Abstract
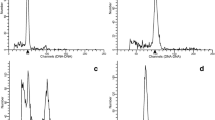
This paper elucidates a procedure for isolating homogeneous triploid and tetraploid progeny from mixoploids, which are the most desirable genetic resources to develop genetically stable seedless variety in pointed gourd (Trichosanthes dioica Roxb.) as seeds are unpalatable. All the colchicine concentrations (0.05, 0.1, 0.5%) effectively led to the production of mixoploid for 48 and 72 h exposure time whereas 24 h did not response to induce mixoploid. These mixoploids (female and male) exhibit cross compatability with diploid (female and male) parents for F1 seed generation. Interestingly, mixoploid parents (either female or male) produced a mixture of normal diploid size seeds and some abnormally large ones, almost twice normal size. Density plot and principal component analysis (PCA) of 603 seeds resulted in separation of diploid, mixoploid and tetraploid accessions involved in different ploidy crosses. To develop a method for the isolation of sexually derived triploid and tetraploid progeny from the induced mixoploids, we examined the ploidy level of F1 populations by flow cytometry where 18.7% F1 seedlings were confirmed as triploid and tetraploid progeny when female mixoploid crossed with male mixoploid while 16.7% triploids were isolated crossed with male diploid. These findings suggest that mixoploid female parents were the best options for developing triploid and tetraploid progeny. Overall, the results of this study provide a framework to explore the genetic basis of polyploids isolated from colchicine induced mixoploids in in vivo conditions.







Similar content being viewed by others
References
Adhikari S, Biswas A, Bandyopadhyay TK, Ghosh PD (2014) A preliminary report on the genetic variation in pointed gourd (Trichosanthes dioica Roxb.) as assessed by random amplified polymorphic DNA. Acta Biol Hung 65(2):156–164
Aleza P, Juarez J, Cuenca J, Ollitrault P, Navarro L (2012) Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent-effect on the length of the juvenile phase. Plant Cell Rep 31:1723–1735. https://doi.org/10.1007/s00299-012-1286-0
Beatson RA, Ferguson AR, Weir IE, Graham LT, Ansell KA, Ding H (2003) Flow cytometric identification of sexually derived polyploids in hop (Humulus lupulus L.) and their use in hop breeding. Euphytica 134(2):189–194. https://doi.org/10.1023/B:EUPH.0000003882.23615.c5
Birchler JA, Veitia RA (2012) Gene balance hypothesis: connecting issues of dosage sensitivity across biological disciplines. Proc Natl Acad Sci USA 109(37):14746–14753. https://doi.org/10.1073/pnas.1207726109
Birchler JA, Riddle NC, Auger DL, Veitia RA (2005) Dosage balance in gene regulation: biological implications. Trends Genet 21(4):219–226. https://doi.org/10.1016/j.tig.2005.02.010
Blasco M, Naval MM, Zuriaga E, Badenes ML (2014) Genetic variation and diversity among loquat accessions. Tree Genet Genomes. https://doi.org/10.1007/s11295-014-0768-3
Burge GK, Morgan ER, John FS (2002) Opportunities for synthetic plant chimera breeding: past and future. Plant Cell Tissue Organ Cult 70:13–21
Cai X, Kang XY (2011) In vitro tetraploid induction from leaf explants of Populus pseudo-simonii Kitag. Plant Cell Rep 30:1771–1778. https://doi.org/10.1007/s00299-011-1085-z
Chen LL, Gao SL (2007) In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci Hortic 112(3):339–344
Dhooghe E, Laere KV, Eeckhaut T, Leus L, Huylenbroeck JV (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Organ Cult 104(3):359–373. https://doi.org/10.1007/s11240-010-9786-5
