Abstract
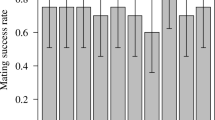
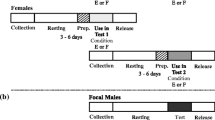
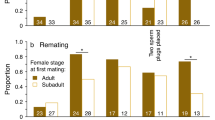
Male Callosobruchus maculatus F. (Coleoptera: Bruchidae) inseminate more sperm than females can effectively store in their spermathecae. This study examines the adaptive significance of “excess” sperm transfer by measuring components of male and female reproductive success in response to manipulating the number of sperm inseminated. The number of sperm transferred during copulation was reduced from 56,000 ±4,462 to 8,700±1,194 by sequentially mating males to virgin females. Reducing the number of sperm inseminated by the first male to mate had no effect on the extent of sperm precedence, but reducing the number of sperm inseminated by the second male resulted in a significant reduction in the extent of sperm precedence. When large numbers of sperm are inseminated the remating refractory period of females is increased. These results indicate that males transferring large numbers of sperm during copulation have a two-fold advantage at fertilization; they are more effective at preempting previously stored sperm and they are likely to father more offspring by delaying the time of female remating. The transfer of “excess” sperm does not appear to serve as nonpromiscuous male mating effort; the number of eggs laid, their fertility and the subsequent survival of zygotes were unaffected by manipulating the number of sperm inseminated. The underlying mechanisms of sperm precedence were also examined. Simple models of sperm displacement failed to accurately predict the patterns of sperm precedence observed in this species. However, the results do not provide conclusive evidence against the models but rather serve to highlight our limited understanding of the movement of sperm within the female's reproductive tract.
Similar content being viewed by others
References
Baker RR, Bellis MA (1988) “Kamikaze” sperm in mammals? Anim Behav 36: 936–939
Baker RR, Bellis MA (1989) Elaboration of the kamikaze sperm hypothesis: a reply to Harcourt. Anim Behav 37: 865–867
Baumann H (1974) Biological effects of paragonial substances PS1 and PS2 on females of Drosophila funebris. J Insect Physio 20: 2347–2362
Bedford JM (1970) The saga of mammalian sperm from ejaculation to syngamy. In: Gibian H, Plotz EJ, (eds) Mammalian reproduction. Springer, Berlin Heidelberg New York, pp 124–182
Brauer A (1944) Influence of population number on egg production in the four spotted pea beetle, Bruchus quadrimaculatus Fabr. Trans K Acad Sci 11: 56–62
Brillard JP, Bakst MR (1991) Quantification of spermatozoa in the sperm-storage tubules of turkey hens and its relation to sperm numbers in the perivitelline layer of eggs. Biol Reprod 43: 271–275
Cohen J (1973) Crossovers, sperm redundancy and their close association. Heredity 31: 408–413
Eady PE (1991) Sperm competition in Callosobruchus maculatus (Coleoptera: Bruchidae): a comparison of two methods used to estimate paternity. Ecol Entomol 16: 45–53
Eady PE (1992) Sperm competition in Callosobruchus maculatus. Ph.D. Thesis, University of Sheffield
Eady PE (1994) Intraspecific variation in sperm precedence in the bruchid beetle Callosobruchus maculatus. Ecol Entomol 19: 11–16
Eady PE (1994b) Sperm transfer and storage in relation to sperm competition in Callosobruchus maculatus. Behav Ecol Sociobiol 35: 123–129
Eberhard WG (1985) Sexual selection and animal genitalia. Harvard University Press, Cambridge
Eberhard WG (1990) Animal genitalia and female choice. Am Sci 78: 134–141
Fox CW (1993) Multiple mating, lifetime fecundity and female mortality of the bruchid beetle Callosobruchus maculatus (Coleoptera: Bruchidae). Fund Ecol 7: 203–208
Gomendio M, Roldan ERS (1993) Mechanisms of sperm competition: linking physiology and behavioural ecology. Trends Ecol Evol 8: 95–100
