Abstract
The aim of this study was to analyse the effects of different date of insecticidal treatment against Noctuinae caterpillars on the technological yield from sugar beet using the additive main effect and multiplicative interaction (AMMI) model. The AMMI model is one of the most widely used statistical tools in the analysis of multiple-environment trials. The results of the analysis of the dependence of the components of the sugar beet yield, carried out separately in individual years (2011–2018) of the experiment, indicate a significant and directly proportional impact of the root mass on the technological yield of sugar in all years. The average sugar content per years also varied from 16.22% (2014) to 19.68% (2015). Potassium molasses from the base of the tested protective treatments varied from 27.27 to 61.43 mmol kg−1. The average sodium molasses per years also varied from 1.196 mmol kg−1 (2015) to 6.692 mmol kg−1 (2018). α-amine-nitrogen of the tested protective treatments varied from 6.03 (for phenological criterion in 2011) to 37.95 mmol kg−1 (for intervention criterion in 2018). Technological yield of sugar beet tested protective treatments varied from 171.4 (for phenological criterion in 2015) to 360.0 t ha−1 (for soil spraying of plants—in 2012) throughout the 8 years, with an average of 280.47 t ha−1. The use of the AMMI model to estimate the interaction of conducted insecticidal treatments based on environmental conditions showed the additivity of the effects of the applied treatments on the effectiveness of the obtained quality features of the technological yield of sugar beet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gross sugar yield is the most important trait for growers, and it depends on the weight of the roots produced per hectare and on the sugar content, i.e., the percentage above-mentioned of sucrose present in the roots. In addition to the gross sugar yield, the extractable sugar must be considered, indicating how much white sugar can be extracted in the factory (Biancardi et al. 2010).
The problem of technological quality of roots is a very important issue both in terms of the sugar yield and the requirements of the sugar industry (Campbell 2002). The influence of agrotechnical factors on the technological sugar yield of sugar beets occurs primarily through the impact on the root yield, and then the sucrose content in the roots, which is most influenced by the habitat conditions (also by cultivars) (Nowakowski and Krüger 1997; Klotz and Finger 2004; Michalska-Klimczak and Wyszyński 2010; Hoffmann and Kenter 2018). The content of molasses-forming compounds (α-amino nitrogen and sodium and potassium ions) in beet roots has a significant impact on the sugar production process. The higher their content, the worse the technological value of beets (Jakubowska et al. 2020a). The increased content of molasses also hinders the sugar production process (Michalska-Klimczak and Wyszyński 2010). Sugar beet is a plant with high habitat and agrotechnical requirements and strongly reacts to various growing conditions. Root and sugar yields, plant morphological features, including ground and underground parts, polarization and molasses content are changed throughout the beet vegetation period under the influence of many factors. In this paper, the weather conditions prevailing during the experiment, soil quality, the date of sowing and harvesting and the insecticide treatments applied at different times were evaluated in relation to selected quality parameters of yield. Sugar beet is attacked by many pests which may directly (feeding on plant tissue) or indirectly (vectors of plant pathogens) affect the plants. Pimentel (2005) estimated the world wide losses (due to weeds, pathogens and insects) as 25–35% pre-harvest and 10–20% post-harvest agricultural plants. Viral diseases and nematodes are a serious problem for beet cultivation, in most parts of the world. Similarly cutworms, or surface caterpillars, damage sugar beet plants and have negative impact on obtained crop yield. Several species are known to damage sugar beet, usually feeding on stem bases or crowns, including larvae of various noctuid moths (Jakubowska et al. 2020b). Many species of cutworms including Agrotis segetum Den. et Schiff. (Turnip moth) and Agrotis exclamationis L. (Heart and dart moth) affect sugar beets in Poland every year. Currently, it is estimated that the harmfulness caused by cutworms in different agricultural crops in Poland ranges from 2 to 30%, depending on the different agricultural crops.
The assessment of the genotype-environmental interaction of sugar beet depends on the importance of the selection of varieties for the stability of raw material production and its quality in different regions of this plant cultivation in Poland (Jaskulska et al. 2017; Hassani et al. 2018) furthermore other different abiotic conditions e.g. temperature of air. One of the most effective and most frequently used methods for analysis genotype-environmental interaction (GEI) based on various modifications of the two-way ANOVA model is the combined regression analysis to determine the significance and strength of correlation and dependence of features called AMMI (Additive Main Effect and Multiplicative Interaction analysis) (Zobel et al. 1988; Gauch and Zobel 1990). The additive main effects and multiplicative interaction (AMMI) model (Gauch 1992) is one of the most widely used statistical methods. It can be used to understand and structure interactions between genotypes and environments. In its essence, the AMMI model applies the singular value decomposition (SVD) to the residuals of an additive two-way analysis of variance (ANOVA) model as applied to the GEI table of means (Gauch, 2013; Rodrigues et al. 2014).
The aim of the study was to evaluate the effects of chemical treatment by year interaction (TYI) for six quantitative traits (root weight, sugar content, potassium molasses, sodium molasses, α-amine nitrogen, and technological yield of sugar beet) depending on different decision-making methods to insecticidal treatments against caterpillars of cutworm using the AMMI model. The following hypothesis was proposed: abiotic conditions, varieties, chemical application of insecticides significantly modifies the content of molasses in root of sugar beet plants.
