Abstract
Environmental pollution by anthropogenic activity is still a highly relevant global problem. Aquatic animals are a specifically endangered group of organisms due to their continuous direct contact with the contaminated environment. Concentrations of selected trace elements in the grass carp (Ctenopharyngodon idella) (n = 36) blood serum/clot were monitored. Possible effects of the elements on selected biochemical and oxidative markers were evaluated. The concentrations of trace elements (Al, Ba, Be, Bi, Cd, Co, Cr, Cu, Fe, Ga, Mn, Mo, Ni, Pb, Sr, Tl, and Zn) were analysed in the fish blood serum and blood clot by inductively coupled plasma optical emission spectrometry (ICP OES). A general scheme of decreasing concentrations of trace elements in the blood serum samples was: Zn ˃ Fe ˃ Sr ˃ Ba ˃ Ni ˃ Al ˃ Cu ˃ Be ˃ Co; < LOQ (below limit of quantification): Bi, Cd, Cr, Ga, Mn, Mo, Pb, Tl; and in the case of the blood clot, the scheme was as follows: Fe ˃ Zn ˃ Sr ˃ Al ˃ Ni ˃ Ba ˃ Cu ˃ Be ˃ Co ˃ Mn; < LOQ (below limit of quantification): Bi, Cd, Cr, Ga, Mo, Pb, Tl. Significant differences among the seasons were detected. The Spearman R correlation coefficients and linear or non-linear regression were used to evaluate direct relationships between trace elements and selected blood biomarkers. The correlation analysis between biochemical parameters (Na, K, P, Mg, AST, ALT, ALP, GGT, TAG, TP, urea, glucose) and trace elements (Al, Ba, Be, Cu, Fe, Ni, Sr, and Zn) concentrations confirmed statistically significant interactions in both seasons (summer and autumn). The regression analysis between oxidative stress markers (ROS, GPx, creatinine, uric acid, and bilirubin) and elements (Al, Ba, Co, Cu, Fe, Ni, and Sr) content confirmed statistically significant interactions. The results point to numerous connections between the observed elements and the physiological parameters of freshwater fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution by anthropogenic activity is a pressing global problem. Aquatic animals are a specifically endangered group of organisms due to their continuous direct contact with the contaminated environment. The water ecosystem is in fact absolutely connected with the waste systems of industry, the energy sector, agriculture, or households. Monitoring environmental pollutants in relation to aquatic organisms’ health status is an important activity that could reflect the effects and risks stemming from contaminants on animal organisms. Trace elements, pesticides, and microplastics are among the most widespread environmental contaminants today (Clasen et al., 2018; Lushchak, 2011; Shahjahan et al., 2022a, 2022b).
Assessment of trace elements in the blood and tissues of fish can prevent any potential food chain risks while serving as an early detection of contamination (Ansel & Benamar, 2018; Hansson et al., 2020; Hinojosa-Garro et al., 2020; Lakra et al., 2022; Monferrán et al., 2016; Ruas et al., 2008). On the other hand, increased concentrations of toxic as well as essential elements result in various physiological and health alterations (Emenike et al., 2022; Khan et al., 2020; Öner et al., 2008; Shahjahan et al., 2022a, 2022b), defects particularly to the liver, and kidney (Banday et al., 2019; Erdoğan et al., 2021; Lakra et al., 2022; Tlenshieva et al., 2022), and the reproductive system (Gárriz et al., 2019; Kovacik et al., 2018; Kročková et al., 2013; Özgür et al., 2019) in animal species. Blood parameters, such as serum chemistry and enzymatic markers, may provide us with the physiological response of the organism in connection with metals (Gopal et al., 1997; Phoonaploy et al., 2019). Another important system for the assessment of environmental contamination or intoxication by trace elements are oxidative stress markers (El-Sharawy et al., 2022; Giarratano et al., 2016; Kovacik et al., 2019; Lushchak, 2011). The production of reactive oxygen species (ROS) or ROS-induced modification of lipids and proteins leads to lipid peroxidation and the production of carbonyl proteins, which allows us to evaluate the range of pathological processes related to free radicals (Lushchak, 2011, 2014). A subsequent evaluation of the total antioxidant capacity (TAC), enzymatic and non-enzymatic endogenous oxidative status markers may indicate an overall association of environmental pollutants and trace elements on the animal organism (Lushchak, 2016; Shahjahan et al., 2022a, 2022b; Tvrdá et al., 2016a, 2016b).
Based on the above, we focused on several objectives in the present study. In our conditions, we chose to collect samples in two seasons: summer and autumn. The primary goal was to assess the levels of selected trace elements (essential, potentially toxic, and toxic elements), such as aluminum (Al), barium (Ba), beryllium (Be), bismuth (Bi), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), gallium (Ga), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), strontium (Sr), thallium (Tl), and zinc (Zn), in the blood serum and blood clot of grass carp (Ctenopharyngodon idella), as well as in water and sediment samples. The next objective was to assess the health status of the fish and evaluate biochemical parameters such as sodium (Na), potassium (K), chloride (Cl), calcium (Ca), phosphorus (P), magnesium (Mg), urea, total proteins (TP), glucose (Glu), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), cholesterol (Chol), triglycerides (TAG), and oxidative status/stress markers like reactive oxygen species (ROS) production, total antioxidant capacity (TAC), protein carbonyls (PC), lipid peroxidation (LPO), glutathione peroxidase (GPx) activity, total bilirubin (Bili), uric acid (UA), and creatinine (Crea). The final goal of this work was to clarify the possible connections between the studied biomarkers and concentrations of trace elements. Our study can provide information about the impact of these pollutants on a complex of biochemical parameters, oxidative stress markers, and potential health consequences in natural conditions.
