Abstract
Landfill sites are subjected to long-term risks of accidental spill of leachate through the soil and consequential contamination of the groundwater. Wide areas surrounding the landfill can seriously be threatened with possible consequences to human health and the environment. Given the potential impact of different coexisting anthropic pollution sources (i.e., agriculture and cattle farming) on the same site, the perturbation of the groundwater quality may be due to multiple factors. Therefore, it is a challenging issue to correctly establish the pollution source of an aquifer where the landfill is not isolated from other anthropic land uses, especially in the case of a karstic coastal aquifer. The present study is aimed at setting in place an integrated environmental monitoring system that included microbiological, chemical, and isotope methods to evaluate potential groundwater pollution in a landfill district in the south of Italy located in Murgia karstic aquifer. Conventional (microbial plate count and physical–chemical analyses) and advanced methods (PCR-ARISA, isotope analysis of δ18O, δ2H, 3H, δ 13C, δ 15N-NO3−, and δ 18O-NO3−) were included in the study. Through data integration, it was possible to reconstruct a scenario in which agriculture and other human activities along with seawater intrusion in the karst aquifer were the main drivers of groundwater pollution at the monitored site. The microbiological, chemical, and isotope results confirmed the absence of leachate effects on groundwater quality, showing the decisive role of fertilizers as potential nitrate sources. The next goal will be to extend long-term integrated monitoring to other landfill districts, with different geological and hydrogeological characteristics and including different sources of pollution, to support the ecological restoration of landfills.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among potential groundwater pollution sources, landfills still represent a major threat. The highly toxic landfill leachate could accidentally or chronically leak from the disposal site and reach the underlying soil and the saturated aquifer, representing a serious pollution source (Abd El-Salam and I. Abu-Zuid 2015). Despite UN Sustainable Development Goals clearly stating a call for environmentally sound disposal facilities, the management of the existing landfill sites still poses many challenges. Even though modern landfills for urban solid waste are designed to minimize environmental impacts (Feng et al., 2020), when they are improperly built (i.e., without engineered liners and/or leachate collection/purification systems) or poorly managed, the risks of underground leachate infiltration increase consequently (Feng et al., 2020; Kjeldsen et al., 2002; Negi et al., 2020).
Evidence of groundwater contamination by landfill leachate has been found in many regions of the world (Guo et al., 2022; Ringle Raja et al., 2023), and researchers have been working hard to develop methodology to monitoring soil pollution and groundwater contamination (Ameloko & Ayolabi, 2018; Maryadi et al., 2020).
In Italy, a new model has been proposed that can be a helpful management tool for monitoring the potential contamination process of groundwater due to the presence of landfills with municipal solid waste, including a significant organic component (Sappa et al., 2023).
Considering the general characteristics of municipal landfill wastes, landfill leachate may be defined as a water-based solution with a high content of dissolved organic matter, inorganic macro-components, heavy metals, and xenobiotic organic compounds (Christensen et al., 1994).
Groundwater is generally oligotrophic open environments that host different micro- and macro-organisms, with ecological functions in the recycling and distribution of energy and organic matter (Danielopol et al., 2003). Specifically, they host microbial communities that are particularly responsive to environmental changes, and the evaluation of their disturbances may act as bio-indicators of ecosystem health (Griebler & Avramov, 2015). Moreover, groundwater quality depends both on natural factors, such as the geochemical and hydrogeological characteristics of the aquifer, biological activity, and aquifer biodiversity, and on anthropogenic factors, mainly related to the type and concentration of released pollutants (Fida et al., 2023; Girvan et al., 2005; Loreau, 2000; McCann, 2000).
The landfill ecosystems are characterized by specific microbial communities involved in the process of waste degradation that may differ in abundance and distribution due to the type and age of landfill, the oxygen content, and switching from aerobic to facultative-anaerobic/anaerobic (from cellulose and hemicellulose hydrolyzing bacteria to methanogenic bacteria and Actinobacteria) (Kjeldsen et al., 2002; Liu et al., 2019). Also, pathogenic bacteria of human or animal origin can be found, associated with urban and farming waste disposal (Javahershenas et al., 2022).
As a direct consequence of the landfill leachate release into groundwater, the aquifer microbiota may show clear signs of disturbance, drastically shifting most of the key taxa (Gu et al., 2022; Lu et al., 2012). Complementary to microbiological monitoring, geochemical approaches that consider each potential nitrogen source and methods using isotopes (mainly oxygen, hydrogen, carbon, and nitrogen) as tracers can be used in the case of groundwater. Hydrogeochemical, isotopic, and microbiological investigations are necessary to elucidate the primary mechanism controlling the biogeochemistry of NO3− in the groundwater environment (Wang et al., 2024).
Different isotopic compositions may indeed characterize nitrates (NO3−) and other pollutants of different origins. Specifically, the δ15N values range from − 4‰ to + 4‰ for synthetic fertilizers, from + 2‰ to + 5‰ for soil organic nitrogen, and from + 10‰ to + 20‰ for human and animal waste nitrate (Kendall et al., 2007). Synthetic fertilizers are also characterized by enriched 18O values (+ 17‰– + 25‰) (Kendall et al., 2007), while high values of tritium (3H) and δ13C are indicators of leachate impacts (Cossu et al., 2018; Wimmer et al., 2013).
This paper proposed an integrated environmental monitoring system to evaluate possible leachate contamination events of groundwater from municipal solid waste landfills using hydrogeochemistry, stable isotopes, and microbiological methods. The use of classical hydrogeological methodologies was integrated with the use of advanced molecular microbial ecology methods and chemical and isotopic analyses.
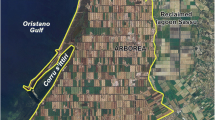
We investigate the presence of pollution from leachate in groundwater in a landfill district located near the town of Conversano, in the province of Bari, the main town of the Apulia region (south-eastern Italy, Fig. 1).
The geological and hydrogeological characteristics of the area make the Murgia karst aquifer vulnerable due to the lack of adequate surface protection and expose it to a very high risk of pollution (Polemio & Limoni, 2006; Polemio et al., 2009).
The inclusion of other potential causes of karstic groundwater quality disturbances is another advantage of our integrated methodologies: public authorities and environmental monitoring agencies could easily fall into misinterpretations of data arising from conventional methods that do not allow a comprehensive scenario. This may induce an underestimation or overestimation of the potential contamination of the site.
Hydrogeological features
The large carbonate hydrogeological structure called Murgia can be distinguished in the Apulia region (Fig. 1). It constitutes a wide coastal karstic aquifer, the high-quality groundwater of which is used for drinking. The Murgia plateau is mainly made of limestone (Mesozoic calcareous and/or calcareous-dolomitic rocks); the hydraulic conductivity is from medium to high but is heterogeneous and anisotropic for karstic and fractured features. It shows surface and deep karstic features, such as poljes, swallow holes, dolines, and dry valleys. The limestone outcrops are widespread below a very thin layer of residual soil in the study area; it is somewhere overlapped by subsequent formations, constituted by calcarenite, sandy clay, and alluvial deposits. The groundwater-saturated flow involves predominantly the limestone formation, which is part of a deep karstic aquifer, involving the whole Murgia hydrogeological structure.