Material and methods
The experiment was carried out in Słupia Wielka (52° 13′ 02″ N, 17° 13′ 04″ E) (COBORU Field Experimental Station) in 2011–2018. The soils on which the research was carried out were podzolic soils developed on light loamy sands and fawn soils. These are soils of a very good rye complex, valuation class IIIa, IIIb and IVa. Three varieties of sugar beet were used: Jagoda, Janusz, Maryna. Soil pH was close to neutral as required for sugar beet, with medium phosphorus (P) content, and high potassium (K) and magnesium (Mg) content. The winter wheat was a fore crop for sugar beet in every year. The experiments were carried out according to the same methodology, assuming the use of generally accepted agrotechnical procedures and treatments for sugar beet. All plots were fertilized with the same dose of nitrogen (120 kg N ha−1). Half of the nitrogen dose was applied before sowing and when sugar beets had four pairs of leaves developed (BBCH 14). One week before sowing the soil was fertilized with P, dose 60 kg of P2O5 ha−1, combined with K. During the growth period standard herbicide and fungicide protection were used. Years of research were characterized by differently weather conditions for the growth and yielding of sugar beet. The amount of precipitation during the growing season (April–October) was a the biggest in 2017 (454.7 mm) and the smallest in 2015 (221.2 mm). The largest rainfall deficiency was observed in July (2011–2012; 2015–2018) and September (2013) as well as in May 2014. The hydrotermical coefficient (K) deficiency occurred in May and August in years 2012–2018 (without 2011) as well as in September in 2011, 2014–2016). The average daily temperature in the study period was greater in July and August in all the years (Table 1).
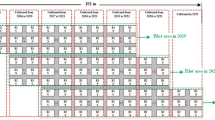
In the experiment, a variety of sugar beet—Beta vulgaris (L.) ssp. vulgaris were used: Jagoda (2011–2013), Janusz (2014–2016), and Maryna (2017–2018). All sugar beet seed varieties were treated with Tachigaren 70 WP fungicide (hymeksazol—active ingredient (a.i.)—700 g kg−1—70%) in dose 40 g per seeds unit·ha−1. The seeds were sown in the first decade of April (between on 4–9 of April) the sowing density was 1.02 the seeding unit per ha−1. The automatic seed drill ZÜRN D82 was used for sowing sugar beet, intended for precise sowing of test plots. The area of the plot for sowing was 13.5 m2 (width—1.8 m, length—7.5 m). The number of plants per plot was 108, when sowing beet seeds every 24.0 cm and with a row spacing of 45.0 cm. The number of rows in the plot was 4. The mean final plant density was 86 sugar beet plants per plot. The decision to use an insecticide treatment was determined based on the following criteria:
-
signaling criterion—S—catches of the first adults (moths),
-
intervention criterion—P based on feeding symptoms,
-
prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence)—PD,
-
phenological criterion—F based on the calculated sums of effective temperatures,
-
control object (without treatment)—K,
-
control object—K1, plants were taken for analysis.
All chemical treatments were carried out with the use of a plot sprayer with the recommended amount of water of about 400–450 L per hectare. The spraying fluid had a pressure of 0.3 MPa. In year 2011–2014 was used Dursban 480 EC insecticide [a.i. chloropiryfos 480 g (44.86%)] at a dose of 1 L per hectare. The next years (2015–2018) was used Pyrinex 480 EC [a.i. chloropiryfos 480 g (44.4%)] at dose of 0.9 l ha−1. The sugar beet root yield and parameters of their technological quality, i.e. the biological content of sugar (polarization) and molasses-forming components (α-amino nitrogen, potassium, and sodium ions) were assessed in the beet technological maturity phase (BBCH 49). The roots were harvested by hand from each plot, from the four middle rows, 6 m long, which was an area of 10.8 m2. The quality of the collected roots was determined on the basis of a sample of 20 roots taken from each plot from the two middle rows (10 consecutive roots). The weight of harvested roots, the sugar content—polarization, nitrogen, sodium, and potassium (K, Na and N-amines) were assessed on the automatic Venema Type II G line at the Środa Wlkp Sugar Plant. The technological sugar yield was calculated according to the formula of Bucholtz et al. (1995) (Górski et al. 2017).
-
(a)
sugar processing losses:
$$ {\text{CT}}\, = \,0.{\text{12}}\, \times \,\left( {{\text{K}}\, + \,{\text{Na}}} \right)\, + \,0.{\text{24}}\, \times \,\left( {{\text{N-}}\alpha {\text{-amines}}} \right)\, + \,{\text{1}}.0{\text{8 }}[\% ] $$ -
(b)
technological sugar expenditure: Pol − CT [%]
-
(c)
technological sugar yield (white):
$$ {\text{Y}}_{{{\text{PCT}}}} \, = \,{\text{Y}}_{{{\text{PK}}}} \, \times \,\left( {{\text{Pol}} - {\text{CT}}} \right)\, \times \,{\text{1}}00^{{{-}{\text{1}}}} [{\text{t}}\,{\text{ ha}}^{{ - {\text{1}}}} ] $$
where CT is the sugar processing losses, Pol is the sugar content in roots—polarization [% fresh weight], K, Na, N-α-amines are the potassium, sodium, and nitrogen content α-amino [mmol (100 g fresh roots)−1], YPK is the root yield [t ha−1], YPCT is thetechnological sugar yield [t ha−1].