Material and methods
Fish and experimental design
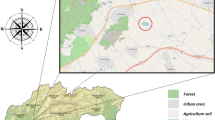
This work was realized during the summer and autumn seasons. Adult (4-year-old) grass carps were harvested from a university experimental pond in Kolíňany—West Slovak Lowland—Slovak Republic (48°21′14.0″N 18°13′02.8″E) (Fig. 1). The location selected for this study is characterized by intensive agricultural activity; the university experimental fishpond also serves as a catchment area for the wastewater treatment plant. Fish stocking was realized one year before the first sampling. The freshwater fish (Ctenopharyngodon idella) were caught using a seine net, according to our previous study (Kovacik et al., 2019). In total, 36 fish were collected. After catching, the fish were promptly transferred to the laboratory in polyethylene bags within 20 min for the collection of blood samples. Blood collection was realized following a standard ichthyology assessment [total length (TL), standard length (SL), and weight measurements] (Table 1). The animals were carefully managed by a capable individual in compliance with the regulations specified in the national law. All experiments were conducted in adherence to the guidelines set forth by the Ethics Committee for Protection of Animals Used for Scientific and Teaching Purposes of the Slovak University of Agriculture in Nitra. The Ethics Committee of the Slovak University of Agriculture in Nitra (SUA in Nitra) for the protection of animals used for scientific and educational purpose confirms that no special ethical approval was required for this type of experiment. During biological material collection, sampling of water and sediment from the experimental pond was also realized. To facilitate comparison with other authors who provided the total length (TL) of fish, the transformation equation is presented as follows: TL = 9.56889 + 1.15693*SL (r = 0.9932; r2 = 98.64%).
Sample collection
The sampling of fish (n = 36) was realized in the summer (n = 18) and autumn (n = 18) seasons from adult fish (4-year-olds). Blood samples were collected from the aorta ventralis of each individual and placed into three collection tubes. The samples were allowed to coagulate and subsequently centrifuged for 20 min at 1006 × g and 20 °C. Collected blood serum and blood clot samples were stored at -20 °C until analyses at the Institute of Applied Biology (SUA in Nitra, Slovakia).
Water and sediment samples were collected during fish harvesting. Sterile bottles were used for water sampling and were rinsed with pond water at the sampling site. About 200 mL of water was collected by immersing it to a depth of about 25 cm. Sediment samples were collected using a Snapper sediment sampler (about 150 g). All samples were collected from the inflow, middle, and dam parts of the pond. In each parts 4 samples of water and 4 samples of sediment were collected, covering litoral and pelagic zones. We took one extra sample of water from the inflow part of the pond due to the stronger water flow in this part. Samples were collected in duplicate and stored in a portable refrigerator at a temperature of 4 °C.
Measurements of serum biochemistry parameters and oxidative status markers
Sodium (Na), potassium (K), and chloride (Cl) ions were measured using the EasyLite analyser (Medica, Bedford, MA, USA) provided with an ion-selective electrode. Calcium (Ca), phosphorus (P), magnesium (Mg), urea, total proteins (TP), glucose (Glu), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), total bilirubin (Bili), cholesterol (Chol), triglycerides (TAG), uric acid (UA), and creatinine (Crea) were analysed using standard commercial kits from DiaSys (Diagnostic Systems GmbH, Holzheim, Germany) on the semi-automated clinical chemistry analyser Randox RX Monza (Randox Laboratories, Crumlin, UK) (Kovacik et al., 2017, 2019; Massanyi et al., 2014). Total bilirubin, creatinine, and uric acid values were recalculated to grams of total protein; thus, the outcomes are presented in terms of μmol/g total protein (Bili and Crea) and mg/g total protein (UA).
Reactive oxygen species (ROS) production was quantified by the chemiluminescence assay according to Kashou et al. (2013) and optimized for our laboratory conditions as in our previous studies (Kovacik et al., 2018, 2019; Tvrdá et al., 2016a, 2016b; Tvrdá et al., 2016a, 2016b). The results are expressed as relative light units (RLU)/s/g total protein.
Total antioxidant capacity (TAC) was assessed using the improved chemiluminescence antioxidant assay, which utilizes the horseradish peroxidase conjugate and luminol according to Muller et al. (2013) and is optimized for our laboratory conditions as in our previous studies (Kovacik et al., 2018, 2019). The outcomes are presented in terms of μmol of Trolox Eq./g of total protein.
The quantification of the carbonyl group was accomplished using the conventional 2,4-dinitrophenylhydrazine (DNPH) method. The expression of protein carbonyls is given in units of nmol per gram of total protein (Kovacik et al., 2019; Tvrda et al., 2017; Weber et al., 2015).
Lipid peroxidation (LPO) was measured by the quantification of malondialdehyde (MDA) production with the help of the TBARS assay, according to Tvrdá et al., 2016a, 2016b; Tvrdá et al., (2016a, 2016b). MDA concentrations are expressed as μmol per gram of total protein (Kovacik et al., 2019).
