition dependence and sexual dimorphism in Drosophila prolongata, Dryad, Dataset. https://doi.org/10.5061/dryad.zcrjdfnhd
Pomiankowski A (1987) Sexual selection: the handicap principle does work - sometimes. Proc R Soc B 231:123–145
Punzalan D, Rodd FH, Rowe L (2010) Temporally variable multivariate sexual selection on sexually dimorphic traits in a wild insect population. Am Nat 175:401–414
R Development Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rittschof CC (2010) Male density affects large-male advantage in the golden silk spider, Nephila clavipes. Behav Ecol 29:536–548
Rohlf FJ (2016) tpsDig ver. 2.32. SUNY: Department of Ecology and Evolution. Stony Brook, NY, USA
Rohner PT, Blanckenhorn WU (2018) A comparative study of the role of sex-specific condition dependence in the evolution of sexually dimorphic traits. Am Nat 192:E202–E215
Rohner PT, Pitnick S, Blanckenhorn WU, Snook RR, Bächli G, Lüpold S (2018a) Interrelations of global macroecological patterns in wing and thorax size, sexual size dimorphism, and range size of the Drosophilidae. Ecography 41:1707–1717
Rohner PT, Teder T, Esperk T, Lüpold S, Blanckenhorn WU (2018b) The evolution of male-biased sexual size dimorphism is associated with increased body size plasticity in males. Funct Ecol 32:581–591
Rometsch SJ, Torres-Dowdall J, Machado-Schiaffino G, Karagic N, Meyer S (2021) Dual function and associated costs of a highly exaggerated trait in a cichlid fish. Ecol Evol 11:17496–17508
Rowe L, Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc B 263:1415–1421
Setoguchi S, Takamori H, Aotsuka T, Sese J, Ishikawa Y, Matsuo T (2014) Sexual dimorphism and courtship behavior in Drosophila prolongata. J Ethol 32:91–102
Setoguchi S, Kudo A, Takanashi T, Ishikawa Y, Matsuo T (2015) Social context-dependent modification of courtship behaviour in Drosophila prolongata. Proc R Soc B 282:20151377
Shingleton AW, Frankino WA (2018) The (ongoing) problem of relative growth. Curr Opin Insect Sci 25:9–19
Simmons LW, Tomkins JL (1996) Sexual selection and the allometry of earwig forceps. Evol Ecol 10:97–104
Singh BK, Gupta JP (1977) Two new and two unrecorded species of the genus Drosophila fallen (Diptera: Drosophilidae) from Shillong, Meghalaya, India. Proc Zool Soc Calcutta 30:31–38
Somjee U, Woods HA, Duell M, Miller CW (2018) The hidden cost of sexually selected traits: the metabolic expense of maintaining a sexually selected weapon. Proc R Soc B 285:20181685
Spieth HT (1974) Courtship behavior in Drosophila. Annu Rev Entomol 19:385–405
Toda MJ (1991) Drosophilidae (Diptera) in Myanmar (Burma) VII. The Drosophila melanogaster species-group, excepting the D. montium species-subgroup. Orient Insects 25:69–94
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine Publishing Company, Chicago, pp 136–179
Vedenina VY, Ivanova TI, Lazebny OE (2013) Analysis of courtship behavior in closely related species of Drosophila virilis group: a new approach arises new questions. J Insect Behav 26:402–415
Voje KL (2016) Scaling of morphological characters across trait type, sex, and environment: a meta-analysis of static allometries. Am Nat 187:89–98
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) Smart 3- an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183
Wright LI, Tregenza T, Hosken DJ (2008) Inbreeding, inbreeding depression and extinction. Conser Genet 9:833–843
Acknowledgements
We thank Prof. Tran Phuong and Prof. Nguyen Duc Khanh for the assistance during the fieldwork to collect flies and Prof. Takashi Matsuo for sharing his isofemale lines with us. In addition, we are also thankful to Dr. Sonja Sbilordo, Jeannine Roy, Valérian Zeender, Tyler Figueira, and Dr. Monica Anderson Berdal for their help with the fly dissections and measurements, and all members of the Lüpold lab for their support.
Funding
Open access funding provided by University of Zurich. This work was supported by the University of Zurich Forschungskredit (FK-16-089 to J.P.F.), GRC Travel Grant (to J.P.F.), the Georges and Antoine Claraz Donation (to J.P.F), and the Swiss National Science Foundation (PP00P3_170669 and PP00P3_202662 to S.L.).
Author information
Authors and Affiliations
Contributions
JPF and SL designed the experiments; JPF collected the data; JPF and PTR analyzed the data; JPF drafted the manuscript, and all authors edited and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perdigón Ferreira, J., Rohner, P.T. & Lüpold, S. Strongly sexually dimorphic forelegs are not more condition-dependent than less dimorphic traits in Drosophila prolongata. Evol Ecol 37, 493–508 (2023). https://doi.org/10.1007/s10682-022-10226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10226-0