The experiment was based on a randomized block system. One-factor field experiments were carried out in a design in four replications of the objects. The analyses were performed for a completely randomized system.
Obtained data were analysed using additive main effects and multiplicative interaction (AMMI) model (Gauch and Zobel 1990) for each trait, independently. The AMMI model first fits additive effects for the main effects of treatments (T) and years (Y) followed by multiplicative effects for treatment by year interaction (TYI) by principal component analysis (PCA). Results of AMMI analysis was presented by biplot graphs. The AMMI model (Nowosad et al. 2016) is given by:
where yge is the trait mean of treatment g in year e, μ is the grand mean, αg is the treatment mean deviations, βe is the year mean deviations, N is the number of PCA axis retained in the adjusted model, λn is the eigenvalue of the PCA axis n, γgn is the treatment score for PCA axis n, δen is the score eigenvector for PCA axis n, Qge is the residual, including AMMI noise and pooled experimental error. The AMMI stability value (ASV) was used to compare the stability of treatment as described by Purchase et al. (2000):
where SSIPCA1 is the sum of squares for first component from interactive principal component analysis (IPCA1), SSIPCA2—the sum of squares for second component from interactive principal component analysis (IPCA2), the IPCA1 and IPCA2 scores are the treatment scores in the AMMI model, IPCA means interactive principal component analysis. Lower ASV score indicate a more stable treatment across years (Nowosad et al. 2017). The rank of the ASV (rASV) for the particular treatment was calculated for each trait. The total rank of the ASV (TrASV) was calculated for each treatment as the sum of the rASVs for all six traits (Bocianowski et al. 2021a; Bocianowski and Prażak 2022). The total of ASV (TASV) for the particular treatment was calculated for each treatment as the sum of the ASVs for all six traits. Finally, the rank of the TASV (rTASV) was calculated for each treatment. All the analyses were conducted using the GenStat v. 18 statistical software package.
Results
AMMI model represents observations into a systematic component that consists of main effect and interaction effect through multiplication of interactions components, apart from random errors component (Sa’diyah and Hadi 2016). The year was statistically significant for all six observed traits of sugar beet (Table 2). The protective treatment was significant important for root weight and yield, while TYI was significant for root weight, sodium molasses and yield (Table 2).
Root weight—tests
The sum of squares for year main effect represented 53.48% of the total root weight variation. The differences between the protective treatments explained 2.25% of the total root weight variation, while the effects of TYI explained 10.78% (Table 2). Values for the two principal components were also significant and accounted jointly for 71.11% of the whole effect it had on the variation of root weight; the IPCA1 and IPCA2 accounted for 45.39% and 25.72%, respectively, of the variation caused by interaction. Root weight of the tested protective treatments varied from 9.2 (for PD variant in 2015) to 21.32 Mg ha−1 (for PD in 2016) throughout the 8 years, with an average of 16.42 Mg ha−1 (Table 3). The protective treatment PD variant had the highest average root weight (17.22 Mg ha−1), and the F (phenological treatment) had the lowest (15.32 Mg ha−1). The average root weight per years also varied from 10.26 in 2015, to 19.25 Mg ha−1 in 2014 (Table 3). The stability of tested protective treatments can be evaluated according to biplot for root weight (Fig. 1). Sugar beet protective treatments interacted differently with climate conditions in the observed years. The P variant interacted positively with the 2011, 2013, 2014, 2015 and 2018, but negatively with the 2016 (Fig. 1). The analysis showed that some protective treatments have high adaptation; however, most of them have specific adaptability. AMMI stability values (ASV) revealed variations in root weight stability among the six protective treatments (Table 3). According to Purchase et al. (2000), a stable protective treatment is defined as one with ASV value close to zero. Consequently, the protective treatment K1- control variant, with ASV of 0.069 was the most stable, while the treatment PD (3.428) was the least stable (Table 3).
Biplot for protective treatments by year interaction of root weight in the protective treatments in 8 years, showing the effects of primary and secondary interactive principal components (IPCA1 and IPCA2, respectively). F—phenological criterion—based on the calculated sums of effective temperatures, K—control object—without treatment, K1—control object—plants were taken for analysis, P—intervention criterion—P based on foraging symptoms, PD—prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence), S—signaling criterion—catches of the first adults (moths)
Sugar content—tests
The sum of squares for year main effect represented 93.39% of the polarization (Table 2). Values for the two principal components were significant and accounted jointly for 89.17% of the whole effect it had on the variation of polarization. The IPCA1 accounted for 59.24% of the variation caused by interaction, while IPCA2 accounted for 29.94%. Polarization of the tested protective treatments varied from 16.11 (for K1 in 2014) to 20.16% (for PD in 2015) throughout the 8 years, with an average of 17.87% (Table 4). The F had the highest average polarization (17.99%), and the K the lowest (17.78%). The average polarization per years also varied from 16.22% in 2014, to 19.68% in 2015. The protective treatment S interacted positively with the 2012, but negatively with the 2013 (Fig. 2). The protective treatment F interacted positively with the 2018; PD positively with 2015, while K with 2014. The treatment S with ASV of 0.234 was the most stable, while the protective treatment PD (1.129) was the least stable (Table 4).