Glutathione peroxidase (GPx) activity was assessed using the Randox commercial kits (Randox Laboratories, Crumlin, Great Britain) and the semiautomated analyser Randox RX Monza (Randox Laboratories, Crumlin, UK). The findings are reported in units per gram of total protein (Tvrdá et al., 2016a, 2016b).
Determination of trace elements
The levels of selected trace elements (Al, Ba, Be, Bi, Cd, Co, Cr, Cu, Fe, Ga, Mn, Mo, Ni, Pb, Sr, Tl, and Zn) in various matrices (blood serum, blood clot, water, and sediment) were analysed using inductively coupled plasma optical emission spectrometry (ICP Thermo ICAP 7000 Dual (Thermo Fisher Scientific, Waltham, Massachusetts, USA)).
Pre-analytical procedure for ICP OES
Sample (blood serum and blood clot, about 500 mg) preparation for ICP OES analysis was carried out by closed-vessel microwave acid digestion with the high-performance microwave digestion system Ethos UP (Milestone Srl, Sorisole, BG, Italy) according to our previous studies (Kovacik et al., 2018, 2019). The same conditions for mineralization (amount of chemicals, time, temperature, and power) were used for sediment samples (about 500 mg). For this procedure, all the chemicals used had high purity grade to minimize contamination.
Water samples were measured directly after filtration using Whatman® qualitative filter paper, Grade 595 (basis weight 68 g/m2, thickness 150 µm, pore size 4–7 µm).
ICP OES analysis
Elemental determination of selected elements (Al, Ba, Be, Bi, Cd, Co, Cr, Cu, Fe, Ga, Mn, Mo, Ni, Pb, Sr, Tl, and Zn) was carried out using the spectrophotometer ICP Thermo ICAP 7000 Dual (Thermo Fisher Scientific, Waltham, Massachusetts, USA). A calibration curve was created using different dilutions (1:5, 1:25, 1:100, and 1:500) of Multielement Standard Solution V for ICP (Sigma-Aldrich Production GmbH, Switzerland) (Kovacik et al., 2018, 2019). The instrumental parameters of the determination with which it was carried out are listed in Table 2. Detection limits (LOD) of elements for blood serum (mg/kg), blood clot (mg/kg), sediment (mg/kg), and pond water (mg/L) were Al 0.0002; Ba 0.004; Be 0.001; Bi 0.020; Cd 0.0004; Co 0.003; Cr 0.017; Cu 0.003; Fe 0.002; Ga 0.026; Mn 0.0004; Ni 0.0002; Pb 0.009; Sr 0.002; Tl 0.005; and Zn 0.0006. Quantification limits (LOQ) of elements for blood serum (mg/kg), blood clot (mg/kg), sediment (mg/kg), and pond water (mg/L) were Al 0.001; Ba 0.013; Be 0.002; Bi 0.064; Cd 0.001; Co 0.008; Cr 0.055; Cu 0.011; Fe 0.052; Ga 0.085; Mn 0.013; Ni 0.001; Pb 0.032; Sr 0.007; Tl 0.016; and Zn 0.002. In all analyses, we used certified reference material for verification (CRM-ERM CE278K, Sigma-Aldrich Production GmbH, Switzerland).
Statistics
Before statistical analyses, we subjected the obtained data to normality tests using Kolmogorov–Smirnov. Subsequently, we evaluated the significance of the differences between the experimental groups divided based on the season in different matrices (blood serum or blood clot) using an unpaired t-test. Results are presented as the mean ± SD (standard deviation). Spearman R correlations were used to determine mutual associations between trace elements and biochemical markers. We also subjected the possible associations between selected trace elements and oxidative status markers to correlation analysis. The statistically significant correlations of this relationship were evaluated using regression analysis. A p value below 0.05 was set as statistically significant. STATGRAPHICS Centurion (© StatPoint Technologies, Inc., USA), GraphPad Prism 6.01 (GraphPad Software Incorporated, San Diego, California, USA), and Minitab® statistical software (Minitab Ltd., Coventry, UK) were used for statistical evaluation.
Results
In the present study, we used four matrices (blood serum, blood clot, water from the sampling area, and sediment from the sampling area) to quantify selected trace elements. Minimum and maximum concentrations are presented in Table 3. The most accumulated elements were Zn in the blood serum, Fe in the blood clot, Sr in the water, and Al in the sediment. The general trend of decreasing levels of trace elements in the blood serum samples was as follows: Zn ˃ Fe ˃ Sr ˃ Ba ˃ Ni ˃ Al ˃ Cu ˃ Be ˃ Co; < LOQ (below limit of quantification): Bi, Cd, Cr, Ga, Mn, Mo, Pb, and Tl; and in case of the blood clot, the scheme was as follows: Fe ˃ Zn ˃ Sr ˃ Al ˃ Ni ˃ Ba ˃ Cu ˃ Be ˃ Co ˃ Mn; < LOQ (below limit of quantification): Bi, Cd, Cr, Ga, Mo, Pb, and Tl. In the water samples, almost all monitored elements were below the limit of quantification, except for Al, Ba, Cu, and Sr. In the sediment samples, the values of the monitored elements were generally the highest, and the scheme was as follows: Al ˃ Fe ˃ Mn ˃ Ba ˃ Sr ˃ Zn ˃ Cr ˃ Cu ˃ Ni ˃ Pb ˃ Co ˃ Be ˃ Cd; < LOQ (below limit of quantification): Bi, Ga, Mo, and Tl.