These karstic features of the Murgia aquifer determine a wide range of groundwater vulnerability, from low to very high, as assessed with multiple methods applied in a test area located not far from the Conversano landfill district (CLD) (Polemio et al., 2009).
The recharge area includes inland portions of the site, and the outflow goes along the Adriatic coast and the Ionian coast. Serious seawater intrusion effects are known for this coastal aquifer (Polemio, 2016). The salinity threshold of pure fresh groundwater in the aquifer was assessed to be equal to 0.5 g/L. The threshold was defined by discussing chemical analyses of 500 groundwater samples, recognizing samples free from seawater intrusion mixing, and discussing statistically the salinity variability of this subset of samples; for these samples (defined as “pure fresh groundwater”), the salinity variability is mainly due to water-carbonate rock interaction (Polemio, 2016; Polemio et al., 2009). The coastal strip of Murgia, where the groundwater salinity is higher than the law potability limit (1.5 g/L), is about 3 km wide, as close to CLD, up to 6 km. The main human activity in the area is agriculture, and the most common crops are vineyards, orchards, olive groves, and arable crop cultivations.
The CLD includes five municipal solid waste (MSW) landfills, two of which are still operating (Zuffianò et al., 2018). The local groundwater flow is toward the Adriatic Sea (6 km away, north-northeast). The water table depth is so high, generally not less than 120 m from the ground surface, that it complicates any type of hydrogeological survey and sampling. Groundwater overexploitation for irrigation promotes groundwater–seawater intrusion mixing (Casarano et al., 2019; Polemio, 2016).
Materials and methods
Sampling
The groundwater and landfill leachate samples were taken concurrently with monthly samplings carried out in December 2017 and January 2018 in the CLD, as shown in Fig. 1.
The chemical study focused on the major ions together with some minor ions: potassium (K+), sodium (Na+), calcium (Ca2+), magnesium (Mg2+), boron (B−), fluoride (F−), bromide (Br−), chloride (Cl−), sulfate (SO42−), ammonium (NH4+), nitrate (NO3−), nitrite (NO2−), and bicarbonate (HCO3−) to define the geochemical characteristics.
The isotope characterization of groundwater and leachate was focused on δ18O, δ2H, 3H, δ 13C, δ 15N-NO3−, and δ 18O-NO3−.
During the groundwater sampling, specific measurements in the field were performed using a multiparametric probe (Hydrolab-Quanta G): electrical conductivity (EC), temperature (T), pH, dissolved oxygen (DO), and redox potential (Eh). The determination of NH4+ and NO2− was carried out by means of a photometric field method. All water samples were collected and stored in high-density polyethylene bottles (500 mL) with watertight caps. Samples for cation analysis were acidified by the addition of nitric acid (HNO3−) to a pH < 2, while water samples for metals determination were filtered through a cellulose acetate membrane (pore size 0.45 µm) and then were acidified by HNO3− to a pH < 2.
The sample for the dissolved carbonate δ13CDIC was acidified with orthophosphoric acid (H3PO4), according to Atekwana and Krishnamurthy (1998). The sample for δ15N-NO3− and δ18O-NO3− was acidified with hydrochloric acid (HCl) to a pH < 2.
Two liters of groundwater, one used for the bacteriological counts and one for the extraction of microbial DNA, respectively, were taken from groundwater wells after 5 min of outflow and sterilization of the outlet taps by an alcohol-soaked wipe and then by a portable Bunsen burner. Groundwater samples and samples of the leachate from the landfill, collected directly from the landfill collection tank, were placed in pre-sterilized Pyrex glass bottles. Both the water and leachate samples were then transported in refrigerated bags and analyzed within 4 h for microbial counts or stored at − 20 °C for subsequent DNA extraction.
Bacterial count
The total mesophilic and Escherichia coli bacterial counts were conducted according to the Italian APAT-IRSA standard methods (APAT, IRSA-CNR 2003) that refer to the APHA methods (APHA, AWWA, WEF 2005).
One hundred milliliters of each water sample was filtered under sterile conditions on 45 mm cellulose acetate membranes with a 0.45 μm pore size (Millipore). The membranes were subsequently placed on PCA (Oxoid, Basingstoke, UK) culture medium for total mesophilic bacterial counts and on chromogenic medium TBX agar (Oxoid) for the microbial count of E. coli. The plates were incubated at 30 and 37 °C, respectively, for total mesophilic and E. coli counts. The results were expressed as CFU (colony-forming units)/100 mL.
DNA extraction
The DNeasy PowerSoil Kit (Qiagen) was used for the extraction of DNA from samples, as follows: 500 mL of water well sample and 50 mL of landfill leachate sample were previously filtered under vacuum on isopore polycarbonate membranes (Whatman) with a diameter of 0.45 μm within 8 h of the sample collection. The filters were aseptically cut into approximately 0.5 cm2 pieces and inserted into the tubes for the mechanical and chemical lysis of microbial cell walls, following the manufacturer’s protocol. Then, the extracted DNA was stored at − 20 °C prior to molecular biology analyses.
The concentration and quality of the DNA were determined by fluorometric analysis and the agarose gel electrophoresis. For the fluorometric analysis, the Qubit 2.0 Fluorometer and dsDNA HS Kit (Thermo Fisher Scientific) were used.
PCR and target organisms
The detection and amplification of Bacteroides/Prevotella spp., Bifidobacterium spp., and Enterococcus spp. (including eight species of different human/animal origin: E. avium, E. gallinarum, E. saccharolyticus, E. faecium, E. faecalis, E. hirae, E. casseliflavus, and E. durans) were performed to identify possible fecal contamination sources. The references to the PCR methods are reported in Table 1. Furthermore, to evaluate the possible origin of Bacteroides spp. identified among our sample positive results and discriminate specific gene sequences for Bacteroides and Bifidobacterium strains of bovine origin (222 bp–313 bp, respectively) rather than human (119 bp–142/152 bp, respectively), the PCR products obtained in the amplification underwent enzymatic digestion with the restriction enzyme HaeIII (Bernhard & Field, 2000). As a positive control, a DNA sample taken from an urban wastewater treatment plant, naturally rich in Bacteroides spp. of human origin, was used. For Enterococcus faecalis and faecium, DNA from a collection strain of Enterococcus faecalis, DSMZ 2570, acted as a positive and negative control for the first and second gene amplifications, respectively.