Biplot for protective treatments by year interaction of polarization in the protective treatments in 8 years, showing the effects of primary and secondary interactive principal components (IPCA1 and IPCA2, respectively). F—phenological criterion—based on the calculated sums of effective temperatures, K—control object—without treatment, K1—control object—plants were taken for analysis, P—intervention criterion—P based on foraging symptoms, PD—prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence), S—signaling criterion—catches of the first adults (moths)
Potassium molasses—tests
The sum of squares for year main effect represented 89.48% of the total potassium molasses variation (Table 2). Value for the first principal component was significant and accounted for 61.75% of the whole effect it had on the variation of potassium molasses. Potassium molasses from the base of the tested protective treatments varied from 27.27 (for F in 2016) to 61.43 mmol kg−1 (for S in 2015) throughout the 8 years, with an average of 42.88 mmol kg−1 (Table 5). The protective treatments S had the highest average potassium molasses (43.88 mmol kg−1), and the F had the lowest (42.17 mmol kg−1). The average potassium molasses per years also varied from 27.93 mmol kg−1 in 2016, to 55.95 mmol kg−1 in 2015. The stability of tested protective treatments can be evaluated according to biplot for potassium molasses (Fig. 3). The protective treatments F and K1 interacted positively with the 2017, but negatively with the 2018 (Fig. 3). The S interacted positively with the 2015, but negatively with the 2013 and 2014. The P interacted positively with the 2018, but negatively with the 2017 (Fig. 3). The protective treatment P and K1 with ASV of 1.016 and 1.247, respectively, were the most stable, while the S (8.155) was the least stable (Table 5).
Biplot for protective treatments by year interaction of potassium molasses in the protective treatments in 8 years, showing the effects of primary and secondary interactive principal components (IPCA1 and IPCA2, respectively). F—phenological criterion—based on the calculated sums of effective temperatures, K—control object—without treatment, K1—control object—plants were taken for analysis, P—intervention criterion—P based on foraging symptoms, PD—prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence), S—signaling criterion—catches of the first adults (moths)
Sodium molasses—tests
The sum of squares for year main effect represented 92.71% of the total sodium molasses variation, while the effects of TYI explained 1.42% (Table 2). Values for the two first principal components were significant and accounted jointly for 83.50% of the whole effect it had on the variation of sodium molasses (IPCA1: 52.43% and IPCA2: 31.07%). Sodium molasses of the tested protective treatments varied from 1.125 (for K1 in 2015) to 7.000 mmol kg−1 (for F and K in 2018) throughout the 8 years, with an average of 3.019 mmol kg−1 (Table 6). The protective treatment F had the highest average sodium molasses (3.153 mmol kg−1), and the K1 had the lowest (2.888 mmol kg−1). The average sodium molasses per years also varied from 1.196 mmol kg−1 in 2015, to 6.692 mmol kg−1 in 2018. The stability of tested protective treatments can be evaluated according to biplot for sodium molasses (Fig. 4). The protective treatments S and K1 interacted positively with the 2013, 2015, 2016 and 2017, but negatively with the 2018 (Fig. 4). The protective treatments F and K interacted positively with the 2011. The P interacted positively with the 2012. The protective treatments PD and K1 with ASV of 0.318 and 0.448, respectively, were the most stable, while the protective treatment P (1.455) was the least stable (Table 6).
Biplot for protective treatments by year interaction of sodium molasses in the protective treatments in 8 years, showing the effects of primary and secondary interactive principal components (IPCA1 and IPCA2, respectively). F—phenological criterion—based on the calculated sums of effective temperatures, K—control object—without treatment, K1—control object—plants were taken for analysis, P—intervention criterion—P based on foraging symptoms, PD—prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence), S—signaling criterion—catches of the first adults (moths)
α-Amin-nitrogen—tests
In the ANOVA, the sum of squares for years main effect represented 87.92% of the total α-amin-nitrogen, and only this factor was significant on the α-amin-nitrogen. α-amin-nitrogen of the tested protective treatments varied from 6.03 (for F in 2011) to 37.95 mmol kg−1 (for P in 2018) throughout the 8 years, with an average of 16.67 mmol kg−1 (Table 7). The S had the highest average α-amin-nitrogen (17.16 mmol kg−1), and the F had the lowest (15.99 mmol kg−1). The average α-amin-nitrogen per years also varied from 6.83 mmol kg−1 in 2011 to 36.58 mmol kg−1 in 2018. The stability of tested lines can be evaluated according to biplot for α-amin-nitrogen (Fig. 5). The protective treatment K interacted positively with the 2011 and 2017, but negatively with the 2018 (Fig. 5). The PD interacted positively with the 2016, but negatively with the 2015. The protective treatment P with ASV of 1.307 was the most stable, while the protective treatment PD (2.614) was the smallest stable (Table 7).
