Abstract
Research has conclusively demonstrated the potential for dispersal evolution in range expansions and shifts, however the degree of dispersal evolution observed has varied substantially among organisms. Further, it is unknown how the factors influencing dispersal evolution might impact other ecological processes at play. We use an individual-based model to investigate the effects of the underlying genetics of dispersal and mode of reproduction in range expansions and shifts. Consistent with predictions from stationary populations, dispersal evolution increases with sexual reproduction and loci number. Contrary to our predictions, however, increased dispersal does not always improve a population’s ability to track changing conditions. The mate finding Allee effect inherent to sexual reproduction increases extinction risk during range shifts, counteracting the beneficial effect of increased dispersal evolution. Our results demonstrate the importance of considering both ecological and evolutionary processes for understanding range expansions and shifts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Range expansions and shifts have become ubiquitous features of modern biomes. For centuries, humans have initiated the range expansions of invasive species into new areas through travel, commerce, agriculture, and other routes, a trend that has only increased with further globalization (Hulme 2009). In recent decades, anthropogenic climate change has led to additional range expansions in a wide variety of taxa as species move to track changing climatic conditions (Parmesan 2006). Further, range expansions are common in conservation settings as successfully reintroduced species expand throughout their former habitats (Smeraldo et al. 2017). Given the widespread occurrences of range expansions and their importance for conservation, dynamics of range expansions have been studied intensively from both ecological and evolutionary perspectives (Miller et al. 2020). How these underlying ecological and evolutionary dynamics interact to shape the outcomes of range expansions remains an open question with great potential to shape our predictions of changes in biodiversity in the coming decades (Miller et al. 2020).
One area with potential for ecological and evolutionary processes to interact during range expansion is dispersal. Ecological theory has long demonstrated the key role that dispersal plays in determining the rate of expansion (Skellam 1951). However, recent research has demonstrated that dispersal can evolve during range expansion, even over relatively short time scales (Miller et al. 2020). Range expansions can introduce multiple selective pressures for increased dispersal ability, including a release from intraspecific competition at the expansion front (Perkins et al. 2013), kin competition among closely related founding populations (Kubisch et al. 2013), and spatial sorting of highly dispersive individuals at the expansion front (Shine et al. 2011). Simultaneously, environmental variability and Allee effects can introduce costs (Shaw and Kokko 2015; Shaw et al. 2014) that could make evolution of heightened dispersal less likely (Travis and Dytham 2002).
Laboratory studies have generally confirmed that range expansions lead to evolutionary increases in dispersal, thus increasing the rate of expansion (Weiss-Lehman et al. 2017; Ochocki and Miller 2017; Williams et al. 2016). However, the degree to which expansions accelerated varied widely among, and sometimes within, study organisms (Miller et al. 2020). Increased rates of expansion can be problematic in the context of invasive species (Phillips et al. 2006), but it could be beneficial to species expanding their ranges in response to climate change as increased dispersal abilities allow species to better track changing climatic conditions (Boeye et al. 2013). However, given the increasing rate of climate change (Chen et al. 2017) and the already substantial lag of certain species in tracking changing climatic conditions (Devictor et al. 2008), it is unclear if dispersal evolution will be enough to rescue dispersal-limited populations. Some theoretical models suggest that dispersal evolution could indeed provide a buffer, allowing populations otherwise doomed to extinction to persist (Boeye et al. 2013), but others show it may be insufficient by itself to prevent the extinction of struggling populations (Weiss-Lehman and Shaw 2020). Therefore, it is critical to understand the factors underlying dispersal evolution in range expansions and in what contexts it should lead to increased spread rates.
Empirical studies have demonstrated a wide array of genetic architecture underlying dispersal (Saastamoinen et al. 2018). However, until recently theoretical models have typically used relatively simple genetic assumptions (e.g. single-locus control, asexual reproduction) when modeling dispersal, despite evidence that dispersal is typically a polygenic trait (Saastamoinen et al. 2018). Factors such as reproductive mode (sexual vs. asexual) and the number of loci contributing to dispersal likely influence its evolutionary dynamics. As with other traits, sexual reproduction and increased numbers of loci might be expected to increase the rate of evolution due to the larger potential for recombination (Goddard et al. 2005; Pritchard et al. 2010). However, in a previous simulation study, increasing the number of loci defining dispersal led to slower evolutionary responses to a habitat fragmentation event (Saastamoinen et al. 2018). It is thus unclear how the number of loci defining dispersal might shape evolution during a range expansion or shift. Additionally, these factors could interact with the demographic processes of range expansion to shape dispersal evolution. For example, sexual reproduction is expected to lead to faster dispersal evolution, but it can also lead to mate finding Allee effects in range expansions which could reduce the strength of selection acting on dispersal (Travis and Dytham 2002) and reduce overall expansion speed (Shaw and Kokko 2015). Asexual organisms or those with the ability to self-fertilize might escape the negative impacts of mate finding Allee effects, but simultaneously lose some of the benefit to dispersal evolution caused by sexual reproduction. Understanding how these factors interact to shape the overall dynamics of range expansion will be critical for predicting which species may be able to shift their ranges successfully in response to climate change and which may be left behind (Hargreaves and Eckert 2014).