Dzialuk A, Chybicki I, Welc M, Sliwinska E, Burczyk J (2007) Presence of triploids among oak species. Ann Bot 99:959–964
Frost HB, Soost RK (1968) Seed reproduction: development of gametes and embryos. In: Reuther W, Batchelor LD, Webber HB (eds) The citrus industry, vol 2. University of California, Barkley, pp 290–324
Fujishige I, Tanaka R, Taniguchi K (1996) Efficient isolation of nonchimeric tetraploids artificially induced in a stable culture of Haplopappus gracilis. Theor Appl Genet 92(2):157–162
Guo QG, Li XL, Wang WX, He Q, Liang GL (2007) Occurrence of natural triploids in loquat. In: Proceedings of the second international symposium on Loquat, Guangzhou, China, pp 128–128
Han Z, Geng X, Du K, Xu C, Yao P, Bai F, Kang X (2018) Analysis of genetic composition and transmitted parental heterozygosity of natural 2n gametes in Populus tomentosa based on SSR markers. Planta 247(6):1407–1421. https://doi.org/10.1007/s00425-018-2871-4
Harbard JL, Griffin AR, Foster S, Brooker C, Kha LD, Koutoulis A (2012) Production of colchicine induced autotetraploids as a basis for sterility breeding in A. mangium Willd. Forestry 85:427–436. https://doi.org/10.1093/forestry/cps041
Hassan J, Miyajima I, Ozaki Y, Mizunoe Y, Sakai K, Zaland W (2020) Tetraploid induction by colchicine treatment and crossing with a diploid reveals less-seeded fruit production in pointed gourd (Trichosanthes dioica roxb.). Plants. https://doi.org/10.3390/plants9030370
Hassan J, Miyajima I (2019a) Flowering habit and fruit setting of pointed gourd (Trichosanthes dioica Roxb.) influenced by seasonal temperatures. J Fac Agric Kyushu Univ 64(2), 177–182. http://hdl.handle.net/2324/2339051.
Hassan J, Miyajima I (2019b) Induction of parthenocarpy in pointed gourd (Trichosanthes dioica Roxb.) by application of plant growth regulators. J Horticult Plant Res 8:12–21
Hassan J, Miyajima I (2019c) Morphological and ecological characteristics of pointed gourd (Trichosanthes dioica Roxb.). http://hdl.handle.net/2324/2339052.
Hassan J, Miyajima I (2020) Breeding techniques and management approaches for the improvement of pointed gourd (Trichosanthes dioica Roxb.). In: Pointed Gourd (Trichosanthes dioica): a promising dioecious cucurbit, pp 1–11. LAMBERT Academic Publishing, 17 Meldrum Street, Beau Bassin 71504, Mauritius
Hazra P (2001) Induced polyploidy as a breeding approach in pointed gourd. J Breed Genet 33:47–48
Johnsson H (1940) Cytological studies of diploid and triploid Populus tremula and of crosses between them. Hereditas 26(3–4):321–352
Kagan-Zur V, Yaron-Miron D, Mizrahi Y (1991) A study of triploid tomato fruit attributes. J Am Soc Hortic Sci 116(2):228–231
Kang XY (2002) Mechanism of 2n pollen occurring in Chinese white poplar. J Beijing Univ 24(5/6):67–70
Kang XY, Zhu ZT (1997) A study on the 2n pollen vitality and germinant characteristics of White populars. Acta Bot Yunnanica 19(4):402–406
Khan ASMMR, Rabbani MG, Islam MS, Rashid MH, Alam AKMM (2009) Genetic diversity in pointed gourd (Trichosanthes dioica Roxb) revealed by random amplified polymorphic DNA (RAPD) markers. Thai J Agric Sci 42(2):61–69
Kihara H (1951) Tetraploid watermelons. Proc Am Soc Hort Sci 58:217–230
Koutoulis A, Roy AT, Price A, Sherriff L, Leggett G (2005) DNA ploidy level of colchicine-treated hops (Humulus lupulus L.). Sci Hortic 105:263–268
Kumar S, Singh BD (2012) Pointed gourd: botany and horticulture. Hort Rev 39:203–238
