Abstract
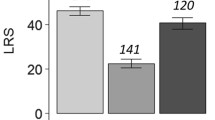
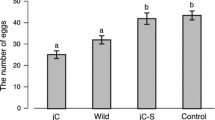
Reproductive interference (RI) is a negative interspecific interaction caused by reproductive activities. Previous studies have implied that male-male competition increases RI capability, consisting of the male harm-inflicting capability of RI by males (RI infliction) and the female susceptibility to heterospecific male harm (RI resistance). Geographical populations show variations in the intensity of male-male competition and sexual conflict due to the influence of biotic/abiotic factors. We made two predictions: (1) RI capability would vary among conspecific populations, and (2) the degree of RI infliction by males would match that of female RI resistance in geographical strains. To test these predictions, we measured the fecundity of once-mated females in two scenarios: housed with heterospecific males and without males. We performed experiments using six strains from each of two bean weevil species, Callosobruchus maculatus and C. chinensis. The results demonstrated intraspecific variation in RI capability within both Callosobruchus species, but the RI infliction of males and RI resistance of females within a geographical strain did not consistently coincide. Our results suggest that RI varies depending on geographical strains, even within species, but we did not obtain evidence that sexual conflict molds RI capability, indicating traits driving RI do not follow the sexual conflict in both Callosobruchus species.





Similar content being viewed by others
References
Arnqvist G (1992) Spatial variation in selective regimes: sexual selection in the water strider, Gerris odontogaster. Evolution 46:914–929
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press
Arnqvist G, Nilsson T, Katvala M (2005) Mating rate and fitness in female bean weevils. Behav Ecol 16:123–127
Bateman A (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368
Boiteau G (1985) Bionomics and genetics of a black mutant colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Ann Entomol Soc Am 78:663–666
Chapman T, Liddle L, Kalb J et al (1995) Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373:241–244
Chapman T, Miyatake T, Smith H, Partridge L (1998) Interactions of mating, egg production and death rates in females of the mediterranean fruitfly, Ceratitis capitata. Proc R Soc Lond B 265:1879–1894
Dougherty L, van Lieshout E, McNamara K et al (2017) Sexual conflict and correlated evolution between male persistence and female resistance traits in the seed beetle Callosobruchus maculatus. Proc Royal Soc B: Biol Sci 284:20170132
Eady P (1995) Why do male Callosobruchus maculatus beetles inseminate so many sperm? Behav Ecol Sociobiol 36:25–32
Eady P (2000) Copulating with multiple mates enhances female fecundity but not egg-to-adult survival in the Bruchid beetle Callosobruchus maculatus. Evolution 54:2161–2165
Endler J (1977) Geographic variation, speciation and clines. (MPB-10), volume 10. Princeton University Press http://www.jstor.org/stable/j.ctvx5wbdg
Fox C (1993) Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). Funct Ecol 203–208
Gröning J, Hochkirch A (2008) Reproductive interference between animal species. Q Rev Biol 83:257–282
Harano T, Yasui Y, Miyatake T (2006) Direct effects of polyandry on female fitness in Callosobruchus chinensis. Anim Behav 71:539–548
Hotzy C, Arnqvist G (2009) Sperm competition favors harmful males in seed beetles. Curr Biol 19:404–407
Hotzy C, Polak M, Rönn J, Arnqvist G (2012) Phenotypic engineering unveils the function of genital morphology. Curr Biol 22:2258–2261
Kashiwagi M, Utida S (1972) A new mutant in Callosobruchus chinensis L. (Coleoptera: Bruchidae). Appl Entomol Zool 7:95–96
Kishi S, Nishida T, Tsubaki Y (2009) Reproductive interference determines persistence and exclusion in species interactions. J Anim Ecol 78:1043–1049
Kondo N, Tuda M, Toquenaga Y et al (2011) Wolbachia infections in world populations of bean beetles (Coleoptera: Chrysomelidae: Bruchinae) infesting cultivated and wild legumes. Zoolog Sci 28:501–509
Kyogoku D (2021) Seed beetles as a modern model system of interspecific competition. Ecol Res 36:580–589
Kyogoku D, Nishida T (2013) The mechanism of the fecundity reduction in Callosobruchus maculatus caused by Callosobruchus chinensis males. Popul Ecol 55:87–93
Kyogoku D, Sota T (2015) Does heterospecific seminal fluid reduce fecundity in interspecific copulation between seed beetles? J Insect Physiol 72:54–60