A second set of PCR analyses was conducted to evaluate the potential influence of soil nitrification or leachate contamination in the presence of nitrites and/or nitrates in the groundwater. Nitrobacter spp. and Nitrospira spp., ammonium-oxidizing archaea (AOA), and ammonium-oxidizing bacteria (AOB) were the target microbial groups. For Nitrobacter spp. and Nitrospira spp., DNA extracted from a sludge nitrification tank of a wastewater treatment plant was used as a positive control. AOA and AOB were detected from digested and purified synthetic plasmids (Geneart, Thermo Fisher Scientific, Regensburg, GmbH) containing a sequence 100% homologous with the AmoA gene of Archaea and Bacteria, respectively, used as controls.
The thermal amplification programs used, the reaction conditions, and the primers set for all the methods and microbial populations considered in the analysis are shown in supplementary materials (Table 2), along with relative references. In all cases, Dream Taq Master Mix (Thermo Fisher Scientific) was used, and primers were synthesized by Macrogen Europe (The Netherlands).
PCR-ARISA
The PCR-ARISA reaction was performed according to the method of Cardinale et al. (2004) using 0.75 μL of 0.25 mM (each) ITSF (5′-GTCGTAACAAGGTAGCCGTA-3′)/ITSReub (5′-GCCAAGGCATCCACC-3′) primers, targeting bacterial ITS (Cardinale et al., 2004), in a reaction mixture containing 5 μL of 5X PCR buffer, 0.25 μL of 1.5U Taq DNA polymerase (Phusion HF DNA Polymerase—Thermo Fisher Scientific), and 0.5 μL of 0.2 mM (each) deoxynucleoside triphosphate and PCR grade water in a final volume of 25 μL and performed 35 times. Primer ITSReub was 5′-labeled with HEX fluorochrome in order to detect the ITS fragments.
The results obtained by the reaction were visualized by the 1.5% agarose gel electrophoresis run and then through a VersaDoc transilluminator (Bio-Rad, Hercules, Ca, USA).
After agarose-gel electrophoresis, the PCR ARISA products were quantified at a Qubit 2.0 fluorimeter and sent to the fragment analysis service provided by STAB-VIDA (Caparica, Portugal) to be subjected to capillary electrophoresis.
The data were analyzed using the Peak Scanner v1.0 software program (Applied Biosystem), according to the methods of Brusetti et al., 2018.
Chemical and isotopic analyses
Chemical and isotopic compositions in groundwater and leachate followed standard procedures: (a) ion chromatography (IC) for anions (B−, F−, Br−, Cl−, SO42−, NO3−, and NO2−) and ammonium (NH4+); (b) volumetric titration for HCO3−; (c) ICP-OES spectrometry for K+, Na+, Ca2+, and Mg2+; (d) Wavelength-Scanned Cavity Ring Down Spectroscopy technology for stable isotope values of δ18O and δ2H (the uncertainty of the measurements is ± 0.2 d ‰ for δ18O and ± 1 d ‰ for δ2H); (e) mass spectrometry IRMS with a Finnigan MAT250 for the isotopic ratio δ13CDIC (the uncertainty of the measurements is ± 0.2 δ ‰); (f) liquid scintillation counting (LSC) for 3H level (the analytical precision for tritium was 0.5 TU, 1r criterion/analytical errors); (g) IRMS (Finnigan MAT 250) for δ15N-NO3− and δ18O-NO3− (the 1σ analytical precisions for δ15N-NO3− and δ18O-NO3− are ± 0.5‰ and ± 1‰, respectively). The isotopic content of δ15N-NO3− and δ18O-NO3− was determined also considering the main used commercial fertilizers, using the result of land use analysis (Cossu et al., 2018; Zuffianò et al., 2018).
Statistical analyses
The cluster analysis was carried out using the number and position of the ARISA peaks of the samples as an index of the presence or absence of a given taxon and the height of the corresponding peaks as an index of the abundance of each taxon (Brown et al., 2005; Hewson & Fuhrman, 2006). From these data, a matrix was obtained by measuring the dissimilarity using the Bray–Curtis algorithm and then applying the Jaccard index. Non-metric multidimensional scaling (NMDS) analysis was realized by Past 4.07 software (Hammer et al. 2001), using the Bray–Curtis distance matrix calculated from PCR-ARISA. Environmental variables that included chemical and microbiological analyses were represented by vectors in the 2D plot generated. Diversity indexes were also calculated based on PCR-ARISA data (Brusetti et al. 2018), namely, Shannon–Wiener diversity, dominance, and evenness.
Results and discussion
Chemical characterization
Groundwater on-site physical and chemical parameters are summarized in Table 3. As expected, the leachate had consistently higher pH (8.2) and EC (12,347.0 mS/cm) values than groundwater. Among the groundwater samples, few differences were found: the sample of well 14 differed from others by a higher level of EC and lower DO. More generally, the EC values showed higher values in wells with a high discharging rate and/or with a higher boring depth, as is usual in a coastal aquifer, for the upcoming effect of seawater intrusion.
Fresh groundwater is characterized by variable chemical compositions (Moujabber et al., 2006), and its EC and salinity could be influenced by rainfall and high recharge events, seawater intrusion, evapotranspiration (for shallow aquifers), and anthropogenic effects due to land use and urbanization (Polemio & Zuffianò, 2020).
The DO values observed were consistent with those normally found in fresh groundwater, which is rich in DO, both due to the infiltration of meteoric water and the enrichment in the unsaturated area, as is common in this aquifer (Polemio, 2016).
Table 4 summarizes the results of the analyses of the main cations and anions determined for the groundwater and the leachate. Regarding inorganic nitrogen content, the nitrate concentration of groundwater fell in the range of 17.2–56.0 mg/L; the parameter exceeded the limit value of 50 mg/L as NO3− (European Directive 91/676/EEC) only in well 3.
NO2− values were always below detectable levels (< 0.1 mg/L), while NH4+ was below the detection limit and sporadically detected in groundwater samples (< 0.5 mg/L of ammonia nitrogen as the ammonia limit value) and at high levels in landfill leachate. Mono and divalent ions were found to be considerably different from leachate to groundwater, being Na+, K+, SO42−, and Cl− significantly higher in leachate, while Ca2+ and Mg2+ were lower.
The distribution of the main ion concentrations in the sampled waters is shown with a Schoeller diagram (Fig. 2). The geochemical features of the samples are compared with two typical reference compositions (Polemio et al., 2009): seawater and pure fresh groundwater of the carbonate aquifer, meaning they are not affected by the seawater intrusion (sampled in the recharge aquifer area, outside and upward of the study area).
The relative abundance of major ions was mainly Ca2+ > Mg2+ > Na+ + K+ for cations and HCO3− > Cl− > SO42−. The groundwater within carbonate aquifers is generally characterized by a predominance of calcium and bicarbonate ions due to the dissolution of calcite and dolomite. The leachate sample has completely different geochemical characteristics from those of groundwater samples (Table 4).