To explore the interplay between evolutionary and ecological factors shaping dispersal evolution and range expansion dynamics, we constructed an individual-based model to explore the role of the number of loci defining dispersal and the mode of reproduction (asexual vs. sexual and the role of self-fertilization) in dispersal evolution during range expansions. We explored the role of these factors in unbounded range expansions and in range shifts (range expansion at one edge coupled with range contraction at the opposite edge). By using a single, common framework, we directly compared the effect of each factor on the rate of dispersal evolution and related them to the extinction risk faced by populations shifting their ranges in response to climate change. We predicted that sexual reproduction and more loci would lead to greater increases in the dispersal trait due to the increased role for recombination. Further, we hypothesized that this increased rate of dispersal evolution would lead to lower extinction risk in populations shifting their ranges in response to simulated climate change.
Methods
Model overview
Our model examined population dynamics of a single species within a 1-dimensional landscape consisting of discrete habitat patches. We implemented a life cycle consisting of non-overlapping generations in which individuals first dispersed among patches and then reproduced within their patch. An individual’s dispersal trait was defined by a variable number of loci, each contributing additively to the overall trait value. Reproduction occurred either asexually or sexually with individuals defined as haploid or diploid accordingly. Under sexual reproduction, individuals could be dioecious or monoecious with variable levels of self-fertilization in monoecious populations. For our experimental scenarios, we varied (1) the number of dispersal loci, (2) the mode of reproduction, and (3) the level of self-fertilization in sexually reproducing monoecious populations. All simulations were performed in R (version 3.5.3 (R Core Team 2019)) and run on the Teton Computing Environment, Intel x86_64 cluster (Advanced Research Computing Center 2018). All model code is available through GitHub (https://github.com/tpweiss06/DispersalEvolution).
Environment
Landscapes consisted of linear, 1-dimensional arrays of discrete habitat patches. While modeling only a single dimensional landscape could impact the evolutionary dynamics of dispersal, previous research has shown that dispersal evolution proceeded similarly in 1- and 2-dimensional simulated range expansions (Phillips 2015). Additionally, the reduced computational burden of simulating a 1-dimensional landscape allowed us to more fully explore a wide range of scenarios and parameter combinations (described below). Environmental conditions in each patch (x) were defined by the carrying capacity K(x), which could range from 0 (uninhabitable) to a maximum value of \(K_{max}\). To simulate range boundaries, we defined two additional parameters: \(\tau \) defined the width of the range core, in which \(K(x) = K_{max}\), and \(\gamma \) was the rate of decline in K(x) at the range edges. More precisely, the carrying capacity of each patch was given by
in which \(\beta (t)\) defined the center of the range. When initiating simulations, \(\beta (0) = 0\) but it changed linearly in some scenarios to simulate range shifts due to climate change (see Experimental scenarios below).
Dispersal
In each generation, individuals first dispersed among the discrete habitat patches making up the landscape. Each individual dispersed according to an exponential dispersal kernel defined by the individual’s dispersal trait. The dispersal trait for an individual i was the expected dispersal distance for an individual (\(d_i\)), given by
where \(\hat{d}\) was the maximum expected dispersal distance in terms of discrete patches, \(\rho \) and \(\lambda \) were constants determining the slope and location of the transition between 0 and \(\hat{d}\), and the summation was taken across all l alleles contributing to dispersal. While alleles can take on negative values, this function restricts dispersal to positive values less than \(\hat{d}\). By incorporating a maximum expected dispersal distance, we attempted to capture the reality that dispersal traits will be limited for many species by either physiological constraints or trade-offs with other traits (Rees 1993). Importantly, as individual dispersal distances were drawn from an exponential dispersal kernel, realized dispersal could exceed \(\hat{d}\). A visual representation of the dispersal function along with further explanation of each of the parameters is presented in Figure S1. The number of alleles contributing to dispersal in each simulation depended on both the number of loci (L) used in the simulation and the number of chromosomes (i.e. in diploid individuals, \(l = 2L\)). Thus, alleles were assumed to contribute additively with no dominance or epistasis. The expected dispersal distance, \(d_{i}\), was then used to draw a realized distance from an exponential dispersal kernel and direction (forward or backward in the linear landscape) was chosen by a single draw from a Bernoulli distribution with \(p = 0.5\) (i.e. a coin flip).
Population dynamics
Following dispersal, reproduction occurred in each discrete patch according to a stochastic implementation of the classic Ricker model (Ricker 1954). Importantly, this model can account for asexual reproduction or sexual reproduction with explicit males and females (Leach et al. 2020). In the relatively simple case of an asexual population, the expected population size in patch x at time \(t+1\) was given by
where \(N_{t,x}\) was the current population size of patch x, R was the intrinsic growth rate, and K(x) was the carrying capacity as defined above. This equation also applied to a sexually reproducing population of monoecious individuals. To expand the model to sexually reproducing populations of dieocious individuals we introduced a new parameter, \(\psi \), defining the expected proportion of females produced each generation (i.e. \(\psi = 0.5\) corresponded to an even sex ratio on average). The expected population growth then became
in which \(F_{t,x}\) was the number of females in patch x at time t. To account for demographic stochasticity, these expected population sizes were then used to draw the realized population sizes from a Poisson distribution (\(N_{t+1,x}\sim Poisson(\hat{N}_{t+1,x})\)). Similarly, to allow for stochasticity in sex ratios for dioecious populations, the number of females in each generation was drawn from a binomial distribution (\(F_{t+1,x}\sim Binomial(N_{t+1,x}, \psi )\)). Thus, mating and density-dependent competition occurred locally, within each patch, similar to the dynamics of annual plants or other semelparous organisms (Jerde et al. 2009).