Kyogoku D, Sota T (2015) Exaggerated male genitalia intensify interspecific reproductive interference by damaging heterospecific female genitalia. J Evol Biol 28:1283–1289
Kyogoku D, Sota T (2017) The evolution of between-species reproductive interference capability under different within-species mating regimes. Evolution 71:2721–2727
Mano H, Toquenaga Y (2008) Wall-making behavior as a proximate mechanism to generate variation in larval competition in Callosobruchus maculatus (Coleoptera: Bruchidae). Evol Ecol 22:177–191
Messina F, Mitchell R (1989) Intraspecific variation in the egg-spacing behavior of the seed beetleCallosobruchus maculatus. Journal of Insect Behavior 2:727–742
Miyatake T, Matsumura F (2004) Intra-specific variation in female remating in Callosobruchus chinensis and C. maculatus. J Insect Physiol 50:403–408
Nishida S, Hashimoto K, Kanaoka M et al (2017) Variation in the strength of reproductive interference from an alien congener to a native species in Taraxacum. J Plant Res 130:125–134
Numajiri Y, Kondo N, Toquenaga Y (2017) Melanic mutation causes a fitness decline in bean beetles infected by Wolbachia. Entomol Exp Appl 164:54–65
Pascoal S, Mendrok M, Wilson A et al (2017) Sexual selection and population divergence II. Divergence in different sexual traits and signal modalities in field crickets (Teleogryllus oceanicus). Evolution 71:1614–1626
Price T, Leonard A, Lancaster L (2017) Warp-speed adaptation to novel hosts after 300 generations of enforced dietary specialisation in the seed beetle Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae). Eur J Entomol
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/
Rönn J, Katvala M, Arnqvist G (2007) Coevolution between harmful male genitalia and female resistance in seed beetles. Proc Natl Acad Sci 104:10921–10925
Shimomura K, Mimura T, Ishikawa S et al (2010) Variation in mate recognition specificities among four Callosobruchus seed beetles. Entomol Exp Appl 135:315–322
Takano M, Toquenaga Y, Fujii K (2001) Polymorphism of competition type and its genetics in Callosobruchus maculatus (Coleoptera: Bruchidae). Popul Ecol 43:265–273
Terry M (2022)A package for survival analysis in https://CRAN.R-project.org/package=survival
Terry M, Patricia M (2000) Modeling survival data: extending the Cox model. Springer, New York
Thanthianga C, Mitchell R (1990) The fecundity and oviposition behavior of a south indian strain of Callosobruchus maculatus. Entomol Exp Appl 57:133–142
Toquenaga Y (1993) Contest and scramble competitions in Callosobruchus maculatus (Coleoptera: Bruchidae) II. Larval competition and interference mechanisms. Popul Ecol 35:57–68
Toquenaga Y, Fujii K (1991) Contest and scramble competitions in callosobruchus maculatus (Coleoptera: Bruchidae). Popul Ecol 33:199–211
Ursprung C, Den Hollander M, Gwynne D (2009) Female seed beetles, Callosobruchus maculatus, remate for male-supplied water rather than ejaculate nutrition. Behav Ecol Sociobiol 63:781–788
Utida S (1941) Studies on experimental population of the azuki bean weevil, Callosobruchus chinensis (L.) I. The effect of population density on the progeny populations. Mem College Agric, Kyoto Imp Univ 48:1–30
Van Haren M, Rönn J, Schilthuizen M, Arnqvist G (2017) Postmating sexual selection and the enigmatic jawed genitalia of Callosobruchus subinnotatus. Biology Open 6:1008–1012
Yassin A, David J (2016) Within-species reproductive costs affect the asymmetry of satyrization in Drosophila. J Evol Biol 29:455–460
Yun L, Agrawal A, Rundle H (2021) On male harm: How it is measured and how it evolves in different environments
Funding
This study was supported, in part, by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Nos. 14405003, 17405005, 17570014, 20405006, 23405008, 23570017, 26304016, and 17H04612 to Y.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We declare we have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We are grateful to Koichi Fujii, Masakazu Shimada, and Frank Messina for dispensing the strains of bean weevils which we used. We would like to thank Editage (www.editage.com) for English language editing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukaimine, W., Toquenaga, Y. Intraspecific variation of reproductive interference capability in Callosobruchus species. Evol Ecol 37, 531–544 (2023). https://doi.org/10.1007/s10682-022-10223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10223-3