The mean seasonal levels of trace elements in the blood serum and blood clot are presented in Fig. 2 (mean ± SD). Significant differences between the seasons were detected for concentrations of Ba, Co, and Zn in the blood serum and of Be, Co, Cu, Fe, and Sr in the blood clot. Overall, Ba, Be, Cu, Ni, and Sr content was higher in the blood serum, while Al, Co, Fe, Mn, and Zn content was higher in the blood clot.
Mean concentrations of blood biochemical parameters are reported in Table 4. Mg, Na, Cl, TP, glucose, AST, ALT, cholesterol, and triglyceride concentrations were significantly higher in the autumn season (p < 0.05). K level was significantly higher in the summer season (p < 0.05). Non-significant differences were found in the cases of Ca, P, urea, and ALP levels. The mean (± SD) concentrations of selected biomarkers (ROS, TAC, GPx, PC, MDA, UA, Bili, and Crea) among seasons are presented in Fig. 3. We did not record significant differences in this group of evaluated parameters.
The Spearman R correlation coefficients and linear or non-linear regression were used to evaluate direct relationships between trace elements and selected blood biomarkers. Statistically significant correlations between trace elements and serum biochemistry parameters (divided according to the season and matrices) are presented in Table 5.
In the summer season, negative statistically significant correlations between the serum Al, Ba, Fe, Ni, clot Sr concentrations, and Na were found. Positive (statistically significant) correlations were found between serum Al, Ba, Ni, Sr, and clot Cu, Sr vs. K. P positively correlated with serum Al, Ba, Cu, Ni, Sr, Zn, and clot Ba. Zn in the serum was positively correlated with Mg. Enzymes such as AST positively correlated with serum Al, Ba, Be, Fe, Sr, and clot Ni, and ALT positively correlated with serum Al, Fe, Ni, Sr, and clot Sr. Concentrations of ALP were positively correlated with serum Fe and Sr. Fe in the serum and Sr in the blood clot were positively correlated with TAG levels. Al and Be in the blood clot positively correlated with GGT levels. TP was positively correlated with the serum elements (Ba, Cu, Fe, Sr, and Zn). Positive statistically significant correlations between the serum Ni, clot Ba, Be, and Sr concentrations vs. urea were confirmed as well.
The correlation analysis in the autumn season showed a significant negative association between Cl and Be (clot). Cu in the blood clot is negatively correlated with glucose, Mg, AST, ALT, and urea. Glucose levels were also positively correlated with Fe in the serum and Al, Ba, and Be in the blood clot. Positive (statistically significant) correlations were found between serum Zn vs. P, Mg, Ca, AST, ALT, cholesterol, TP, and urea. Be in the blood clot significantly correlated with ALT, TAG, and urea. Serum Fe was also positively correlated with AST and urea levels. Positive and significant relationships were observed between Ni (clot) and P and Al (clot) and ALP.
Linear and non-linear regression analysis was used to explain the dependencies between trace element concentrations and oxidative status markers. The results of the regression analysis for the monitored parameters in the summer season are listed in Fig. 4. Strong statistically significant correlations were detected between Crea and serum Ni (0.612), as well as Fe (-0.576), Co (-0.609), and Cu (0.508) in the blood clot. A positive statistically significant correlation was observed between bilirubin and Al in the blood clot (0.574). A moderately negative (-0.497) significant association was detected between ROS and Cu in the blood clot.
Dependencies between trace element concentrations and RedOx status parameters in the autumn season are presented in Fig. 5. Strong or moderate statistically significant correlations were detected between UA and Ni (0.550), Fe (0.565) in the serum, and Al (0.719), Ba (0.754), Cu (-0.469), Mn (0.472), and Sr (0.563) in the blood clot. A moderately positive and significant association was detected between bilirubin and Sr in the blood clot (0.464), as well as GPx and Al in the blood clot (0.505). Creatinine levels were significantly associated with serum Fe (-0.637), Ba (-0.546), and Cu (0.613) in the blood clot.
Discussion
Due to the persistent pollution of the environment with various contaminants, it is increasingly important to monitor the concentration of dangerous substances in living and non-living nature. There are numerous studies based on biomonitoring of the content of trace elements in various fish matrices (blood, muscle, gills, liver, kidney, or spermatozoa) (Ansel & Benamar, 2018; Hansson et al., 2020; Karjalainen et al., 2020; Malik et al., 2010; Monferrán et al., 2016; Rajeshkumar et al., 2018). On the other hand, there is a relatively small amount or even a lack of studies describing possible associations of pollutants with various animal blood biomarkers in natural conditions. As such, the present study is dealing with the monitoring of trace element content in the blood serum, blood clots of grass carp, and in the pond water, and sediment. A substantial part of our study looks for relevant associations between the animal organism and trace elements, health status parameters, and markers of oxidative stress. For this type of study, we consider freshwater animals to be an ideal biological material, as they are in constant contact with their surrounding environment.