The results of the isotopic composition are shown in Table 5. Stable isotopic compositions, comparable for all groundwater samples and completely different with respect to leachate samples, range from − 6.53 to − 6.42‰ for δ18O and from − 42.89 to − 39.13‰ for δ2H in groundwater, while the value is − 4.29‰ for δ18O and 4.70‰ for δ2H in leachate.
Figure 3a shows the δ18O/δ2H values compared with the Global Meteoric Water Line (GMWL; Craig, 1961) and the Mediterranean Meteoric Water Line (MMWL; Gat & Carmi, 1970).
The δ18O/δ2H diagram shows that the samples of groundwater are closer to the Global Meteoric Water Line than the leachate data. This indicates a rapid infiltration of meteoric water to recharge coastal aquifers in a temperate climate.
The absence of a linear distribution of the points prevents the reconstruction of a Local Meteoric Water Line (LMWL), also denoting that the sampled groundwater belongs to a geographically limited area, likely coming from the same aquifer with the same recharge area.
The isotopic variability of δ13C in groundwater is rather narrow (− 7.44 ÷ − 11.88‰, Table 5, Fig. 3b) and is characteristic of uncontaminated groundwater flowing in carbonate aquifers (Clark & Fritz, 1997). In the leachate sample, the concentration is 23.24‰ (Table 5). The positive δ13C value and deuterium enrichment in leachate are attributed to the process of methanogenesis (Grossman, 1997; Hackley et al., 1996; Wimmer et al., 2013).
Leachate differs markedly from groundwater for the tritium content, which shows a value of 231.6 TU, while for groundwater, the maximum value is 1.7 TU.
The groundwater isotopic compositions of dissolved nitrates range between + 2.84 and + 10.92‰ vs. AIR in δ15N-NO3− and between + 6.17 and + 9.56‰ vs SMOW in δ18O-NO3− (Table 5).
Microbiological characterization
Microbial counts and PCR detection of the different target microorganisms are summarized in Table 6. The total mesophilic count of the groundwater showed average values of 512 (± 1.019) CFU/100 mL, with values ranging from 3 (well 13) to 3200 (well 15). E. coli was not isolated in any groundwater sample, except for well 14, where the count was 3 CFU/100 mL. The mesophilic bacterial count for all the groundwater samples was in line with what was reported in the literature in other studies (Keesari et al., 2015) and consistent with the range assessed in a regional study focused on the regional area that was studied in the present work (De Giglio et al., 2016). Regarding the fecal indicator E. coli, it should ideally not be present in the groundwater sample, as it is an indicator of recent fecal contamination. Considering that only one groundwater sample was found positive for E. coli, with a low count and according to the other fecal contamination microbial markers (as described below in the paragraph), it can be stated as sporadic contamination. In a previous study on a different landfill site, Grisey et al. (2010) reported a higher level of fecal coliforms and enterococci in the inspection wells than in the landfill leachate itself, due to the contamination of a septic tank of the toilet system located in the landfill plant.
The PCR targeting indicators of fecal contamination of the groundwater showed the following results: Bacteroides spp. was detected in well samples 12, 14, and 15 since a target amplicon of 676 base pairs was generated. Similarly, the amplicon produced by the leachate sample had a molecular weight comparable to what was expected for Bacteroides spp. Subsequent enzymatic digestion with HaeIII of the above-reported amplicons generated non-specific 190 bp and 460 bp fragments for wells samples 12, 14, and 15, while no fragment was produced for leachate DNA from the starting PCR product (Fig. 4).
A Electrophoretic PCR visualization realized with primer for Bacteroides/Prevotella spp. (16SrDNA target gene, expected amplicon size 676 bp, and Bifidobacterium spp., expected amplicon size 453 bp). M1 = molecular weight marker 1 kb (Invitrogen), Samples of wells 11, 12, 13, 14, and 15; P1, P2 = DNA extracted from leachate coming from the landfill; C − = no template control (white); C + = DNA extracted from purifying sludge; M2 = molecular weight marker 1 Kb (Promega); 2–5 = samples of wells 2, 5, 3, and 8; C − = no template control (white); C + = positive DNA control from sewage sludge. B Electrophoretic visualization of enzymatic digestion with HaeIII on PCR products of Bacteroides spp. positive samples. (16SrDNA target gene, expected fragment size for human/bovine, 119/222 bp)
No samples were found positive for Bifidobacterium spp., since the PCR targeting eight different species of Enterococcus spp. related to specific animal sources was negative for all groundwater samples and for the leachate (Fig. 5).
Therefore, a general confirmation of the absence of markers of fecal contamination was observed. The only exception was the Bacteroides/Prevotella. We adopted a method able to potentially discriminate between human or zoonotic origin of Bacteroides (Bernhard & Field, 2000). Despite positive results for Bacteroides spp. obtained from leachate and from 4 wells, the restriction profile of the positive amplicons in the wells was clearly different from the leachate. In both cases, we could not attribute a potential host, even though we could exclude the human origin for both samples since no specific restriction fragment was obtained for all the samples.
According to the microbiological analyses carried out with both cultivation and molecular methods, it is possible to exclude groundwater pollution linked to fluid urban waste (e.g., wastewater treatment plants and sewage pipes) or intensive breeding and manure in the sampled site. Similarly, it is possible to exclude the hypothesis of an intake of fecal microorganisms by landfill leachate.
A second set of PCR assays (Table 6) was made to detect nitrogen cycling-related microorganisms, particularly ammonia-oxidizing, nitrite-oxidizing, and denitrifying, as indicators of the occurrence of nitrification–denitrification processes in the groundwater environment.
Interestingly, in the case of ammonia-oxidizing bacteria and archaea (AOB and AOA), leachate tested positive for the PCR targeting the AmoA (ammonium monooxygenase) gene of AOB, while all groundwater samples were negative. Conversely, the AmoA gene of the AOA was detected in wells 12, 13, 14, and 15 but was absent in the leachate. Wells 12, 13, and 14 are upward of both the two active landfills and four of the five district landfills (Fig. 1). These data confirmed the presence of different nitrifying communities in groundwater and leachate. A previous study also reported the dominance of nitrifying archaeal populations in groundwater environments with low ammonia levels (Zheng et al., 2017). The presence of different taxa between leachate (presence of AOB only) and groundwater (presence of AOA only) is noteworthy and underscores the absence of direct interaction between groundwater and leachate. While both environments may support nitrifying bacterial activity, the distinctive features observed in each suggest incompatibility for potential microbial contamination of water by leachate. As reported in a recent review (Meyer-Dombard et al., 2020), there is still little knowledge about nitrogen cycling gene activity in landfill environments. Although our results referred to a limited dataset, we found distinct nitrifier communities in the landfill leachate compared to the ones from all groundwater samples. Our results align with a previous study (Zhu et al., 2007) that underlined specific nitrifier populations in ammonia-rich landfill leachate environments.