For both monoecious and dioecious populations, we assumed a relatively simple mating system in which individuals could mate multiple times. Monoecious populations only experienced a mate finding Allee effect when self-fertilization was prohibited (obligatory outcrossing), meaning there had to be at least two individuals in a patch for reproduction. In dioecious populations, patches had to contain at least one individual of each sex for successful reproduction. However, as individuals could mate multiple times, if these conditions were met all individuals were able to reproduce. Thus, the mate finding Allee effects for both types of sexually reproducing populations were minimal.
Inheritance
For each individual produced for the next generation, parentage was assigned randomly according to the mode of reproduction. Under asexual reproduction, a single parent was drawn randomly (with replacement) from the local population. Under sexual reproduction in dioecious populations, a male and female were drawn randomly from the local population. In monoecious populations, a single parent was first drawn and then a second was drawn with probability \(1-\omega \) so that \(\omega \) was the probability of self fertilization. Offspring then inherited alleles from their parent(s) assuming no linkage among loci and a mutation process defined by two parameters: the probability of at least one mutation across the genome (U) and the standard deviation of mutational effects (\(\sigma \)). The per allele probability of mutation (\(\phi \)) was calculated as \(\frac{U}{l}\). Thus, when a mutation occurred with probability \(\phi \), the new allele value was drawn from a normal distribution with mean equal to the parental allele value and a standard deviation of \(\sigma \). This ensured mutational dynamics were roughly equivalent among scenarios with different numbers of loci.
Simulation initiation
Each simulation began with a 50000 generation burn-in period to minimize the role of initial conditions. Each patch was populated with a number of individuals equal to the patch’s carrying capacity (K(x)). Individuals were assigned random genotypes assuming normally distributed allele frequencies. The range of possible allele values defining the initial populations was jointly determined by L and \(\sigma \), such that simulations with fewer loci drew from a larger range of possible allele values. Thus, initial alleles for simulations with few loci tended to have larger values on average compared to the initial alleles in simulations with many loci. This ensured that the mean and variability of dispersal phenotypes was equivalent regardless of the number of dispersal loci, allowing for dispersal evolution to proceed comparably across simulations. Ranges were stationary during the burn-in period, with \(\beta (t) = 0\) for the first 50000 generations of the simulation, after which different experimental scenarios were imposed.
Experimental scenarios
In our simulations, we varied the mode of reproduction to explore (1) asexual populations, (2) dioecious populations, (3) monoecious populations with obligate selfing (\(\omega = 1\)), (4) monoecious populations with partial selfing (\(\omega = 0.5\)), and (5) monoecious populations with obligate outcrossing (\(\omega = 0\)). We also varied the number of loci defining dispersal by powers of 2 (1, 2, 4, 8, 16, or 32 loci; diploid populations then had twice this number of alleles contributing to dispersal), yielding a total of 30 scenarios for our simulations (5 modes of reproduction by 6 possible numbers of loci defining dispersal). After the 50000 generation burn-in period for each simulation, we examined dispersal evolution in two contexts: (1) unbounded range expansions and (2) simultaneous range expansion at one edge and contraction at the opposite range edge (hereafter referred to as range shifts). In unbounded range expansions, the carrying capacities of all patches in the landscape were set to \(K_{max}\) in generation 50001 and populations were allowed to expand in both directions. After 200 generations, we recorded the distance spread in both directions and the mean dispersal phenotype and additive genetic variance of each local population within 50 patches of the last occupied patch in either direction (equivalent to the number of patches in which \(K(x) < K_{max}\) on either end of the stable range; hereafter referred to as the edge population). In range shifts, the center of the range changed linearly with time according to \(\beta (t)=\nu t\) so that \(\nu \) defined the rate of simulated climate change. In these simulations, patch carrying capacities declined away from the range center according to equation 1. Therefore, populations had to expand to track the viable habitat and avoid extinction. Range shifting populations were also tracked for 200 generations, after which mean dispersal phenotype and additive genetic variance were recorded for all patches in extant populations. We also recorded the overall proportion of simulated populations to go globally extinct during the range shift across all scenarios. Each scenario was explored with 1000 simulations for each population type. For both scenarios, we also performed 1000 simulations in which dispersal evolution was prevented and individuals in each generation were randomly assigned allele values drawn from the population at generation 50000 (i.e. from the end of the burn-in period). Thus, we quantified evolutionary changes in dispersal via comparisons between populations before and after 200 generations of either unbounded range expansion or range shift and we further quantified the impact of dispersal evolution on the observed dynamics by comparisons to the respective simulations in which dispersal evolution was prevented. A full list of the parameter values used for these simulations is given in Table 1. We additionally performed sensitivity analyses to determine the role of key parameters governing the mutation process (U and \(\sigma \)), population growth (\(K_{max}\) and R), and dispersal (\(\hat{d}\) and \(\rho \)) in our results. We describe the sensitivity analyses in detail and present the results in the Supplementary Materials.