Biplot for protective treatments by year interaction of N-α-amin-amine-α-nitrogen in the protective treatments in 8 years, showing the effects of primary and secondary interactive principal components (IPCA1 and IPCA2, respectively). F—phenological criterion—based on the calculated sums of effective temperatures, K—control object—without treatment, K1—control object—plants were taken for analysis, P—intervention criterion—P based on foraging symptoms, PD—prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence), S—signaling criterion—catches of the first adults (moths)
Technological yield—tests
The three sources of variation for the yield were significant. The sum of squares for year effect represented 49.68% of the total yield variation, the differences between protective treatments explained 2.22% of the total yield variation, while the effects of TYI explained 11.41% (Table 2). Values for the two principal components were also significant and accounted jointly for 70.52% of the whole effect it had on the variation of yield. The first principal component (IPCA1) accounted for 41.24% of the variation caused by interaction, while IPCA2 accounted for 29.28%. Yield of the tested protective treatments varied from 171.4 (for F in 2015) to 360.0 t ha−1 (for PD in 2012) throughout the 8 years, with an average of 280.47 t ha−1 (Table 8). The PD had the highest average yield (294.7 t ha−1), and the F had the lowest (264.0 t ha−1). The average yield per years also varied from 187.7 t ha−1 in 2015, to 341.6 t ha−1 in 2013. The stability of tested protective treatments can be evaluated according to biplot for yield (Fig. 6). The protective treatments P interacted positively with the 2011, 2013, 2014, 2015 and 2018 (Fig. 6). The protective treatment K1 with ASV of 0.604 was the most stable, while the PD (11.618) was the least stable (Table 8).
Biplot for protective treatments by year interaction of yield in the protective treatments in 8 years, showing the effects of primary and secondary interactive principal components (IPCA1 and IPCA2, respectively). F—phenological criterion—based on the calculated sums of effective temperatures, K—control object—without treatment, K1—control object—plants were taken for analysis, P—intervention criterion—P based on foraging symptoms, PD—prophylactic criterion treatment in the form of soil spraying of plants (after sugar beet emergence), S—signaling criterion—catches of the first adults (moths)
The rank of the ASV, the total rank of the ASV, the total of ASV, the rank of the TASV
The best ASV (rASV equal to 1) was observed (two times) for K1 (root weight and yield) and P (potassium molasses and N-α-amin-amine-α-nitrogen) (Table 9). The best total rank of the ASV was observe for K1 treatment (TrASV = 14), and the worst for F and PD treatments (TrASV = 433). The total AMMI stability value varied from 5.177 (for K1) to 23.155 (for PD). The total rank of the ASV (TrASV) and the rank of the TASV (rTASV) were very similar (Table 9). K1 and P treatments were the best stability if both ranks, while F and PD were the worst in both ranks (Table 9).
Discussion
The use of AMMI for the analysis of our findings provided more information on the interaction (TYI) between the applied variants of protective treatments against soil pests in individual years of study, and the obtained components of the white sugar yield over 8 years of the trial. Many authors have demonstrated that the AMMI analysis can be used to identify specific treatments with the highest yields in different years (Hoffmann and Kluge-Severin 2011; Bocianowski et al. 2018).
The relationship between the effect of quantitative characteristics on the technological yield of sugar has been studied by many authors (Märländer et al. 2003; Kenter et al. 2006; Bzowska-Bakalarz and Banach 2009; Rezbova et al. 2013; Moliszewska 2015; Jakubowska et al. 2020a). Kamali and Zand-Parsa (2017) confirmed that in the absence or elimination of pests and diseases, the reduction of the genetic potential of sugar beet depends on regional weather conditions. The technological yield of sugar increases with the length of the growing season, i.e. the number of days between sowing and harvesting.
Effect of environmental conditions on biological and commercial traits of sugar beet is very significant. The results of field trials demonstrated the impact of weather conditions (especially influence of temperature and precipitation in development of plants), environment and varieties on the yield of sugar beet. The obtained results show that the influence of varieties, years, and related weather conditions during the growing season of sugar beet have a huge impact on the root yield, sugar content and the formation of assimilates in the plant growth and development process. Malnou et al. (2006) concluded that a greater number of organic compounds produced at the beginning of vegetation is used for leaf growth, which in turn reduces the initial share of roots in the total plant mass. Similarly, Milford et al. (1985), who in their research proved that there is a high variability between genotypes regarding the number, shape and surface of leaves, a similar root yield can be obtained for different morphological features of the leaves. Lemaire et al. (2008) believe that there is a close relationship between the rate of growth and the production of larger leaf rosettes and air temperature. Until the inter-rows are covered, a close relationship is observed between