About nitrite-oxidizing communities, samples from wells 12 and 14 were positive for both Nitrobacter spp. and Nitrospira spp., while samples from wells 15 and 3 and the leachate were positive for Nitrobacter spp. only (Fig. 6). It is assumed that the origin of these nitrifying microorganisms is attributable to the surrounding soil or soil fertilizers applied near the wells, considering that the chemical analysis showed a null intake of nitrites in the groundwater.
A Electrophoretic visualization of the PCR carried out on all the samples of the 9 wells for the research of Nitrospira spp. and Nitrobacter spp. (16SrDNA target gene, expected fragment size 397 bp for Nitrobacter spp. and 151 bp for Nitrospira spp.). B Electrophoretic visualization of the PCR conducted on all the samples of the 9 wells for the research of AOB and AOA. (Target gene AmoA, expected fragment size 491 bp for AOB and 624 bp for AOA). C Electrophoretic visualization of the PCR carried out on the samples of the examined wells for the search of denitrifying bacteria. Target NosZ genes, expected fragment dimensions 267 bp and NirK, and expected fragment dimensions 164 bp
Finally, the search for denitrifying microbial populations completed the evaluation of the potential origin and fate of the inorganic nitrogen forms present in the area examined. Denitrifiers were found in the leachate, as well as in wells 12 and 14. Other groundwater samples were all negative, with none showing a positive result for the NirS gene (data not shown). The functional role of denitrifiers’ community both in groundwater and landfill leachate environments are complex and very different, as reported in previous studies (Cao et al., 2019; Cerminara et al., 2020; Heffernan et al., 2012; Utom et al., 2020). In our study, we can confirm that leachate hosts a denitrifying population that is compatible with a reducing environment in which nitrate can be used as an alternative electron acceptor by denitrifiers. The denitrifying population presence is negligible in the groundwater, stressing the inconsistency of the hypothetical contamination of groundwater by landfill leachate. It is important to note that denitrification is a process observed both in natural soil and instances of excessive nitrate intake after leaching, whether in water or through percolating leachate from MSW. These phenomena are not interrelated, and an adequate supply of organic carbon and a limited presence of oxygen can occur in the presence of high amounts of nitrates, regardless of their source of origin. Given the limited number of samples analyzed in the present study, we cannot make further assumptions about their ecological role.
The similarity matrix generated from the PCR-ARISA fragment of bacterial communities was used for cluster analysis, to evaluate possible influence of leachate on the bacterial communities of the aquifer.
The ARISA profiles of bacterial communities (Fig. 7) revealed that three clusters are clearly distinguishable (reported with brackets and Roman numbers) and evidence a major difference in bacterial communities in different sites of the sample area.
Cluster analysis based on ARISA patterns obtained from bacterial community of the groundwater samples (well number replicates a–b) and the leachate. Bray–Curtis similarity index is reported on the axis. The three clusters are numbered with roman numbers. Numbers on the nodes are the results of bootstrap analysis (5.000 repetitions)
Particularly, the wells close to the main group of landfills, named 11–12-13–14 (internal to the landfill district) and 15 (downward of the district), clustered together (cluster I). Cluster II grouped the microbial community of groundwater sampled from wells 2, 5, 3, and 8, located far from the landfill district, both upward and downward. Interestingly, the leachate bacterial community was found to be highly dissimilar to all the groundwater samples and grouped separately in a specific cluster (cluster III). The microbial communities of the wells are strictly influenced only by their location in space and human activity (anthropized and non-anthropized), without a relation to the distance or the upward or downward location with respect to the landfill district.
The alpha diversity analysis was also reported (Fig. 8), to evaluate the richness of the groundwater bacterial communities and the abundance variation within sampled sites.
Box plot of the diversity indexes of leachate and groundwater bacterial communities, based on the ARISA analysis. Central bar represents the median, and rectangles represent first and third quartiles. A Shannon–Wiener, B evenness, and C dominance. Samples are grouped and numbered with roman numbers according to the clustering reported in Fig. 7
The Shannon–Wiener diversity index (Fig. 8A) depicted a general trend of higher bacterial diversity in the wells sampled far away from the landfill district (group II), where agricultural use of the land is the exclusive activity. Wells sampled in the landfill district showed a lower diversity (group I). Despite its proximity to landfill zone inspection wells, the landfill leachate showed a higher diversity than the closest wells. The differentiation among abundance trends in the different samples was mostly explained by the evenness of the bacterial communities, as reported in Fig. 8B, C, where Shannon’s evenness and dominance indexes are reported. While the dominance was relatively low in all samples, the evenness of samples far from the landfill (group II) was considerably higher than both leachate and wells in the proximity of the landfill site, which was also highly variable. A possible explanation is that the anthropic influence of the landfill environment (concrete pavement, buildings, truck handling, etc.) may affect the underground water environment, shaping a different bacterial assembly than in the surrounding environment, where agriculture is the major activity. Leachate bacterial communities were different both in terms of alpha and beta-diversity from the groundwater sampled both close and far from the landfill site, confirming that no direct influence of potential leachate infiltration could be drawn.
Correlation of microbial community with environmental variables
Finally, to evaluate if and how microbial groundwater communities were differentially shaped by environmental variables, a non-metric multidimensional scaling method (NMDS) analysis was also conducted (Fig. 9).
The NMDS confirms a high distance among a bacterial community assembly close to and far from the landfill site. Leachate sample coherently with previous results, clustered separately by all groundwater sample points. In particular, the figure outlined how physical parameters like EC and pH and chemical parameters (mostly EC, NH4+, B−, and Cl−) were the main drivers of leachate bacterial community differentiation in space. On the opposite, the differentiation of groundwater clusters was mainly driven by divalent cations (Mg+ and Ca+) and seawater intrusion mixing parameters (EC and Cl−) but it was also related to the differential presence of NO3− and the N cycling microbial key groups (AOA, Nitrobacter spp., and Nitrospira spp.).
Figure 10 shows the values of δ18O and δ15N of the groundwater nitrate, including the “Nitrophoska Special” fertilizer, widely used in the areas around the site under study for agricultural purposes, in order to identify the different sources of nitrate. The graph shows that the isotopic delta of most groundwater samples falls to the mineralized NH4-NO3 fertilizer area. That indication confirms the hypothesis of active nitrification in the soil and its possible impact on the nitrate content of the groundwater derived from agriculture and microbial activity rather than any other possible source (Nestler et al., 2011).
Stable isotope composition of dissolved nitrates in groundwater (blue dots) and fertilizer (green triangle). The rectangles and the oriented lines highlight the main types of nitrate sources and the effect of the processes respectively (modified after Clark & Fritz, 1997)
The groundwater samples 14 and 15 show a higher δ15N-NO3− isotopic signature as an effect of partial denitrification. Those results are coherent with previous studies on CLD that highlighted the effect of the denitrification process in the groundwater sampled (Cossu et al., 2018).