Results
After the 50000 generation burn-in period, asexual and monoecious populations evolved to approximately the same average dispersal phenotype across all numbers of loci with dioecious populations exhibiting a slightly higher average phenotype (Fig. 1b). This agrees with previous results that have shown increased selection for dispersal due to kin competition in sexually reproducing populations (Hamilton and May 1977), which can be enhanced in dioecious populations (Heilbuth et al. 2001), as well as the risk of reproductive failure under a skewed local sex ratio in dioecious populations (Lawrence 1987). Despite these similar initial conditions, evolution of increased dispersal during unbounded range expansions and range shifts varied greatly among scenarios (Figs. 1, 2). In scenarios with no genetic mixing among individuals (asexual and obligately selfing populations), the increase in dispersal phenotypes was small and constant across different numbers of loci (Figs. 1a, 2a). In scenarios with at least some genetic mixing, on the other hand, increases in average dispersal phenotypes were positively correlated with the number of loci defining dispersal (Figs. 1b, 2b). The magnitude of this relationship depended on the degree to which genetic mixing occurred among individuals in the populations. Dioecious populations experienced the greatest increases in dispersal phenotypes while monoecious populations with partial selfing experienced the lowest increases among this group. However, even in populations with partial self-fertilization, the increase in dispersal phenotypes compared to populations with no genetic mixing among individuals was dramatic.
Effects of 200 generations of unbounded range expansion on evolution of the dispersal trait. Panels a and b show the average dispersal phenotypes of edge populations after 200 generations (filled points) compared to the starting populations (hollow points) across different numbers of loci defining the dispersal trait. Panels c and d show the average additive genetic variance of edge populations after expansion (filled points) compared to average additive genetic variance of initial populations (hollow points) with loci number again on the x axis. Panels a and c show results for scenarios in which there is no genetic mixing among individuals (asexual and obligately self-fertilizing populations) and panels b and d show results for the other scenarios. In all panels, the color and shape of points correspond to the population type as indicated in the legends on panels a and b. Points are the means across replicate simulations and line segments show the interquartile ranges. Additive genetic variance of asexual and obligately selfing populations (c) is essentially 0 for all numbers of loci, indicating the dominance of only a few or even one genotype after expansion
Effects of 200 generations of range shifts on evolution of the dispersal trait. Panels a and b show the average dispersal phenotypes of surviving populations after 200 generations (filled points) compared to the starting populations (hollow points) across different numbers of loci defining the dispersal trait. Panels c and d show the average additive genetic variance of surviving populations after expansion (filled points) compared to average additive genetic variance of initial populations (hollow points) with loci number again on the x axis. Panels a and c show results for scenarios in which there is no genetic mixing among individuals (asexual and obligately self-fertilizing populations) and panels b and d show results for the other scenarios. In all panels, the color and shape of points correspond to the population type as indicated in the legends on panels a and b. Points are the means across replicate simulations and line segments show the interquartile ranges. Additive genetic variance of asexual and obligately selfing populations (c) is essentially 0 for all numbers of loci, indicating the dominance of only a few or even one genotype after the range shift
Initial additive genetic variance (i.e. after the 50,000 generation burn-in) was broadly similar across loci for scenarios with no genetic mixing (Figs. 1c and S2) and increased with loci number in all other scenarios (Fig. 1d) but was then greatly reduced during range expansions and shifts, consistent with directional evolution of a trait. In unbounded range expansions (Fig. 1c, d), all scenarios exhibited similar reductions in genetic variance of edge populations when dispersal was defined by only a few loci. However, at large numbers of loci, scenarios with genetic mixing (Fig. 1d) were able to maintain higher levels of additive genetic variance after expansion, with obligately outcrossing populations maintaining the highest levels. The reduced additive genetic variance in asexual and obligately self-fertilizing populations likely reflects the absence of any recombination, meaning new genetic combinations would be entirely dependent on mutation. This also explains the slightly higher initial additive genetic variance in asexual populations as the per allele probability of mutation (\(\phi \)) is higher due to a smaller number of alleles in the haploid, asexually reproducing populations (see Methods). The reduction in variance in dioecious populations compared to other populations with recombination is likely a result of the stochastic sex ratios in these populations. Deviations from even sex ratios in dioecious populations can reduce the effective population size (Nunney 1993), thus reducing the genetic diversity these populations can support. In range shifts (Fig. 2d), both monoecious, obligately outcrossing and dioecious populations were able to maintain greater levels of genetic variance compared to partially self-fertilizing populations. However, this pattern is most likely driven by numerical effects as far fewer of the dioecious and obligately outcrossing populations survived the range shifts (Fig. 3).
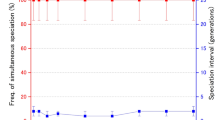
Extinction risk of evolving populations undergoing climate driven range shifts for 200 generations. The y axis shows the proportion of replicate simulations in each category to go extinct during the 200 generations. The x axis shows the number of loci defining dispersal in each simulation. As in previous graphs, the color and shape of points correspond to the population type as indicated in the legend
Observed increases in average dispersal phenotypes of edge populations in unbounded range expansions corresponded to increases in the distance spread by these populations compared to scenarios in which evolution was prevented (Fig. 4). Following the same trends as dispersal phenotypes, populations with no genetic mixing experienced a small and constant increase in distance spread while others showed a positive correlation between the increase in distance spread and the number of dispersal loci. However, despite dioecious and monoecious, obligately outcrossing populations experiencing the greatest increases in dispersal phenotypes at the expansion edge, they showed reduced increases in distance spread compared to monoecious, partially selfing populations. This is likely due to the ecological consequences of these mating systems, as outcrossing individuals must co-colonize a patch with another individual whereas self-fertilizing individuals can successfully colonize a new patch regardless of the presence of a potential mate.
