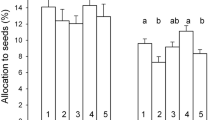
Abstract
Unlike other species of the genus Blechnum, the fern Blechnum chilense occurs in a wide range of habitats in Chilean temperate rainforest, from shaded forest understories to abandoned clearings and large gaps. We asked if contrasting light environments can exert differential selection on ecophysiological traits of B. chilense. We measured phenotypic selection on functional traits related to carbon gain: photosynthetic capacity (A max), dark respiration rate (R d), water use efficiency (WUE), leaf size and leaf thickness in populations growing in gaps and understorey environments. We assessed survival until reproductive stage and fecundity (sporangia production) as fitness components. In order to determine the potential evolutionary response of traits under selection, we estimated the genetic variation of these traits from clonally propagated individuals in common garden experiments. In gaps, survival of B. chilense was positively correlated with WUE and negatively correlated with leaf size. In contrast, survival in shaded understories was positively correlated with leaf size. We found positive directional fecundity selection on WUE in gaps population. In understories, ferns of lower R d and greater leaf size showed greater fecundity. Thus, whereas control of water loss was optimized in gaps, light capture and net carbon balance were optimized in shaded understories. We found a significant genetic component of variation in WUE, R d and leaf size. This study shows the potential for evolutionary responses to heterogeneous light environments in functional traits of B. chilense, a unique fern species able to occupy a broad successional niche in Chilean temperate rainforest.


Similar content being viewed by others
References
Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, Sandquist DR, Geber MA, Evans AS, Dawson TE, Lechowicz MJ (2000) The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50:979–995
Aldasoro JJ, Cabezas F, Aedo C (2004) Diversity and distribution of ferns in sub-Saharan Africa, Madagascar and some islands of the South Atlantic. J Biogeogr 31:1579–1604
Almeyda E, Sáez F (1958) Recopilación de datos climáticos de Chile y mapas sinópticos respectivos. Ministerio de Agricultura, Santiago, Chile
Arntz A, Delph L (2001) Pattern and process: evidence for evolution of photosynthetic traits in natural populations. Oecologia 127:455–467
Brodribb TJ, Holbrook NM (2004) Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytol 162:663–670
Chapin FS, Autumn K, Pugnaire F (1993) Evolution of suites of traits in response to environmental stress. Am Nat 142:S78–S92
Chazdon R (1992) Photosynthetic plasticity of two rain forest shrubs across natural gaps transects. Oecologia 92:586–595
Chiariello N (1984) Leaf energy balance in the wet lowland tropics. In: Medina E, Mooney H, Vázquez-Yanes C (eds) Physiological ecology of plants of the wet tropics. Dr. W. Junk, The Hague, Netherlands, pp 85–98
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annu Rev Ecol Syst 18:431–451
Dudley SA (1996) Differing selection on plant physiological traits to environmental water availability: a test of adaptive hypotheses. Evolution 50:92–102
Ehleringer JR (1993) Gas exchange implications of isotopic variation in arid-land plants. In: Smith JA, Griffith H (eds) Plant responses from cell to community. Bios Scientific Publishers, Oxford, UK, pp 265–284
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton, NJ, USA
Falster DS, Westoby M (2003) Leaf size and angle vary widely across species: what consequences for light interception? New Phytol 158:509–525
Geber M, Dawson T (1990) Genetic variation in and covariation between leaf gas exchange, morphology, and development in Polygonum arenastrum, an annual plant. Oecologia 85:153–158
Geber M, Griffen LR (2003) Inheritance and natural selection on functional traits. Int J Plant Sci 164:S21–S42
Givnish TJ (1987) Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol 106:131–160
Givnish TJ (1988) Adaptation to sun and shade: a whole plant perspective. Aust J Plant Physiol 15:63–92
Grime JP (1965) Shade tolerance in flowering plants. Nature 208:161–163
Huber A, López D (1993) Hydric balance changes in an adult Pinus radiata stand (Valdivia, Chile). Bosque 14:11–18
Janzen FJ, Stern HS (1998) Logistic regression for empirical studies of multivariate selection. Evolution 52:1564–1571
Karst J, Gilbert B, Lechowicz MJ (2005) Fern community assembly: the roles of chance and the environment at local and intermediate scales. Ecology 86:2473–2486
Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P (2001) The strength of phenotypic selection in natural populations. Am Nat 157:245–261
Kitajima K (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Lambers H, Chapin FS, Pons T (1998) Plant physiological ecology. Springer-Verlag, New York, USA
Lande R, Arnold S (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Loach K (1967) Shade tolerance in tree seedlings. I. Leaf photosynthesis and respiration in plants raised under artificial shade. New Phytol 66:607–621
Ludwig F, Rosenthal LF, Johnston JA, Kane N, Gross BL, Lexer C, Dudley SA, Riesenberg LH, Donovan LA (2004) Selection on leaf ecophysiological traits in a desert hybrid Helianthus species and early-generation hybrids. Evolution 58:2682–2692
Lusk CH (2002) Leaf area accumulation helps juvenile evergreen trees tolerate shade in a temperate rainforest. Oecologia 132:188–196
Lusk CH, Reich P (2000) Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 123:318–329
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Associates Inc. Publishers, MA, USA
Marticorena C, Rodríguez R (1995) Flora de Chile. vol I. Universidad de Concepción, Edit. Anibal Pinto, Concepción, Chile
Mitchell-Olds T, Shaw RG (1987) Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41:1149–1161
Page CN (2002) Ecological strategies in fern evolution: a neopteridological overview. Rev. Pelaeobot. Palynol 119:1–33
Pérez-García B, Mendoza A, Ricci M (1996) Morfogénesis de la fase sexual de Blechnum chilense y Blechnum cycadifolium (Pterophyta: Blechnaceae). Rev Biol Trop 44:491–497
Phillips P, Arnold S (1989) Visualizing multivariate selection. Evolution 43:1209–1222
Primack RB, Kang H (1989) Measuring fitness and natural selection in wild plant populations. Annu Rev Ecol Syst 20:367–396
Reich P, Walters M, Ellsworth D, Vose J, Volin J, Gresahm C, Bowman W (1998) Relationship of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life span: a test across biomes and functional groups. Oecologia 114:471–482
Raven PH, Evert RF, Eichhorn SE (1999) Biology of plants, 6th edn. W. H. Freeman and Company, New York, USA
Robinson J (1994) Speculations on carbon dioxide starvation, Late Tertiary evolution of stomatal regulation and floristic modernization. Plant Cell Environ 17:345–354
Rodríguez R (1973) Morfología de los protalos y esporofitos jóvenes de algunas especies chilenas de Blechnum (Polypodiaceae s. l.). Gayana 22:3–30
Saldaña A, Lusk CH (2003) Influencia de las especies del dosel en la disponibilidad de recursos y regeneración avanzada en un bosque templado lluvioso del sur de Chile. Rev Chil Hist Nat 76:639–650
Saldaña A, Gianoli E, Lusk CH (2005) Physiological and morphological responses to light availability in three Blechnum species (Pteridophyta, Blechnaceae) of different ecological breadth. Oecologia 145:252–257
Simms E, Rausher M (1992) Uses of genetics for studying the evolution of plant resistance. In: Fritz R, Simms E (eds) Plant resistance to herbivores and pathogens. Ecology, evolution and genetics. University of Chicago Press, Chicago, USA
Smith TM, Huston ML (1989) A theory of the spatial and temporal dynamics of plant communities. Vegetatio 83:49–69
Spencer W, Teeri J, Wetzel R (1994) Acclimation of photosynthetic phenotype to environmental heterogeneity. Ecology 75:301–314
Sultan SE, Wilczek A, Bell D, Hand G (1998) Physiological response to complex environments in annual Polygonum species of contrasting ecological breadth. Oecologia 115:564–578
Walters M, Field C (1987) Photosynthetic light acclimation in two rainforest Piper species with different ecological amplitudes. Oecologia 72:449–456
Woodhouse R, Nobel P (1982) Stipe anatomy, water potentials and xylem conductances in seven species of ferns (Filicopsida). Am J Bot 69:135–142
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin FS, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Acknowledgements
We are grateful to Flor, Susana and Nicol Fuentes for help in field sampling and common gardens setup. This research was funded by CONICYT project AT-4040099. The first author was supported by a CONICYT doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saldaña, A., Lusk, C.H., Gonzáles, W.L. et al. Natural selection on ecophysiological traits of a fern species in a temperate rainforest. Evol Ecol 21, 651–662 (2007). https://doi.org/10.1007/s10682-006-9143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-006-9143-7