Monitored trace elements in different matrices
We monitored the content of various microelements over two seasons. Samples were not collected in the winter due to the hibernation of the fish, while we let the fish spawn in the university's experimental pond in the springtime. For these reasons, fishing took place from summer to autumn. We quantified Al, Ba, Cu, and Sr in water samples. Contamination of surface waters occurs primarily from natural sources, but anthropogenic sources of pollution (combustion processes, industrial activity, intensive agriculture, waste disposal, mining, and smelting) are the main reason for increased levels of toxic elements in the environment (Kolarova & Napiórkowski, 2021). The collected sediments from the selected site contained all monitored elements in measurable concentrations except for Bi, Ga, Mo, and Tl. In general, sediments form an ideal reservoir for the deposition of toxic elements, which is subsequently manifested in the accumulation of these substances in living organisms (Emenike et al., 2022). The basic determination of the content of microelements in blood serum may be counterploted with our previous study carried out on Cyprinus carpio (Kovacik et al., 2019). Similarly to our collected data, Zn and Fe were among the most accumulated, which is logical due to their general biological relevance and occurrence in the animal organism. A positive finding of this study lies in the undetected levels of traditional toxic elements (Cd and Pb) in the blood serum and blood clot of grass carp in comparison to our previous study conducted on the common carp. At the same time, concentrations of other elements such as Bi, Cr, Ga, Mo, and Tl were below the quantification limits. Our data confirm a seasonal impact on the contents of Ba, Be, Cu, and Sr (increased in the summer season) as well as Co, Fe, and Zn (increased in the autumn season). Currently, there is a lack of studies monitoring the content of trace elements in the blood of freshwater animals. Most biomonitoring studies are concerned with the metal content in tissues (especially in muscle) due to their potential health risks for consumers (Bosch et al., 2016; Dhar et al., 2023; Varol et al., 2022) without further biological relevance or physiological and/or pathological interactions. One of the few studies published to this date describes seasonal variations of selected elements in the muscle, gill, and liver of the freshwater fish Esox lucius. While the tendencies were different in comparison to our results, if we focus on the liver, the highest accumulation of most metals (Al, Ba, Cr, Cu, Fe, Pb, and Zn) was recorded in the spring and summer with a gradual decrease in autumn and winter (Erdoğan et al., 2021). Nevertheless, focusing not only on the tissues but also on the blood of fish, the content of elements and biomarkers can reveal various physiological indications and pathological changes (Emenike et al., 2022; Shahjahan et al., 2022a, 2022b).
Trace elements and serum biochemistry parameters
Previous studies primarily describe the accumulation of trace elements in water or in tissues associated with the haematological and biochemical functions of the organism. Khan et al. (2020) confirmed a direct association between increased liver enzymes (AST, ALT, ALP, and CK) and selected haematological parameters and a rise in the content of Cr, Mn, Fe, Ni, Cu, and Zn in the muscle, liver, and kidney of Oreochromis niloticus (Nile tilapia). Tlenshieva et al. (2022) observed genotoxic effects and histopathological changes in the liver of grass carp (Ctenopharyngodon idella) in metal (Pb, Co, Mg, Cd, Cu, Zn, and Fe) contaminated locations. The authors suggested a direct association of these changes (especially gill lesions) with the level of water contamination. In our study, we may discuss the associations of Al, Ba, Fe, Ni, Sr, Cu, or Zn with the mineral profile of the blood (Na, K, Mg, and P) in the summer season, as well as a possible response of liver enzymes (AST, ALT, ALP, and GGT). We also recorded an increased content of AST, ALT, cholesterol, and triglycerides in the autumn season, confirming association with concentrations of Fe, Zn, Cu, Be, and Al. The Zn content was significantly higher in the autumn period, while at the same time, its associations with increased concentrations of TP and urea were observed. Increased liver enzymes (ALT and AST) and cholesterol due to higher Cu levels were also noted by Öner et al. (2008) in Oreochromis niloticus. The effect of Zn on biochemical markers was not confirmed in their study, except for the association with increased cholesterol in the blood. In our already mentioned previous study carried out on common carp (Kovacik et al., 2019), several associations between blood minerals (Ca, Mg, Na, K, Cl) and trace elements were confirmed in the spring (Sr, Zn, Cd, Fe, Mn) and summer (Zn, Cr, Cu, Fe, Hg, Ni, As) seasons, followed by a negative correlation of Ni with the content of TP, Chol, and TG, or by a positive association of Cu vs. Chol or TG. According to Fırat and Kargın (2010), a single and combined metals exposure, represented by 5.0 mg/L Zn, 1.0 mg/L Cd, and 5.0 mg/L Zn + 1.0 mg/L Cd mixtures, was tested on a freshwater fish, Oreochromis niloticus, for 7 and 14 days, leading to an increase in TP, ALT, AST, albumin, transferrin, ceruloplasmin, cortisol, and glucose, and a decrease in cholesterol concentration. Banday et al. (2019) analysed the effects of trace elements on the health status of freshwater Clarias gariepinus. The authors obtained the fish from a river contaminated by several sources of contamination (power plants, agricultural waste, and domestic waste). The study monitored the content of Cd, Ni, Cu, and Cr in the integument, liver, blood, spleen, head-kidney, and thymus, where the highest content of the elements was found in the liver and blood. The authors noted changes in serum chemistry, haematology, hormonal levels, severe pathological alterations in immune organs, or elevations in hepatic and renal enzymes. In the meantime, Abdel-Tawwab et al. (2016) reported that exposure to sublethal Zn concentrations significantly increased glucose, AST, ALT, creatinine, cortisol, proteins, and lipid levels in Oreochromis niloticus (L.). On the other hand, Atli et al. (2015) recorded a decrease in the activity of ALP, cholesterol, and triglyceride levels due to acute (10 μM, 2 d) and chronic (20 μM, 20 d) exposure to Cd and Pb. Furthermore, the authors noted an increase in the concentration of glucose levels, partially due to the impact of Cd. Nevertheless, the results of serum chemical markers did not seem to be consistent enough. This may hinder the comparison of studies, especially because of the number of freshwater and marine species used in studies. Nevertheless, we consider blood biochemical markers to be a very important diagnostic tool for the early detection of various physiological changes and diseases caused by contaminants (Shahjahan et al., 2022a, 2022b).