Conclusions
Our study is aimed at proposing a multidisciplinary approach for environmental monitoring of a potentially polluted aquifer. The approach integrates chemical and isotopic analyses with both culture based and molecular microbiology methods. It offered explanations of all the measured variability in the monitored site that led to robust conclusions about absence of leachate pollution events in the surveyed period.
The proposed approach confirmed that leachate had no measurable influence on groundwater microbial community, showing other anthropic activities and seawater intrusion explain well the measured variability of the microbial populations. On the other hand, the chemical and isotopic results confirmed the absence of leachate effects on the groundwater samples, showing the decisive role of fertilizers as potential nitrate sources, as confirmed by N-cycling bacterial population features evidenced by molecular methods.
According to the results, this multi-integrated method clearly shows the absence of leachate effects on groundwater in the Conversano landfill district despite the inherent difficulty of operating in a karstic aquifer affected by seawater intrusion and with very deep groundwater. The integrated methods also give explanation of all observed variabilities in both chemical and microbiological parameters.
Compared to previous studies, the proposed approach shows advantages considering site-specific factors concerning natural ecology and potential anthropic disturbances related to landfills, agriculture, and other potential pollution sources. The next aim will be extended to a broader sampling period and different sites, providing a solid scientific basis for the effective waste management control and ecological restoration of landfills, in complex sites constituted by more landfills and including different pollution sources.
References
Abd El-Salam, M. M., & Abu-Zuid, G. I. (2015). Impact of landfill leachate on the groundwater quality: A case study in Egypt. Journal of Advanced Research, 6(4), 579–586. https://doi.org/10.1016/j.jare.2014.02.003
Ameloko, A. A., & Ayolabi, E. A. (2018). Geophysical assessment for vertical leachate migration profile and physicochemical study of groundwater around the Olusosun dumpsite Lagos, south-west Nigeria. Applied Water Science, 8(5), 142. https://doi.org/10.1007/s13201-018-0775-x
APAT, IRSA-CNR. (2003). Metodi analitici per le acque. Manuali e linee guida. http://www.irsa.cnr.it/Docs/Capitoli/1000.pdf (web archive link, 15 January 2017).
APHA, AWWA, WEF. (2005). Standard methods for the examination of water and wastewater (XX.). American Publishing Health Association,APHA, AWWA, WEF (2005) Standard Methods for the Examination of Water and Wastewater, XX ed. American Publishing Health Association.
Atekwana, E. A., & Krishnamurthy, R. V. (1998). Seasonal variations of dissolved inorganic carbon and δ13C of surface waters: Application of a modified gas evolution technique. Journal of Hydrology, 205(3–4), 265–278. https://doi.org/10.1016/S0022-1694(98)00080-8
Bernhard, A. E., & Field, K. G. (2000). Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Applied and Environmental Microbiology, 66(4), 1587–1594. https://doi.org/10.1128/AEM.66.4.1587-1594.2000
Brown, M. V., Schwalbach, M. S., Hewson, I., & Fuhrman, J. A. (2005). Coupling 16S-ITS rDNA clone libraries and automated ribosomal intergenic spacer analysis to show marine microbial diversity: Development and application to a time series. Environmental Microbiology, 7(9), 1466–1479. https://doi.org/10.1111/j.1462-2920.2005.00835.x
Brusetti, L., Ciccazzo, S., Borruso, L., Bellucci, M., Zaccone, C., & Beneduce, L. (2018). Metataxonomy and functionality of wood-tar degrading microbial consortia. Journal of Hazardous Materials, 353, 108–117. https://doi.org/10.1016/j.jhazmat.2018.03.041
Cao, Q., Liu, X., Ran, Y., Li, Z., & Li, D. (2019). Methane oxidation coupled to denitrification under microaerobic and hypoxic conditions in leach bed bioreactors. Science of the Total Environment, 649, 1–11. https://doi.org/10.1016/j.scitotenv.2018.08.289
Cardinale, M., Brusetti, L., Quatrini, P., Borin, S., Puglia, A. M., Rizzi, A., et al. (2004). Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Applied and Environmental Microbiology, 70(10), 6147–6156. https://doi.org/10.1128/AEM.70.10.6147-6156.2004
Casarano, D., Dragone, V., & Polemio, M. (2019). Groundwater resources at salinisation risk: Effects of climate and utilisation changes in the case of Apulian coastal aquifers (Southeastern Italy). Acque Sotterranee - Italian Journal of Groundwater. https://doi.org/10.7343/as-2019-374
Cébron, A., & Garnier, J. (2005). Nitrobacter and Nitrospira genera as representatives of nitrite-oxidizing bacteria: Detection, quantification and growth along the lower Seine River (France). Water Research, 39(20), 4979–4992. https://doi.org/10.1016/j.watres.2005.10.006
Cerminara, G., Raga, R., Hirata, O., & Pivato, A. (2020). Denitrification of low C/N landfill leachate in lab-scale landfill simulation bioreactors. Waste Management, 113, 236–243. https://doi.org/10.1016/j.wasman.2020.05.041
Christensen, T. H., Kjeldsen, P., Albrechtsen, H., Heron, G., Nielsen, P. H., Bjerg, P. L., & Holm, P. E. (1994). Attenuation of landfill leachate pollutants in aquifers. Critical Reviews in Environmental Science and Technology, 24(2), 119–202. https://doi.org/10.1080/10643389409388463
Clark, I., & Fritz, P. (1997). Environmental isotopes in hydrogeology (Lewis Publishers, Boca Raton.).
Cossu, R., Zuffianò, L. E., Limoni, P. P., De Giorgio, G., Pizzardini, P., Miano, T., et al. (2018). How can the role of leachate on nitrate concentration and groundwater quality be clarified? An approach for landfills in operation (Southern Italy). Waste Management, 77, 156–165. https://doi.org/10.1016/j.wasman.2018.05.014
Craig, H. (1961). Isotopic variations in meteoric waters. Science, 133(3465), 1702–1703. https://doi.org/10.1126/science.133.3465.1702
Danielopol, D. L., Griebler, C., Gunatilaka, A., & Notenboom, J. (2003). Present state and future prospects for groundwater ecosystems. Environmental Conservation, 30(2), 104–130. https://doi.org/10.1017/S0376892903000109
De Giglio, O., Barbuti, G., Trerotoli, P., Brigida, S., Calabrese, A., Di Vittorio, G., et al. (2016). Microbiological and hydrogeological assessment of groundwater in southern Italy. Environmental Monitoring and Assessment, 188(11), 638. https://doi.org/10.1007/s10661-016-5655-y
Degrange, V., & Bardin, R. (1995). Detection and counting of Nitrobacter populations in soil by PCR. Applied and Environmental Microbiology, 61(6), 2093–2098. https://doi.org/10.1128/aem.61.6.2093-2098.1995
Dionisi, H. M., Layton, A. C., Harms, G., Gregory, I. R., Robinson, K. G., Sayler, G. S., & Gregory, I. R. (2002). Quantification of Nitrosomonas oligotropha -like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR quantification of Nitrosomonas oligotropha-like ammonia- oxidizing bacteria and Nitrospira spp. Applied and Environmental Microbiology, 68(1), 245–253.