Law CN, Snape JW, Worland AJ (1987) Aneuploidy in wheat and its uses in genetic analysis. In: Lupton FGH (ed) Wheat breeding: its scientific basis. Chapman and Hall, London, pp 71–108
Lê S, Josse J, Husson F (2008) FactoMineR: An R package for multivariate analysis. J Stat Softw 25:1–18
Liu P, Zhao ZH, Dai L, Liu XY, Peng JY, Peng SQ, Zhou ZJ (2009) Genetic variations of Ziziphus cultivar ‘Zanhuangdazao’ by using RAPD technique. Acta Hortic 840:149–154
Liu W, Zheng Y, Song S, Huo B, Li D, Wang J (2018) In vitro induction of allohexaploid and resulting phenotypic variation in Populus. Plant Cell Tiss Organ Cult 134:183–192
Liu W, Song S, Li D, Lu X, Liu J, Zhang J, Wang J (2020) Isolation of diploid and tetraploid cytotypes from mixoploids based on adventitious bud regeneration in Populus. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-019-01705-4
Maletskii SI, Maletskaya EI (1996) Self-fertility and agamospermy in sugarbeet, Beta vulgaris L. Russian J Genet 32:1643–1650
Ollitrault P, Dambier D, F, Luro & Y. Froelicher. (2008) Ploidy manipulation for breeding seedless triploid citrus. Plant Breed Rev 20:323–354. https://doi.org/10.1002/9780470380130.ch7
Ozaki Y, Narikiyo K, Fujita C, Okubo H (2004) Ploidy variation of progenies from inter and intra-ploidy crosses with regard to trisomic production in Asparagus (Asparagus officinalis L.). Sex Plant Reprod 17:157–164. https://doi.org/10.1007/s00497-004-0229-5
Pandit MK, Hazra P (2008) Pointed gourd. In: Rana MK (ed) Scientific cultivation of vegetables. Kalyani Publ, New Delhi, pp 218–228
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tiss Org 64:185–210. https://doi.org/10.1023/A:1010623203554
Rai PK, Jaiswal D, Rai DK, Sharma B, Watal G (2008) Effect of water extract on Trichosanthes dioica fruits in streptozotocin induced diabetic rats. Indian J Clin Biochem 23:387–390
Ramanna M, Jacobsen E (2003) Relevance of sexual polyploidization for crop improvement: a review. Euphytica 133:3–18
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Ranjbar M, Karamian R, Nouri S (2011) Diploid-tetraploid mixoploidy in a new species of Astragalus (Fabaceae) from Iran. Ann Bot Fennici 48:343–351
Rao JY, Liu YF, Huang HW (2012) Analysis of ploidy segregation and genetic variation of progenies of different interploidy crosses in Actinidia chinensis. Acta Horticult Sin 39(8):1447–1456
Rose JB, Kubba J, Tobutt KR (2000) Induction of tetraploidy in Buddleia globosa. Plant Cell Tiss Organ Cult 63(2):121–125
Roy AT, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20(6):489–495. https://doi.org/10.1007/s002990100364
Rugini E, Pannelli G, Ceccarelli M, Muganu M (1996) Isolation of triploid and tetraploid olive (Olea europaea L.) plants from mixoploid cv. ‘Frantoio’ and ‘Leccino’ mutants by in vivo and in vitro selection. Plant Breed 115(1):23–27. https://doi.org/10.1111/j.1439-0523.1996.tb00865.x
Shi QH, Liu P, Liu MJ, Wang JR, Xu J (2015) A novel method for rapid in vivo induction of homogeneous polyploids via calluses in a woody fruit tree (Ziziphus jujuba Mill.). Plant Cell Tiss Organ Cult 121:423–433
Shomotsuma M, Matsumoto K (1957) Comparative studies on the morphology of polyploidy watermelon seeds. Seiken Ziho 8:67–74
Simmonds NW, Sheperd K (1955) The taxonomy and origins of the cultivated bananas. Bot J Linn Soc 55:302–312. https://doi.org/10.1111/j.1095-8339.1955.tb00015.x
Singh BP, Whitehead WF (1999) Pointed gourd: Potential for temperate climates. In: Janick J (ed) Perspectives on new crops and new uses. ASHS Press, Alexandria, pp 397–399