Trace elements and oxidative status markers
RedOx (oxidative status/stress) markers are nowadays widely used to measure the extent of toxicity caused by environmental pollutants such as trace elements (Hinojosa-Garro et al., 2020; Kovacik et al., 2019; Shahjahan et al., 2022a, 2022b), endocrine disruptors (Jambor et al., 2018; Lee et al., 2019), pesticides (Clasen et al., 2018; Shahjahan et al., 2022a, 2022b), or microplastics (Alomar et al., 2017; Capó et al., 2022). This interpretation is based on the fluctuations of several markers, such as ROS, TAS, and enzymatic and non-enzymatic antioxidant defence systems. In our study, we monitored an entire complex of markers. As the main parameters, we evaluated ROS and TAC, supported by enzymatic (GPx) and non-enzymatic endogenous markers of oxidative stress (PC, MDA, Bili, UA, and Crea). Based on our findings, we may deduce a slight decrease in all monitored markers in the autumn season except for GPx, but without statistical evidence, which may indicate a relative resilience of the RedOx markers towards the external environment. Some interesting insights were provided by the correlation analysis evaluated by linear and/or non-linear regression, where the ROS levels were affected by the Cu content in the blood clot in the summer season. In general, several studies describe the redox potential of copper, which may be divided into two groups. The first one talks about the lack of copper in the organism, as a result of which oxidative stress can occur, whereas the second one describes increased concentrations of copper, followed by higher levels of ROS as well as changes in the enzymatic antioxidant system in aquatic animals (Baldissera et al., 2020; Do Carmo et al., 2018; El-Sharawy et al., 2022; Jiang et al., 2011; Lushchak, 2016; Machado et al., 2013; Nunes et al., 2015). Lakra et al. (2022) tested the overall health status of catfish (Clarias batrachus) reared in wastewater from a coal mine. When compared to the control group, the authors recorded increased concentrations of metals (Fe, Zn, Cu, Mn, Ni, Cd, Pb, and Cr) in all tested tissues (skin, air-breathing organ, gills, liver, kidney, and brain). Subsequent analyses revealed a negative effect on the content of all monitored biomarkers (glucose, glycogen, lipid, and protein), as well as on enzymatic parameters (AST, ALT, and ALP) and markers of oxidative stress (superoxide dismutase, catalase, and lipid peroxidation). Subsequently, after transferring the experimental fish to tap water, they observed a decrease in the activity of antioxidant enzymes as well as other health markers. In a different study monitoring Astyanax aeneus as a bioindicator of pollution exposed to the natural environment, in addition to the content of trace elements (Zn, Cd, Pb, Cu, Cr, Fe, Al, Mn, Hg, As, and V), the authors also observed associations with selected biomarkers (Hinojosa-Garro et al., 2020). Furthermore, the study revealed increased activities of glutathione S-transferase, metallothionein, catalase, and TBARs in selected locations, which could be directly related to the increased metal content.
In our study, we also noted associations between Ni, Co, Fe, and Cu and creatinine content, while Al was associated with bilirubin in the summer season. On the other hand, correlations amongst Ni, Fe, Al, Ba, Mn, Sr, and Cu with UA concentrations were confirmed; Sr was correlated with bilirubin; Fe, Ba, and Cu with creatinine; and Al with GPx in the autumn season. Seasonal variations of metal contents (Cr, Cd, Cu, Zn, Mn, and Fe) in water and their subsequent association with cichlid blood biomarkers were monitored by Ruas et al. (2008). In the contaminated site, the authors recorded increased levels of Cr, Cu, Zn, and Fe in the spring when compared to the autumn. These increased concentrations were subsequently associated with increased lipid peroxidation as well as SOD activity, accompanied by a partial decrease in the enzymatic activity of catalase and GPx and a reduction of glutathione. Giarratano et al. (2016) confirmed seasonal variations of Cd, Cu, Cr, Pb, Zn, Ni, and Al in tissues collected from the crab Neohelice granulata. Furthermore, they also found associations between these elements and markers of oxidative stress, specifically through the induction of glutathione in connection with Fe content or a significant effect of Al on MDA (lipid peroxidation).