Effect of evolution on distance spread in unbounded range expansions. The y axis shows the change in distance spread between simulations with evolution compared to simulations without evolution in units of discrete patches. Positive values indicate an increased distance spread due to evolution. The dashed grey line at 0 corresponds to no change in distance spread due to evolution. When evolution was prevented, simulated populations spread about 470 patches, on average, in 200 generations. Spread rates did not differ among population types or numbers of loci when evolution was prevented. The x axis shows the number of loci defining dispersal in each simulation. As in previous graphs, the color and shape of points correspond to the population type as indicated in the legend. Points are the among simulation means and line segments show the interquartile ranges
These ecological consequences of the different mating systems were even more important during range shifts. Populations with no genetic mixing displayed a constant extinction probability across different numbers of loci while other populations showed decreasing extinction probability with increasing numbers of loci (Fig. 3). This mirrors the patterns seen in dispersal phenotypes (Fig. 1a) and distance spread in unbounded range expansions (Fig. 4). Importantly, though, the difference was stark between monoecious, partially selfing populations and populations with no self-fertilization (monoecious, obligately outcrossing and dioecious populations). The reproductive need for two individuals, even under a relatively simple mating system, imposed a mate finding Allee effect so severe that monoecious, obligately outcrossing and dioecious populations experienced the highest extinction probabilities during range shifts, regardless of the number of dispersal loci. In contrast, monoecious, partially selfing populations experienced the lowest extinction probabilities, likely because they achieved the benefits of increased dispersal evolution from sexual reproduction without bearing the cost of a mate finding Allee effect.
Importantly, though, all populations still experienced a reduced extinction probability due to evolution of dispersal compared to no evolution scenarios (Fig. 5). Monoecious, partially selfing populations experienced the largest reduction in extinction risk due to dispersal evolution, and those reductions increased with the number of loci defining dispersal. When dispersal was defined by a single locus, monoecious, obligately outcrossing and dioecious populations experienced only a small reduction in extinction risk. However, as the number of loci defining dispersal increased, these populations experienced greater reductions in extinction risk, eventually matching the modest reductions seen in populations with no genetic mixing, but also no mate finding Allee effect.
Change in extinction risk due to evolution in climate driven range shifts. The y axis shows the change in the proportion of replicate simulations to go extinct in scenarios with evolution compared to scenarios without evolution. Negative values indicate a reduced extinction risk due to evolution. The dashed grey line at 0 corresponds to no change in extinction risk due to evolution. The x axis shows the number of loci defining dispersal in each simulation. As in previous graphs, the color and shape of points correspond to the population type as indicated in the legend
Finally, the sensitivity analyses revealed that, while variation in parameter values leads to variation in the absolute change in dispersal phenotypes or extinction risk, the qualitative patterns among scenarios reported here are robust. For example, as the mutation rate U and effect size of mutations \(\sigma \) increased, populations experienced greater changes in dispersal phenotype (Figs. S3–S6), but comparison among different population types revealed that the qualitative patterns observed in the main simulations remained consistent across different combinations of U and \(\sigma \). Similarly, lower values of R increased extinction risk and reduced distance spread for all populations (Figs. S7–S10), but relative differences among different population types remained. Finally, increasing values of \(\hat{d}\) increased the magnitude of changes in dispersal phenotypes across all scenarios, but dioecious and obligately outcrossing populations always displayed the highest change in dispersal phenotypes while still facing comparatively high extinction risks due to mate finding Allee effects (Figs. S11–S14).
Discussion
Dispersal evolution during range expansions and shifts has been observed in a variety of taxa (Phillips et al. 2006; Weiss-Lehman et al. 2017; Ochocki and Miller 2017; Williams et al. 2016). However, the magnitude of the change in dispersal behavior and the degree to which it influences population dynamics in range expansions have been variable across studies (Miller et al. 2020). Here, we demonstrate one reason for this observed variability could be underlying differences in the genetics of dispersal traits and differences in mating systems among taxa. Further, we show the importance of these differences in dispersal evolution to the dynamics of populations undergoing unbounded range expansions and climate-driven range shifts. While sexual reproduction and greater numbers of loci defining the dispersal trait led to greater evolved increases in dispersal, the accompanying ecological consequences of sexual reproduction (namely mate finding Allee effects) led to slower than expected spread in range expansions and heightened extinction risk in range shifts.