the structure of the leaf and the amount of absorbed solar radiation, on the one hand, and the amount of absorbed radiation, and the yield of root mass, on the other hand, with favorable temperature and humidity conditions (Draycott 2006; Hoffmann and Kluge-Severin 2010, 2011). For this reason, each agrotechnical factor that limits the growth of leaf mass has a negative impact on the final root yield and technological sugar yield. Probably, the combination of various environmental factors in interaction with the genotype influences the distribution of assimilates throughout the plant (Kamali and Zand-Parsa 2017). Another factor that influences the assimilate content, and thus the sugar yield and the root yield, is the availability of nitrogen and water in the soil. Continuous intake of this component has a positive effect on the formation and growth of the leaf mass (Barłóg and Grzebisz 2004; Malnou et al. 2006). Wojciechowski et al. (2002) showed that in many locations and in the years of research, nitrogen uptake was inhibited in mid-August, when the leaf mass reached its maximum and high temperatures at that time caused the root to accumulate assimilates, with the highest sugar content around root head. When water availability is limited, leaf growth is inhibited and assimilates move to the storage root, with molasses accumulating at this time. The course of the weather has a decisive influence on the quality traits of sugar beet roots, although there are also differences between cultivars (Barłóg and Grzebisz 2004; Kenter et al. 2006). Barłóg and Grzebisz (2004) as well as Kenter et al. (2006) also emphasize the complexity of the relationship between the quantitative characteristics and the quality of beetroot roots, mainly the polarization and the content of molasses forming compounds. All the relationships, as for the root mass, were directly proportional (Table 2). In 2011–2014 in Słupia Wielka, the potassium content had an inverse proportionate effect on the technological yield of beet sugar (Table 5). The research by Bzowska-Bakalarz and Banach (2009) conducted in various habitat conditions shows that the concentration of potassium in the roots depends on the abundance of this component in the soil. The technological yield of beet sugar was significantly related to the sodium content only in 2015 in Słupia Wielka (Table 6). The content of α-amino nitrogen determined the beet yield in Słupia Wielka in all years except 2011 and 2017—all these relationships were inversely proportional (Table 2). A negative correlation between the sugar content and the α-amino nitrogen was demonstrated in their studies by Barłóg and Grzebisz (2004). Among the analyzed quality features of sugar beet roots, polarization and the content of α-amino nitrogen and sodium were not significantly differentiated by the effects of the experiment factors in Słupia Wielka. The research of Moradi et al. (2012) show that environmental conditions such as the location and course of weather usually affect the yielding of individual sugar beet genotypes to a varying degree, which should be taken into account when breeding new varieties. TYI is an important and challenging issue for plant breeders especially in developing new improved treatments. Multi-year trials are used to determine treatment-year interaction representing the target year and can identify superior treatments for recommendation to breeders (Biancardi et al. 2010; Chołuj et al. 2014; Podlaski et al. 2017). Differences in environmental/year conditions may cause large TYIs, especially under drought-prone years (Bocianowski et al. 2019a).
The AMMI model provides a useful tool in diagnosing TYI patterns and improving the accuracy of response estimates. It enables clustering of genotypes based on similarity of response characteristics and identifying potential trends in environments (Bocianowski et al. 2018, 2019b, 2019c; Fotso et al. 2018; Bocianowski and Liersch 2022). The suggested strategy could extract more information from the TYI, thereby aiding researchers in identifying specific treatments with competitive yields across diverse years (Paderewski et al. 2016; Podlaski et al. 2017; Bocianowski et al. 2021b).
Conclusions
-
1.
Regarding the AMMI model, the results of the analysis of variance indicated significant treatment × year interaction for all considering physiological traits.2.
-
2.
The technological yield of beet sugar was determined directly proportionally by the mass of roots and polarization with the use of any decision-making method The technological yield of sugar increases with the length of the growing season, i.e. the number of days between sowing and harvesting.
-
3.
The sodium content did not have a statistically significant effect on the technological yield of sugar beet for any of the applied treatments for soil pests.
-
4.
Among SVD-based statistical analyses, AMMI is a unique analysis that completely and always separates assess protective treatments by year interaction as required for most agricultural research purposes.
Availability of data and material
The data presented in this study are available on request from the corresponding authors.
References
Barłóg P, Grzebisz W (2004) Plonotwórcza i diagnostyczna ocena nawożenia buraków cukrowych potasem z udziałem sodu i magnezu. Część II. Jakość korzeni i plon cukru. [Yield-generating and diagnostic assessment of potassium fertilization of sugar beet with sodium and magnesium. Part II. Root quality and sugar yield.]. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 234:83–92 ([in polish])