Conclusion
A positive finding of the research task is the very low concentration of classical toxicants under the limits of detection (Cd, Pb, and Tl) in blood serum/clot and water samples. All monitored microelements except Bi, Ga, Mo, and Tl were present in the sediment. We noted differences in concentrations of elements in serum and/or blood clots based on season for Ba, Be, Co, Cu, Fe, Sr, and Zn. Subsequent correlation analysis of elements with biomarkers confirmed a direct positive association of Al, Ba, Be, Fe, Sr, and Ni with the content of liver enzymes, triglycerides, total proteins, and urea in the summer season. This means that with an increased content of these elements, there is an increase in these biomarkers in the blood serum, which we evaluate negatively, especially from the point of view of possible liver damage. In the autumn season, we found a weaker negative effect of elements (Be, Fe, Zn) on liver damage parameters (AST, ALT, ALP, cholesterol, TP), which could be caused by a higher content of essential Cu in the serum in this season, as we recorded a negative correlation of Cu with the content of AST, ALT, and urea. Cu also associated a decrease in reactive oxygen species (ROS) in the summer season, which we rate as highly positive in terms of possible cellular protection against oxidative stress. In the future, we would recommend continuing a similar type of study at other locations, especially with intensive industrial and agricultural activity, or in former mining locations, as well as in breeding ponds, where it is definitely necessary to monitor the concentrations of toxic and essential elements, but also other types of environmental pollutants such as persistent organic pollutants (POPs), which represent risks not only for animals but especially for humans. Another recommendation is to test a larger number of individuals in a group, as well as multispecies studies. However, there may be a problem with the interpretation of the results due to species specificities, such as the tolerance of the species to toxicants or other physiological mechanisms. Analyses of the tissues (gills, kidneys, and liver) of freshwater fish can also provide a deeper evaluation of the impact of toxic environmental elements. We assume that it will be possible to continue experimentally in this area in the future.
Data availability
The authors declare that the datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdel-Tawwab, M., El-Sayed, G. O., & Shady, S. H. (2016). Growth, biochemical variables, and zinc bioaccumulation in Nile tilapia, Oreochromis niloticus (L.) as affected by water-born zinc toxicity and exposure period. International Aquatic Research, 8(3), 197–206. https://doi.org/10.1007/s40071-016-0135-0
Alomar, C., Sureda, A., Capó, X., Guijarro, B., Tejada, S., & Deudero, S. (2017). Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environmental Research, 159, 135–142. https://doi.org/10.1016/j.envres.2017.07.043
Ansel, M. A., & Benamar, N. (2018). Accumulation of heavy metals in muscle, liver, and gonads of little tunny (Euthynnus alletteratus) from the western region of Algeria. Environmental Science and Pollution Research, 25(32), 32640–32648. https://doi.org/10.1007/s11356-018-3254-x
Atli, G., Ariyurek, S. Y., Kanak, E. G., & Canli, M. (2015). Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposures. Environmental Toxicology and Pharmacology, 40(2), 508–515. https://doi.org/10.1016/j.etap.2015.08.001
Baldissera, M. D., Souza, C. F., Barroso, D. C., Pereira, R. S., De Oliveira, F. C., Alessio, K. O., Wagner, R., Bizzi, C. A., Baldisserotto, B., & Val, A. L. (2020). Consequences of oxidative damage on the fatty acid profile in muscle of Cichlasoma amazonarum acutely exposed to copper. Fish Physiology and Biochemistry, 46(6), 2377–2387. https://doi.org/10.1007/s10695-020-00884-8
Banday, U. Z., Swaleh, S. B., & Usmani, N. (2019). Insights into the heavy metal-induced immunotoxic and genotoxic alterations as health indicators of Clarias gariepinus inhabiting a rivulet. Ecotoxicology and Environmental Safety, 183, 109584. https://doi.org/10.1016/j.ecoenv.2019.109584
Bosch, A. C., O’Neill, B., Sigge, G. O., Kerwath, S. E., & Hoffman, L. C. (2016). Heavy metals in marine fish meat and consumer health: A review: Heavy metals in marine fish meat. Journal of the Science of Food and Agriculture, 96(1), 32–48. https://doi.org/10.1002/jsfa.7360
Capó, X., Alomar, C., Compa, M., Sole, M., Sanahuja, I., Soliz Rojas, D. L., González, G. P., Garcinuño Martínez, R. M., & Deudero, S. (2022). Quantification of differential tissue biomarker responses to microplastic ingestion and plasticizer bioaccumulation in aquaculture reared sea bream Sparus aurata. Environmental Research, 211, 113063. https://doi.org/10.1016/j.envres.2022.113063