Despite the increased magnitude of dispersal evolution in sexually outcrossing populations, monoecious, obligately outcrossing and dioecious populations consistently spread less far in unbounded range expansions and experienced higher extinction risk in range shifts compared to monoecious, partially selfing populations (Figs. 3, 4). These partially self-fertilizing populations essentially gained the benefits of sexual reproduction for dispersal evolution but avoided the costs of mate finding Allee effects, thus allowing them to benefit the most from dispersal evolution (Fig. 5). Previous work has demonstrated the importance of mate finding Allee effects in slowing spatial spread (Shaw and Kokko 2015), but the degree to which they impact extinction risk in range shifts is surprising. Our model assumed a simplistic mating system in which individuals could mate multiple times and only required the presence of one other individual (or one individual of the opposite sex in the case of dioecious populations) to successfully reproduce. Thus, we expected Allee effects in our simulations to be negligible and readily overpowered by the benefits of increased dispersal evolution. Our results, however, indicate that even Allee effects brought on by simplistic mating systems can lead to slower spread and increased extinction risk, despite evolution of increased dispersal ability. More complex mating systems and social structures have been shown to lead to higher extinction risk in stationary populations (Leach et al. 2020), and would likely further exacerbate the negative impacts of mate finding Allee effects for range shifting populations.
Influence of model assumptions
While our model represents a step forward in modeling the underlying genetic complexities of dispersal (Saastamoinen et al. 2018), it still relies on several simplifying assumptions that warrant future exploration. First, we did not incorporate any explicit costs associated with dispersal, which can occur for many species in the form of energetic investment or increased risk of mortality (Bonte et al. 2012). Dispersal can still be costly in our model (e.g., if a sexually reproducing individual disperses into an empty patch, they will not be able to mate). However, due to the lack of explicit costs for dispersal, our results could be viewed as a “best case" scenario for many populations moving in response to climate change. Depending on the severity of dispersal-associated costs in natural populations, extinction risk may be substantially higher compared to the results presented here. Second, our model assumed density-independent dispersal, but both positive and negative density dependence have been observed in empirical dispersal patterns (Matthysen 2005). Positive density dependence in dispersal could reduce mate finding Allee effects as it would lead to more migrants simultaneously dispersing from high density patches, making it more likely to arrive in a patch together with a potential mate. However, several experimental tests have shown that dispersal evolution can lead to a reduction in density dependence of dispersal (Weiss-Lehman et al. 2017; Mishra et al. 2020), making it unclear how density-dependence of dispersal might interact with dispersal genetics to shape the dynamics of range expansions and shifts. Third, we modeled dispersal exclusively as a trait defining an individual’s dispersal kernel, but dispersal is a complex trait which can be defined by multiple processes including emigration from a patch, distance traveled, and settling rules in a new patch among other things (Clobert et al. 2009; Saastamoinen et al. 2018). These different aspects of dispersal could help explain the discrepancy between our results and those of Saastamoinen et al. (2018), in which they showed that with more loci, emigration probability evolved more slowly in response to habitat fragmentation. Future work should explore the roles of genetic architecture and evolutionary processes on evolution of these different aspects of dispersal.
In addition to the assumptions made regarding dispersal in our model, other aspects of our simulations could have contributed to our results. First, our model assumed that mating occurred after dispersal, as would be the case for many semelparous species (Jerde et al. 2009). However, if mating occurred prior to dispersal, mate finding Allee effects would be mitigated (Shaw and Kokko 2015) and extinction risk in range shifts might be greatly reduced. Additionally, research has shown that when the timing of mating is allowed to evolve along with dispersal, the strength of stabilizing selection for local conditions is a key factor determining the evolution of pre- vs. post-dispersal mating (Lakovic et al. 2017). It would be interesting to explore whether the context of range expansion interacts with the evolution of the timing of reproduction. Second, we restricted our simulations to single dimensional landscapes and single values of the rate of decline in carrying capacities at the range edge (\(\gamma \)) and the speed of climate change (\(\nu \)). In a previous model, we showed that increasingly stark range edges (higher \(\gamma \)) and faster rates of climate change (higher \(\nu \)) were both associated with increased extinction risk in range shifting, dioecious populations (Weiss-Lehman and Shaw 2020). While this previous work did not explore these parameters in the context of different modes of reproduction or different numbers of loci, we predict the qualitative effects of changing these parameters would hold. Further, this previous model used 2-dimensional landscapes and showed similar qualitative results in terms of dispersal evolution (Weiss-Lehman and Shaw 2020). Combined with previous work that showed similar results when explicitly comparing spatial sorting during range expansions in 1- vs. 2-dimensional landscapes (Phillips 2015), we are confident the qualitative relationships presented here would hold in 2-dimensional landscapes as well.
Model extensions
Range shifts have already been documented in a wide variety of taxa, though the degree to which different species have fully tracked changing climate conditions is quite variable (Parmesan 2006). Our results could help explain some of this variation. For example, bird species in France are shifting their ranges northwards, but are increasingly lagging behind climate indicators (Devictor et al. 2008). Oceanic dinoflagellates, on the other hand, have been able to closely track changing conditions as they shift their ranges with climate change (Chivers et al. 2017). Many factors are likely to impact such discrepancies among taxa, including, but not limited to, local environmental heterogeneity and dispersal limitations (Velo-Antón et al. 2013), other functional traits beyond dispersal (Ash et al. 2017), the impact of other selective processes on dispersal, like habitat fragmentation (Cote et al. 2017), or differences in growth rates and generation times. In addition to these and other factors, our results suggest that mate finding Allee effects could help explain why some asexual species like dinoflagellates more easily track changing conditions (Chivers et al. 2017) while sexually reproducing species like birds lag behind (Devictor et al. 2008).