Feng, S.-J., Zhao, Y., Zhang, X.-L., & Bai, Z.-B. (2020). Leachate leakage investigation, assessment and engineering countermeasures for tunneling underneath a MSW landfill. Engineering Geology, 265, 105447. https://doi.org/10.1016/j.enggeo.2019.105447
Fida, M., Li, P., Wang, Y., Alam, S. M. K., & Nsabimana, A. (2023). Water contamination and human health risks in Pakistan: A review. Exposure and Health, 15(3), 619–639. https://doi.org/10.1007/s12403-022-00512-1
Fiksdal, L., Maki, J. S., LaCroix, S. J., & Staley, J. T. (1985). Survival and detection of Bacteroides spp., prospective indicator bacteria. Applied and Environmental Microbiology, 49(1), 148–150. https://doi.org/10.1128/aem.49.1.148-150.1985
Gat, J. R., & Carmi, I. (1970). Evolution of the isotopic composition of atmospheric waters in the Mediterranean Sea area. Journal of Geophysical Research, 75(15), 3039–3048. https://doi.org/10.1029/JC075i015p03039
Girvan, M., Campbell, C., Killham, K., Prosser, J., & Glover, L. (2005). Bacterial diversity promotes community stability and functional resilience after perturbation. Environmental Microbiology, 7(3), 301–313.
Griebler, C., & Avramov, M. (2015). Groundwater ecosystem services: A review. Freshwater Science, 34(1), 355–367. https://doi.org/10.1086/679903
Grisey, E., Belle, E., Dat, J., Mudry, J., & Aleya, L. (2010). Survival of pathogenic and indicator organisms in groundwater and landfill leachate through coupling bacterial enumeration with tracer tests. Desalination, 261(1–2), 162–168. https://doi.org/10.1016/j.desal.2010.05.007
Grossman, E. (1997). Stable carbon isotopes as indicators of microbial activity in aquifers. In C. Hurst, G. Knudson, M. McInerney, & L. Stetzenbach (Eds.), Manual of Environmental Microbiology (American Society for Microbiology., pp. 565–567).
Gu, Z., Feng, K., Li, Y., & Li, Q. (2022). Microbial characteristics of the leachate contaminated soil of an informal landfill site. Chemosphere, 287, 132155. https://doi.org/10.1016/j.chemosphere.2021.132155
Guo, Y., Li, P., He, X., & Wang, L. (2022). Groundwater quality in and around a landfill in northwest China: Characteristic pollutant identification, health risk assessment, and controlling factor analysis. Exposure and Health, 14(4), 885–901. https://doi.org/10.1007/s12403-022-00464-6
Hackley, K. C., Liu, C. L., & Coleman, D. D. (1996). Environmental isotope characteristics of landfill leachates and gases. Ground Water, 34(5). https://doi.org/10.1111/j.1745-6584.1996.tb02077.x
Hammer, Ø., Harper, D., & Ryan, P. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 1–9.
Harter, J., Krause, H.-M., Schuettler, S., Ruser, R., Fromme, M., Scholten, T., et al. (2014). Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. The ISME Journal, 8(3), 660–674. https://doi.org/10.1038/ismej.2013.160
Heffernan, J. B., Albertin, A. R., Fork, M. L., Katz, B. G., & Cohen, M. J. (2012). Denitrification and inference of nitrogen sources in the karstic Floridan Aquifer. Biogeosciences, 9(5), 1671–1690. https://doi.org/10.5194/bg-9-1671-2012
Henry, S., Bru, D., Stres, B., Hallet, S., & Philippot, L. (2006). Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Applied and Environmental Microbiology, 72(8), 5181–5189. https://doi.org/10.1128/AEM.00231-06
Hewson, I., & Fuhrman, J. A. (2006). Improved strategy for comparing microbial assemblage fingerprints. Microbial Ecology, 51(2), 147–153. https://doi.org/10.1007/s00248-005-0144-9
Javahershenas, M., Nabizadeh, R., Alimohammadi, M., & Mahvi, A. H. (2022). The effects of Lahijan landfill leachate on the quality of surface and groundwater resources. International Journal of Environmental Analytical Chemistry, 102(2), 558–574. https://doi.org/10.1080/03067319.2020.1724984
Keesari, T., Ramakumar, K. L., Prasad, M. B. K., Chidambaram, S., Perumal, P., Prakash, D., & Nawani, N. (2015). Microbial evaluation of groundwater and its implications on redox condition of a multi-layer sedimentary aquifer system. Environmental Processes, 2(2), 331–346. https://doi.org/10.1007/s40710-015-0067-5
Kendall, C., Elliott, E. M., & Wankel, S. D. (2007). Tracing anthropogenic inputs of nitrogen to ecosystems. In R. Michener & K. Lajtha (Eds.), Stable Isotopes in Ecology and Environmental Science (Blackwell Publishing Inc., Oxford UK, 2nd edition., pp. 375–449). Wiley. https://doi.org/10.1002/9780470691854.ch12
Kjeldsen, P., Barlaz, M. A., Rooker, A. P., Baun, A., Ledin, A., & Christensen, T. H. (2002). Present and long-term composition of MSW landfill leachate: A review. Critical Reviews in Environmental Science and Technology, 32(4), 297–336. https://doi.org/10.1080/10643380290813462
Layton, A., McKay, L., Williams, D., Garrett, V., Gentry, R., & Sayler, G. (2006). Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Applied and Environmental Microbiology, 72(6), 4214–4224. https://doi.org/10.1128/AEM.01036-05
Layton, B. A., Walters, S. P., Lam, L. H., & Boehm, A. B. (2010). Enterococcus species distribution among human and animal hosts using multiplex PCR. Journal of Applied Microbiology, 109(2), 539–547. https://doi.org/10.1111/j.1365-2672.2010.04675.x
Leininger, S., Urich, T., Schloter, M., Schwark, L., Qi, J., Nicol, G. W., et al. (2006). Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature, 442(7104), 806–809. https://doi.org/10.1038/nature04983
Liu, S., Xi, B.-D., Qiu, Z.-P., He, X.-S., Zhang, H., Dang, Q.-L., et al. (2019). Succession and diversity of microbial communities in landfills with depths and ages and its association with dissolved organic matter and heavy metals. Science of the Total Environment, 651, 909–916. https://doi.org/10.1016/j.scitotenv.2018.09.267
Loreau, M. (2000). Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos, 91(1), 3–17. https://doi.org/10.1034/j.1600-0706.2000.910101.x
Lu, Z., He, Z., Parisi, V. A., Kang, S., Deng, Y., Van Nostrand, J. D., et al. (2012). GeoChip-based analysis of microbial functional gene diversity in a landfill leachate-contaminated aquifer. Environmental Science & Technology, 46(11), 5824–5833. https://doi.org/10.1021/es300478j