Biancardi E, McGrath JM, Panella LW, Lewellen RT, Steranato P (2010) Root and tuber crops. (Chapter 6 Sugar beet pp 173–219). https://doi.org/10.1007/978-0-387-92765-7_6
Bocianowski J, Liersch A (2022) Multidimensional analysis of diversity in genotypes of winter oilseed rape (Brassica napus L.). Agronomy 12(3):633. https://doi.org/10.3390/agronomy12030633
Bocianowski J, Prażak R (2022) Genotype by year interaction for selected quantitative traits in hybrid lines of Triticum aestivum L. with Aegilops kotschyi Boiss. and Ae variabilis Eig. using the additive main effects and multiplicative interaction model. Euphytica 218(2):11. https://doi.org/10.1007/s10681-022-02967-4
Bocianowski J, Szulc P, Nowosad K (2018) Soil tillage methods by years interaction for dry matter of plant yield of maize (Zea mays L.) using additive main effects and multiplicative interaction model. J Integr Agric 17:2836–2839. https://doi.org/10.1016/S2095-3119(18)62085-4
Bocianowski J, Księżak J, Nowosad K (2019a) Genotype by environment interaction for seeds yield in pea (Pisum sativum L.) using additive main effects and multiplicative interaction model. Euphytica 215:191. https://doi.org/10.1007/s10681-019-2515-1
Bocianowski J, Niemann J, Nowosad K (2019b) Genotype-by-environment interaction for seed quality traits in interspecific cross-derived Brassica lines using additive main effects and multiplicative interaction model. Euphytica 215:7. https://doi.org/10.1007/s10681-018-2328-7
Bocianowski J, Nowosad K, Szulc P (2019c) Soil tillage methods by years interaction for harvest index of maize (Zea mays L.) using additive main effects and multiplicative interaction model. Acta Agric Scand Sect B-S P 69:75–81. https://doi.org/10.1080/09064710.2018.1502343
Bocianowski J, Radkowski A, Nowosad K, Radkowska I, Zieliński A (2021a) The impact of genotype-by-environment interaction on the dry matter yield and chemical composition in timothy (Phleum pratense L.) examined by using the additive main effects and multiplicative interaction model. Grass Forage Sci 76(4):463–484. https://doi.org/10.1111/gfs.12551
Bocianowski J, Tratwal A, Nowosad K (2021b) Genotype by environment interaction for main winter triticale varieties characteristics at two levels of technology using additive main effects and multiplicative interaction model. Euphytica 217:26. https://doi.org/10.1007/s10681-020-02756-x
Bucholtz K, Märländer B, Puke H, Glattkowski H, Thielecke H (1995) Neubewertung des technischen Wertes von Zuckerrüben. Zuckerindustre 120(2):113–121
Bzowska-Bakalarz M, Banach M (2009) Właściwości technologiczne surowca buraczanego produkowanego w zmodyfikowanej technologii nawożenia. Acta Agrophys 14(1):31–40
Campbell LG (2002) Sugar beet quality improvement. In: Basra AS, Randhawa LS (eds) Quality improvement in field crops. Food Products Press, Binghamton, pp 395–413
Chołuj D, Wiśniewska A, Szafrański KM, Cebula J, Gozdowski D, Podlaski S (2014) Assessment of the physiological responses to drought in different sugar beet genotypes in connection with their gene-tic distance. J Plant Physiol 171:1221–1230. https://doi.org/10.1016/j.jplph.2014.04.016
Draycott AP (2006) Introduction. In: Draycott AP (ed) Sugar beet. Blackwell Publishing Ltd, Oxford, pp 1–8
Fotso AK, Hanna R, Kulakow P, Parkes E, Iluebbey P, Ngome FA, Suh C, Massussi J, Choutnji I, Wirnkar VL (2018) AMMI analysis of cassava response to contrasting environments: case study of genotype by environment effect on pests and diseases, root yield, and carotenoids content in Cameroon. Euphytica 214:155. https://doi.org/10.1007/s10681-018-2234-z
Gauch HG (2013) A simple protocol for AMMI analysis of yield trials. Crop Sci 53:1860–1869. https://doi.org/10.2135/cropsci2013.04.0241
Gauch HG, Zobel RW (1990) Imputing missing yield trial data. Theoretical Applied Genetic 79:753–761. https://doi.org/10.1007/BF00224240
Gauch HG (1992) Statistical analysis of regional yield trials: AMMI analysis of factorial designs 1st edition. Elsevier Science. ISBN: 9780444892409
Górski D, Gaj R, Ulatowska A, Piszczek J (2017) Wpływ dolistnego nawożenia krzemem i wapniem na plon i jakość technologiczną buraka cukrowego. Fragmenta Agronomica 34(4):46–58 ([in polish])
Hassani M, Heidari B, Dadkhodaie A, Stevanato P (2018) Genotype by environment interaction components underlying variations in root, sugar, and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica 214:79. https://doi.org/10.1007/s10681-018-2160-0
Hoffmann CM, Kenter C (2018) Yield potential of sugar beet—have we hit the ceiling? Front Plant Sci 9:289. https://doi.org/10.3389/fpls.2018.00289
Hoffmann CM, Kluge-Severin S (2010) Light absorption and radiation use efficiency of autumn and spring sown sugar beet. Field Crop Res 119:238–244. https://doi.org/10.1016/j.fcr.2010.07.014
Hoffmann CM, Kluge-Severin S (2011) Growth analysis of autumn and spring sown sugar beet. Eur J Agron 34:1–9. https://doi.org/10.1016/j.eja.2010.09.001
Jakubowska M, Cyplik A, Bocianowski J, Wielkopolan B (2020a) Wpływ wybranych cech chemicznych na wartość technologiczną plonu buraka cukrowego po zastosowaniu zabiegów na szkodniki glebowe [Effect of selected chemical features on the technological value of sugar beet yield after application of treatments on soil pests]. Prog Plant Prot 60(4):275–282. https://doi.org/10.14199/ppp-2020-029 ([in polish])