As our results demonstrate, ecological and evolutionary processes can have contrasting effects on the overall dynamics of range expansions and shifts. While we show this specifically for dispersal evolution and mate finding Allee effects, it will likely hold true for other ecological and evolutionary processes at play in range expansions and shifts. Further, our model assumed populations would shift in space in response to climate change, but other responses are possible, including adaptation to changing conditions and shifts in phenology (Parmesan 2006). Furthermore, species’ ranges may be structured along existing environmental gradients, with populations adapted to local conditions (Sexton et al. 2009). Adaptation to such a gradient has been shown to lead to increased extinction risk in range shifting, dioecious individuals as the increased dispersal necessary to track changing climate conditions simultaneously increases deviations of individual genotypes from the local optimum values (Weiss-Lehman and Shaw 2020). Future work should consider the contrasting ecological and evolutionary processes at play in these other possible responses to climate change as well as how these different responses could interact with each other. For example, our results suggest self-fertilizing species might be best situated to respond to climate change in the form of a range shift (Fig. 5). However, research has shown that self-fertilization can reduce the adaptive potential of populations (Noël et al. 2017), which could limit the ability of such populations to persist in novel conditions after a range shift or to adapt their phenology or other traits in response to changing conditions. Further, while our work demonstrates the importance of simple, mate finding Allee effects in range shifts, other research has shown the potential for climate change to exacerbate existing Allee effects through temperature induced changes in metabolism or mating rate (Berec 2019). Thus, range shifting populations could face the prospect of multiple, compounding Allee effects hindering their ability to cope with climate change.
Conclusion
In this study, we demonstrated the critical interactions between the evolutionary mechanisms and ecological consequences of dispersal evolution during range expansions and shifts. In particular, we showed that while sexual reproduction leads to greater increases in dispersal ability, these increases cannot fully counter the negative demographic impacts of even the most simplistic mate finding Allee effects during range shifts. Our results suggest a potential strategy to aid range shifting species could focus on mitigating these mate finding Allee effects. This could be accomplished, for example, through the existing strategy of assisted migration for range shifting species in which the migrants could be transplanted in a manner designed to maximize their ability to find mates in the new habitat (Hällfors et al. 2017). However, in addition to potentially disrupting local adaptation (Montwé et al. 2018), such gene flow from the range core to the expanding edge could hinder dispersal evolution by disrupting the spatial sorting of individuals according to dispersal ability. Such a strategy has, in fact, been suggested as a control method for invasive species (Tingley et al. 2017). As our results demonstrate, more work is needed to fully understand the interacting ecological and evolutionary processes at play in species responding to climate change.
References
Advanced Research Computing Center (2018) Teton computing environment, Intel x86\_64 cluster. University of Wyoming, Laramie, Wyoming. https://doi.org/10.15786/M2FY47
Ash JD, Givnish TJ, Waller DM (2017) Tracking lags in historical plant species shifts in relation to regional climate change. Glob Change Biol 23:1305–1315
Berec L (2019) Allee effects under climate change. Oikos 128:972–983
Boeye J, Travis JM, Stoks R, Bonte D (2013) More rapid climate change promotes evolutionary rescue through selection for increased dispersal distance. Evol Appl 6:353–364
Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M et al (2012) Costs of dispersal. Biol Rev 87:290–312
Chen X, Zhang X, Church JA, Watson CS, King MA, Monselesan D, Legresy B, Harig C (2017) The increasing rate of global mean sea-level rise during 1993–2014. Nat Clim Chang 7:492
Chivers WJ, Walne AW, Hays GC (2017) Mismatch between marine plankton range movements and the velocity of climate change. Nat Commun 8:1–8
Clobert J, Le Galliard J-F, Cote J, Meylan S, Massot M (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209
Cote J, Bestion E, Jacob S, Travis J, Legrand D, Baguette M (2017) Evolution of dispersal strategies and dispersal syndromes in fragmented landscapes. Ecography 40:56–73
Devictor V, Julliard R, Couvet D, Jiguet F (2008) Birds are tracking climate warming, but not fast enough. Proc Roy Soc B Biol Sci 275:2743–2748
Goddard MR, Godfray HCJ, Burt A (2005) Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434:636
Hällfors MH, Aikio S, Schulman LE (2017) Quantifying the need and potential of assisted migration. Biol Cons 205:34–41
Hamilton WD, May RM (1977) Dispersal in stable habitats. Nature 269:578–581
Hargreaves AL, Eckert CG (2014) Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Funct Ecol 28:5–21
Heilbuth JC, Ilves KL, Otto SP (2001) The consequences of dioecy for seed dispersal: modeling the seed-shadow handicap. Evolution 55:880–888
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Jerde CL, Bampfylde CJ, Lewis MA (2009) Chance establishment for sexual, semelparous species: overcoming the allee effect. Am Nat 173:734–746
Kubisch A, Fronhofer EA, Poethke HJ, Hovestadt T (2013) Kin competition as a major driving force for invasions. Am Nat 181:700–706