Maryadi, M., Supriyanto, C., & F. M. S., Triananda, I. N., & Fitriana, D. N. (2020). Analysis of groundwater contamination level in residential areas around Cipayung Landfill using ground penetrating radar. IOP Conference Series: Materials Science and Engineering, 874(1), 012013. https://doi.org/10.1088/1757-899X/874/1/012013
McCann, K. S. (2000). The diversity–stability debate. Nature, 405(6783), 228–233. https://doi.org/10.1038/35012234
Meyer-Dombard, D. R., Bogner, J. E., & Malas, J. (2020). A review of landfill microbiology and ecology: A call for modernization with next generation technology. Frontiers in Microbiology, 11, 1127. https://doi.org/10.3389/fmicb.2020.01127
Moujabber, M. E., Samra, B. B., Darwish, T., & Atallah, T. (2006). Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resources Management, 20(2), 161–180. https://doi.org/10.1007/s11269-006-7376-4
Negi, P., Mor, S., & Ravindra, K. (2020). Impact of landfill leachate on the groundwater quality in three cities of North India and health risk assessment. Environment, Development and Sustainability, 22(2), 1455–1474. https://doi.org/10.1007/s10668-018-0257-1
Nestler, A., Berglund, M., Accoe, F., Duta, S., Xue, D., Boeckx, P., & Taylor, P. (2011). Isotopes for improved management of nitrate pollution in aqueous resources: Review of surface water field studies. Environmental Science and Pollution Research, 18(4), 519–533. https://doi.org/10.1007/s11356-010-0422-z
Polemio, M. (2016). Monitoring and management of karstic coastal groundwater in a changing environment (Southern Italy): A review of a regional experience. Water, 8(4), 148. https://doi.org/10.3390/w8040148
Polemio, M., & Limoni, P. P. (2006). Groundwater pollution and risks for the coastal environment (southeastern Italy). In M. Sivapalan, T. Wagener, S. Uhlenbrook, E. Zehe, V. Lakshmi, X. Liang, et al. (Eds.), Predictions in Ungauged Basins: Promise and Progress (Vol. 303, pp. 477–486). IAHS.
Polemio, M., & Zuffianò, L. E. (2020). Review of utilization management of groundwater at risk of salinization. Journal of Water Resources Planning and Management, 146(9), 03120002. https://doi.org/10.1061/(ASCE)WR.1943-5452.0001278
Polemio, M., Dragone, V., & Limoni, P. P. (2009). Monitoring and methods to analyse the groundwater quality degradation risk in coastal karstic aquifers (Apulia, Southern Italy). Environmental Geology, 58(2), 299–312. https://doi.org/10.1007/s00254-008-1582-8
Ringle Raja, S., Kanagaraj, B., & Eunice, S. (2023). Evaluating groundwater contamination: An examination of a municipal solid waste dump yard in southern India’s Manchester City. Resources, Conservation & Recycling Advances, 20, 200196. https://doi.org/10.1016/j.rcradv.2023.200196
Sappa, G., Barbieri, M., & Andrei, F. (2023). Isotope-based early-warning model for monitoring groundwater–leachate contamination phenomena: First quantitative assessments. Water, 15(14), 2646. https://doi.org/10.3390/w15142646
Schauss, K., Focks, A., Leininger, S., Kotzerke, A., Heuer, H., Thiele-Bruhn, S., et al. (2009). Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environmental Microbiology, 11(2), 446–456. https://doi.org/10.1111/j.1462-2920.2008.01783.x
Throbäck, I. N., Enwall, K., Jarvis, Ã., & Hallin, S. (2004). Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiology Ecology, 49(3), 401–417. https://doi.org/10.1016/j.femsec.2004.04.011
Utom, A. U., Werban, U., Leven, C., Müller, C., Knöller, K., Vogt, C., & Dietrich, P. (2020). Groundwater nitrification and denitrification are not always strictly aerobic and anaerobic processes, respectively: An assessment of dual-nitrate isotopic and chemical evidence in a stratified alluvial aquifer. Biogeochemistry, 147(2), 211–223. https://doi.org/10.1007/s10533-020-00637-y
Wang, D., Li, P., Mu, D., Liu, W., Chen, Y., & Fida, M. (2024). Unveiling the biogeochemical mechanism of nitrate in the vadose zone-groundwater system: Insights from integrated microbiology, isotope techniques, and hydrogeochemistry. Science of the Total Environment, 906, 167481. https://doi.org/10.1016/j.scitotenv.2023.167481
Wimmer, B., Hrad, M., Huber-Humer, M., Watzinger, A., Wyhlidal, S., & Reichenauer, T. G. (2013). Stable isotope signatures for characterising the biological stability of landfilled municipal solid waste. Waste Management, 33(10), 2083–2090. https://doi.org/10.1016/j.wasman.2013.02.017
Zheng, L., Zhao, X., Zhu, G., Yang, W., Xia, C., & Xu, T. (2017). Occurrence and abundance of ammonia-oxidizing archaea and bacteria from the surface to below the water table, in deep soil, and their contributions to nitrification. MicrobiologyOpen, 6(4), e00488. https://doi.org/10.1002/mbo3.488
Zhu, S., Chan, G. Y. S., Cai, K.-L., Qu, L.-H., & Huang, L.-N. (2007). Leachates from municipal solid waste disposal sites harbor similar, novel nitrogen-cycling bacterial communities. FEMS Microbiology Letters, 267(2), 236–242. https://doi.org/10.1111/j.1574-6968.2006.00560.x
Zuffianò, L. E., Limoni, P. P., De Giorgio, G., & Polemio, M. (2018). Data to clarify the landfill role in the case of groundwater quality degradation (Southern Italy). Data in Brief, 20, 1489–1499. https://doi.org/10.1016/j.dib.2018.08.201
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
L.B.: research conceptualization, biological data analysis, manuscript editing, and final revision. P.P.L.: field work, data curation, formal analysis, validation, and manuscript editing. F.P.: field work, writing original draft and manuscript editing, lab analysis, and biological data organization and presentation. M.P.: research conceptualization, manuscript editing, isotope data discussion, and supervision. L.E.Z.: field work, geochemical and isotope data discussion, and manuscript editing.
Corresponding author
Ethics declarations
Consent to participate
All authors have given consent to their contribution.
Consent for publication
All authors have agreed with the content and all have given explicit consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beneduce, L., Piergiacomo, F., Limoni, P.P. et al. Microbial, chemical, and isotopic monitoring integrated approach to assess potential leachate contamination of groundwater in a karstic aquifer (Apulia, Italy). Environ Monit Assess 196, 312 (2024). https://doi.org/10.1007/s10661-024-12477-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12477-6