Sun QR, Sun SH, Li LG, Bell RL (2009) In vitro colchicine-induced polyploidy plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar ‘Fertility.’ J Hortic Sci Biotech 84:548–552
Tian J, Wang JH, Dong L, Dai F, Wang J (2015) Pollen variation as a response to hybridisation in Populus L. section Aigeiros Duby. Euphytica 206:433–443
van Breukelen EWM (1982) Competition between 2x and x pollen in styles of Solanum tuberosum determined by a quick in vivo method. Euphytica 31:585–590
Varela-Álvarez E, Loureiro J, Paulino C, Serrão EA (2018) Polyploid lineages in the genus Porphyra. Sci Rep 8:8696. https://doi.org/10.1038/s41598-018-26796-5
Verma P, Maurya SK, Panchbhaiya A, Dhyani S (2017) Studies on variability, heritability and genetic advance for yield and yield contributing characters in Pointed gourd (Trichosanthes dioica Roxb.). J Pharm Phytochem 6(3):734–738
Wang J, Kang X (2009) Distribution of microtubular cytoskeletons and organelle nucleoids during microsporogenesis in a 2n pollen producer of hybrid Populus. Silvae Genet 58(5/6):220–226
Wang J, Huo B, Liu W, Li D, Liao L (2017) Abnormal meiosis in an intersectional allotriploid of Populus L. and segregation of ploidy levels in 2x × 3x progeny. PLoS ONE 12(7):e0181767. https://doi.org/10.1371/journal.pone.0181767
Wu J, Ferguson AR, Murray BG, Duffy AM, Jia Y, Cheng C, Martin PJ (2013) Fruit quality in induced polyploids of Actinidia chinensis. HortScience 48(6):701–707
Xu CP, Zhang Y, Huang Z, Yao PQ, Li Y, Kang XY (2018) Impact of the leaf cut callus development stages of Populus on the tetraploid production rate by colchicine treatment. J Plant Growth Regul 37:635–655
Zhang Z, Kang X (2010) Cytological characteristics of numerically unreduced pollen production in Populus tomentosa Carr. Euphytica 173(2):151–159
Zhang Q, Luo F, Liu L, Guo F (2010) In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tiss Org 101:41–47. https://doi.org/10.1007/s11240-009-9660-5
Zhou Q, Wu J, Sang Y, Zhao Z, Zhang P, Liu M (2020) Effects of colchicine on Populus canescens ectexine structure and 2n pollen production. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00295
Zhu ZT, Lin HB, Kang XY (1995) Studies on allotriploid breeding of Populus tomentosa B301 clones. Sci Silvae Sin 31:499–505
Acknowledgements
Authors are highly grateful to Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh and Kyushu University, Fukuoka 819-0395, Japan for giving necessary administrative, technical and academic supports to collect the plant materials from Bangladesh to Japan through MTA (materials transfer agreement). Authors are also admiring the contributions of all the laboratory staff for their tremendous cooperation to complete this work.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
J. Hassan, conceived the idea of the study, designed experiment, performed the research and data analysis, and wrote the manuscript. I. Miyajima and Y. Ozaki supervised the work, provided suggestions and comments on the manuscript. Y. Mizunoe and K. Sakai assisted in sample preparation and laboratory analysis. All authors have read, edited the manuscript and approved for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hassan, J., Miyajima, I., Ozaki, Y. et al. Homogeneous triploid and tetraploid production through crossing with mixoploid parents in pointed gourd (Trichosanthes dioica Roxb.). Euphytica 218, 17 (2022). https://doi.org/10.1007/s10681-021-02961-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-021-02961-2