Gwynne DT (1984a) Male mating effort, confidence of paternity and insect sperm competition. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, London, pp 117–149
Gwynne DT (1984b) Courtship feeding increases female reproductive success in bush crickets. Nature 307: 361–363
Gwynne DT (1986) Courtship feeding in katydids (Orthoptera: Tettigoniidae): investment in offspring or in obtaining fertilizations. Am Nat 128: 342–352
Harcourt AH (1989) Deformed sperm are probably not adaptive. Anim Behav 37: 863–865
Harcourt AH (1991) Sperm competition and the evolution of nonfertilizing sperm in mammals. Evolution 45: 314–328
Huck UW, Lisk RD, Thierjung C (1985) Stimulus requirements for pregnancy initiation in the golden hamster (Mesocricetus auratus) change with time of mating during the reproductive period. J Reprod Fertil 76: 449–458
Huignard J (1974) Influence de la copulation sur la fonction reproductice femelle chez Acanthoscelides obtectus Say (Coléoptère: Bruchidae). I. Copulation et spermatophore. Ann Sci Nat Zool Paris 16: 361–434
Huignard J, Quesneau-Thierry A, Barbier M (1977) Isolement, action biologique et evolution des substances paragoniales contenues dans le spermatophore d'Acanthoscelides obtectus (Coleoptere). J Insect Physiol 23: 351–357
Lessells CM, Birkhead TR (1990) Mechanisms of sperm competition in birds: mathematical models. Behav Ecol Sociobiol 27: 325–337
Marshall LD, McNeil JN (1989) Spermatophore mass as an estimate of male nutrient investment: a closer look in Pseudaletia unipuncta (Haworth) (Lepidoptera: Noctuidae). Funct Ecol 3: 605–612
Maynard-Smith J (1974) The theory of games and the evolution of animal conflicts. J Theor Biol 47: 209–221
Mukerji D, Hakim Bhuya MA (1973) Reproductive system of the bruchid beetles Bruchus quadrimaculatus Fabr. Bruchus (Callosobruchus) chinensis L., (Bruchidae: Coleoptera). J Morphol 61: 175–221
Ouedraogo AP (1978) Étude de quelques aspects de la biologie de Callosobruchus maculatus F. (Coléoptère: Bruchidae) et de l'influence des facteurs externes stimulants (plante hôte et copulation) sur l'activité reproductrice de la femelle. Thése Université Paul Sabatier; Thése Mme Cycle, Toulouse, France
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45: 525–567
Parker GA (1984) Sperm competition and the evolution of animal mating strategies. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, London, pp 1–60
Parker GA, Simmons LW (1991) A model of constant random sperm displacement during mating: evidence from Scatophaga. Proc R Soc Lond B 249: 107–115
Parker GA, Baker RR, Smith VGF (1972) The origin and evolution of gamete dimorphism and the male-female phenomenon. J Theor Biol 36: 529–553
Parker GA, Simmons LW, Kirk H (1990) Analysing sperm competition data: simple models for predicting mechanisms. Behav Ecol Sociobiol 27: 55–65
Sugawara T (1979) Stretch receptors in the bursa copulatoox of the butterfly, Pieris rapae crucivora, and its role in behaviour. J Comp Physiol 130: 191–199
Svard L, Wiklund C (1991) The effect of ejaculate mass on female reproductive output in the European swallowtail butterfly, Papilio machaon (L.)(Lepidoptera: Papilionidae). J Insect Behav 4: 33–41
Turner ME, Anderson WW (1983) Multiple mating and female fitness in Drosophila pseudoobseura. Evolution 37: 714–723
Wickler W (1985) Stepfathers in insects and their pseudo-parental investment. Z Tierpsychol 69: 72–78
Wilson K, Hill L (1989) Factors affecting egg maturation in the bean weevil Callosobruchus maculatus. Physiol Entomol 14: 115–126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eady, P. Why do male Callosobruchus maculatus beetles inseminate so many sperm?. Behav Ecol Sociobiol 36, 25–32 (1995). https://doi.org/10.1007/BF00175725
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00175725