Lakovic M, Mitesser O, Hovestadt T (2017) Mating timing, dispersal and local adaptation in patchy environments. Oikos 126:1804–1814
Lawrence W (1987) Dispersal: an alternative mating tactic conditional on sex ratio and body size. Behav Ecol Sociobiol 21:367–373
Leach D, Shaw AK, Weiss-Lehman C (2020) Stochasticity in social structure and mating system drive extinction risk. Ecosphere 11:e03038
Matthysen E (2005) Density-dependent dispersal in birds and mammals. Ecography 28:403–416
Miller TE, Angert AL, Brown CD, Lee-Yaw JA, Lewis M, Lutscher F, Marculis NG, Melbourne BA, Shaw AK, Szűcs M, et al (2020) Eco-evolutionary dynamics of range expansion. Ecology e03139
Mishra A, Chakraborty PP, Dey S (2020) Dispersal evolution diminishes the negative density dependence in dispersal. Evolution 74:2149–2157
Montwé D, Isaac-Renton M, Hamann A, Spiecker H (2018) Cold adaptation recorded in tree rings highlights risks associated with climate change and assisted migration. Nat Commun 9:1–7
Noël E, Jarne P, Glémin S, MacKenzie A, Segard A, Sarda V, David P (2017) Experimental evidence for the negative effects of self-fertilization on the adaptive potential of populations. Curr Biol 27:237–242
Nunney L (1993) The influence of mating system and overlapping generations on effective population size. Evolution 47:1329–1341
Ochocki BM, Miller TE (2017) Rapid evolution of dispersal ability makes biological invasions faster and more variable. Nat Commun 8:14315
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Perkins AT, Phillips BL, Baskett ML, Hastings A (2013) Evolution of dispersal and life history interact to drive accelerating spread of an invasive species. Ecol Lett 16:1079–1087
Phillips BL (2015) Evolutionary processes make invasion speed difficult to predict. Biol Invasions 17:1949–1960
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803
Pritchard JK, Pickrell JK, Coop G (2010) The genetics of human adaptation: hard sweeps, soft sweeps, and polygenic adaptation. Curr Biol 20:R208–R215
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rees M (1993) Trade-offs among dispersal strategies in British plants. Nature 366:150–152
Ricker WE (1954) Stock and recruitment. J Fish Board Can 11:559–623
Saastamoinen M, Bocedi G, Cote J, Legrand D, Guillaume F, Wheat CW, Fronhofer EA, Garcia C, Henry R, Husby A et al (2018) Genetics of dispersal. Biol Rev 93:574–599
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Shaw AK, Kokko H (2015) Dispersal evolution in the presence of allee effects can speed up or slow down invasions. Am Nat 185:631–639
Shaw AK, Jalasvuori M, Kokko H (2014) Population-level consequences of risky dispersal. Oikos 123:1003–1013
Shine R, Brown GP, Phillips BL (2011) An evolutionary process that assembles phenotypes through space rather than through time. Proc Natl Acad Sci 108:5708–5711
Skellam JG (1951) Random dispersal in theoretical populations. Biometrika 38:196–218
Smeraldo S, Di Febbraro M, Ćirović D, Bosso L, Trbojević I, Russo D (2017) Species distribution models as a tool to predict range expansion after reintroduction: a case study on Eurasian beavers (castor fiber). J Nat Conserv 37:12–20
Tingley R, Ward-Fear G, Schwarzkopf L, Greenlees MJ, Phillips BL, Brown G, Clulow S, Webb J, Capon R, Sheppard A et al (2017) New weapons in the toad toolkit: a review of methods to control and mitigate the biodiversity impacts of invasive cane toads (rhinella marina). Q Rev Biol 92:123–149
Travis JM, Dytham C (2002) Dispersal evolution during invasions. Evol Ecol Res 4:1119–1129
Velo-Antón G, Parra J, Parra-Olea G, Zamudio K (2013) Tracking climate change in a dispersal-limited species: reduced spatial and genetic connectivity in a montane salamander. Mol Ecol 22:3261–3278
Weiss-Lehman C, Shaw AK (2020) Spatial population structure determines extinction risk in climate-induced range shifts. Am Nat 195:31–42
Weiss-Lehman C, Hufbauer RA, Melbourne BA (2017) Rapid trait evolution drives increased speed and variance in experimental range expansions. Nat Commun 8:14303
Williams JL, Kendall BE, Levine JM (2016) Rapid evolution accelerates plant population spread in fragmented experimental landscapes. Science 353:482–485
Acknowledgements
We thank members of the Theory Under Construction group at the University of Minnesota for thoughtful comments on the model. C.W.-L. was partially supported by start-up funds from the University of Minnesota (to A.K.S.), partially by start-up funds from the University of Wyoming, and partially by an NSF EPSCoR Track 2 RII grant (NSF award EPS-2019528). We acknowledge the Minnesota Supercomputing Institute at the University of Minnesota (http://www.msi.umn.edu) for providing resources that contributed to our results.
Funding
The research presented here was funded through start-up funds from both the University of Minnesota and the University of Wyoming as well as an NSF EPSCoR Track 2 RII grant (EPS-2019528).
Author information
Authors and Affiliations
Contributions
CWL and AKS developed the idea for the manuscript. CWL created the model, ran the simulations, and wrote the first draft of the manuscript. Both authors contributed to edits and revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Both authors consent to the publication of the manuscript.
Availability of data and material
No new data was used in this manuscript.
Code availability
All code necessary to run the model and associated analyses are available on GitHub (https://github.com/tpweiss06/DispersalEvolution).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weiss-Lehman, C., Shaw, A.K. Understanding the drivers of dispersal evolution in range expansions and their ecological consequences. Evol Ecol 36, 181–197 (2022). https://doi.org/10.1007/s10682-022-10166-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10166-9