Jakubowska M, Bocianowski J, Nowosad K, Kowalska J (2020b) Decision support system to improve the effectiveness of chemical control against cutworms in sugar beet. Sugar Tech 22:911–922. https://doi.org/10.1007/s12355-020-00808-z
Jaskulska I, Jaskulski D, Gałęzewski L, Kotwica K, Doroszewski A, Jóźwicki T (2017) Plony i jakość technologiczna korzeni odmian buraka cukrowego oraz ich zmienność w województwach wielkopolskim i kujawsko-pomorskim. Fragmenta Agron 34(2):18–27 ([in polish])
Kamali H, Zanda-Parsa S (2017) Estimation of sugar beet yield and its dry matter partitioning under different irrigation and nitrogen levels. Mod Appl Sci 11(1):141–156. https://doi.org/10.5539/mas.v11n1p141
Kenter C, Hoffmann C, Märländer B (2006) Effects of weather variables on sugar beet yield development (Beta vulgaris L.). Eur J Agron 24:62–69. https://doi.org/10.1016/j.eja.2005.05.001
Klotz KL, Finger FL (2004) Impact of temperature, length of storage and postharvest disease on sucrose catabolism in sugar beet. Postharvest Biol Technol 34(1):1–9. https://doi.org/10.1016/j.postharvbio.2004.05.016
Lemaire S, Maupas F, Cournede PH, Reffye P (2008) A morphogenetic crop model for sugar beet (Beta vulgaris L.). In: International symposium on crop modeling and decision support. ISCMDS, Nanjing, China, 19–22
Malnou CS, Jaggard KW, Sparkes DL (2006) A canopy approach to nitrogen fertilizer recommendations for the sugar beet crop. Eur J Agron 25:254–263. https://doi.org/10.1016/j.eja.2006.06.002
Märländer B, Hoffmann CM, Koch HJ, Ladening E, Merkes R, Petersen J, Stockfish N (2003) Environmental situation and yield performance of the sugar beet crop in Germany: heading for sustainabke development. J Agron Crop Sci 189(4):201–226. https://doi.org/10.1046/j.1439-037X.200300035.x
Michalska-Klimczak B, Wyszyński Z (2010) Plonowanie buraka cukrowego w zmiennych warunkach agrotechnicznych i siedliskowych. Cz. I. Plon i jakość korzeni a technologiczny plon cukru. Fragmenta Agron 27(1):88–97 ([in polish])
Milford GFJ, Pocock TO, Riley J (1985) An analysis of leaf growth in sugar beet. I. Leaf appearance and expansion in relation to temperature under controlled conditions. Annu Appl Biol 106:163–172. https://doi.org/10.1111/j.1744-7348.1985.tb03106.x
Moliszewska E (2015) Cechy morfologiczne buraka cukrowego a jakość plonu. [Morphological features of sugar beet and yield quality]. Adv Agric Sci Probl 582:43–51
Moradi F, Safari H, Jalilian A (2012) Study of genotype x environment interaction for sugar beet monogerm cultivars using AMMI method. J Sugar Beet 28(1):29–35
Nowakowski M, Krüger KW (1997) Wpływ dawek azotu stosowanych w trzech terminach na wielkość i jakość plonu buraka cukrowego. Cz.I. Zawartość azotu mineralnego w profile glebowym w okresie wiosennym. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 202:105–115 ([in polish])
Nowosad K, Liersch A, Popławska W, Bocianowski J (2016) Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica 208:187–194. https://doi.org/10.1007/s10681-015-1620-z
Nowosad K, Liersch A, Poplawska W, Bocianowski J (2017) Genotype by environment interaction for oil content in winter oilseed rape (Brassica napus L.) using additive main effects and multiplicative interaction model. Indian J Genet Plant Breed 77:293–297. https://doi.org/10.5958/0975-6906.2017.00039.6
Paderewski J, Gauch HG, Mądry W, Gacek ES (2016) AMMI analysis of Four-Way Genotype × Location × Management × Year Data from a wheat trial in Poland. Crop Sci 56(5):2157–2164. https://doi.org/10.2135/cropsci2015.03.015
Pimentel D (2005) Environmental and economic costs of the application of pesticides primarily in the United States. Environ Dev Sustain 7:229–252. https://doi.org/10.1007/s10668-005-7314-2
Podlaski S, Chołuj D, Wiśniewska A (2017) Kształtowanie się plonu buraka cukrowego w zależności od wybranych czynników środowiskowych. Zeszyt Problemowy Postępów Nauk Rolniczych 590:59–71. https://doi.org/10.22630/ZPPNR.2017.590.34 ([in polish])
Purchase JL, Hatting H, van Deventer CS (2000) Genotype × environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. S Afr J Plant Soil 17:101–107. https://doi.org/10.1080/02571862.2000.10634878
Rezbova H, Belova A, Skubna O (2013) Sugar beet production in the European Union and their future trends. Agris on-line Papers in Economics and Informatics ISSN 1804-1930, 5(4):1651–1678
Rodrigues PC, Malosetti M, Gauch HG, van Eeuwijk FA (2014) A weighted AMMI algorithm to study genotype-by-environment interaction and QTLby-environment interaction. Crop Sci 54(4):1555–1570. https://doi.org/10.2135/cropsci2013.07.0462
Sa’diyah H, Hadi AF (2016) AMMI model for yield estimation in multi-environment trials: a comparison to BLUP. Agric Agric Sci Procedia 9:163–169. https://doi.org/10.1016/j.aaspro.2016.02.113
Wojciechowski A, Szczepaniak W, Grzebisz W (2002) Effect of potassium fertilization on yields and technological quality of sugar beet Part III. Potassium uptake. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 222:71–77 ([in polish])
Zobel RW, Wright MJ, Gauch HG (1988) Statistical analysis of yield trial. Agron J 80:388–393. https://doi.org/10.2134/agronj1988.00021962008000030002x
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bocianowski, J., Jakubowska, M. & Kowalska, J. The interaction of different abiotic conditions on the value of the component traits of the technological yield of sugar beet. Euphytica 218, 110 (2022). https://doi.org/10.1007/s10681-022-03070-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-022-03070-4