Clasen, B., Loro, V. L., Murussi, C. R., Tiecher, T. L., Moraes, B., & Zanella, R. (2018). Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Science of the Total Environment, 626, 737–743. https://doi.org/10.1016/j.scitotenv.2018.01.154
Dhar, P. K., Tonu, N. T., Dey, S. K., Chakrabarty, S., Uddin, Md. N., & Haque, Md. R. (2023). Health Risk Assessment and Comparative Studies on Some Fish Species Cultured in Traditional and Biofloc Fish Farms. Biological Trace Element Research, 201(6), 3017–3030. https://doi.org/10.1007/s12011-022-03386-1
Do Carmo, T. L. L., Azevedo, V. C., De Siqueira, P. R., Galvão, T. D., Dos Santos, F. A., Dos Reis Martinez, C. B., Appoloni, C. R., & Fernandes, M. N. (2018). Reactive oxygen species and other biochemical and morphological biomarkers in the gills and kidneys of the Neotropical freshwater fish, Prochilodus lineatus, exposed to titanium dioxide (TiO2) nanoparticles. Environmental Science and Pollution Research, 25(23), 22963–22976. https://doi.org/10.1007/s11356-018-2393-4
El-Sharawy, M. E., Mahmoud, A. A., Soliman, A. A., Amer, A. A., Mahmoud, M. A., Alkafafy, M., & Dawood, M. A. O. (2022). Studying the Influence of Copper on the Growth Behavior, Antioxidative Status, and Histology of the Intestine and Liver of Striped Catfish (Pangasianodon hypophthalmus). Biological Trace Element Research, 200(3), 1331–1338. https://doi.org/10.1007/s12011-021-02717-y
Emenike, E. C., Iwuozor, K. O., & Anidiobi, S. U. (2022). Heavy Metal Pollution in Aquaculture: Sources, Impacts and Mitigation Techniques. Biological Trace Element Research, 200(10), 4476–4492. https://doi.org/10.1007/s12011-021-03037-x
Erdoğan, K., Kandemir, Ş, Doğru, M. I., Doğru, A., Şimşek, I., Yılmaz, S., Örün, G., Altaş, L., Yazıcıoğlu, O., Korkmaz, N., & Örün, I. (2021). The effects of seasonal heavy-metal pollution of Ladik Lake on pike fish ( Esox lucius ). Biological Rhythm Research, 52(6), 821–845. https://doi.org/10.1080/09291016.2019.1607215
Fırat, Ö., & Kargın, F. (2010). Individual and Combined Effects of Heavy Metals on Serum Biochemistry of Nile Tilapia Oreochromis niloticus. Archives of Environmental Contamination and Toxicology, 58(1), 151–157. https://doi.org/10.1007/s00244-009-9344-5
Gárriz, Á., Del Fresno, P. S., Carriquiriborde, P., & Miranda, L. A. (2019). Effects of heavy metals identified in Chascomús shallow lake on the endocrine-reproductive axis of pejerrey fish (Odontesthes bonariensis). General and Comparative Endocrinology, 273, 152–162. https://doi.org/10.1016/j.ygcen.2018.06.013
Giarratano, E., Gil, M. N., Marinho, C. H., & Malanga, G. (2016). Metals from mine waste as potential cause of oxidative stress in burrowing crab Neohelice granulata from San Antonio bay. Ecotoxicology and Environmental Safety, 132, 68–76. https://doi.org/10.1016/j.ecoenv.2016.05.029
Gopal, V., Parvathy, S., & Balasubramanian, P. R. (1997). No title found. Environmental Monitoring and Assessment, 48(2), 117–124. https://doi.org/10.1023/A:1005767517819
Hansson, S. V., Desforges, J.-P., Van Beest, F. M., Bach, L., Halden, N. M., Sonne, C., Mosbech, A., & Søndergaard, J. (2020). Bioaccumulation of mining derived metals in blood, liver, muscle and otoliths of two Arctic predatory fish species (Gadus ogac and Myoxocephalus scorpius). Environmental Research, 183, 109194. https://doi.org/10.1016/j.envres.2020.109194
Hinojosa-Garro, D., Osten, J. R., & Dzul-Caamal, R. (2020). Banded tetra (Astyanax aeneus) as bioindicator of trace metals in aquatic ecosystems of the Yucatan Peninsula, Mexico: Experimental biomarkers validation and wild populations biomonitoring. Ecotoxicology and Environmental Safety, 195, 110477. https://doi.org/10.1016/j.ecoenv.2020.110477
Jambor, T., Greifova, H., Kovacik, A., Kovacikova, E., Tvrda, E., Forgacs, Z., Massanyi, P., & Lukac, N. (2018). Parallel effect of 4-octylphenol and cyclic adenosine monophosphate (cAMP) alters steroidogenesis, cell viability and ROS production in mice Leydig cells. Chemosphere, 199, 747–754. https://doi.org/10.1016/j.chemosphere.2018.02.013
Jiang, W.-D., Wu, P., Kuang, S.-Y., Liu, Y., Jiang, J., Hu, K., Li, S.-H., Tang, L., Feng, L., & Zhou, X.-Q. (2011). Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquatic Toxicology, 105(3–4), 543–551. https://doi.org/10.1016/j.aquatox.2011.08.012
Karjalainen, J., Arola, H. E., Wallin, J., Väisänen, A., & Karjalainen, A. K. (2020). Condition and Sperm Characteristics of Perch Perca fluviatilis inhabiting Boreal Lakes Receiving Metal Mining Effluents. Archives of Environmental Contamination and Toxicology, 79(2), 270–281. https://doi.org/10.1007/s00244-020-00752-9
Kashou, A. H., Sharma, R., & Agarwal, A. (2013). Assessment of Oxidative Stress in Sperm and Semen. In D. T. Carrell & K. I. Aston (Eds.), Spermatogenesis (Vol. 927, pp. 351–361). Humana Press. https://doi.org/10.1007/978-1-62703-038-0_30
Khan, Mohd. S., Javed, M., Rehman, Md. T., Urooj, M., & Ahmad, Md. I. (2020). Heavy metal pollution and risk assessment by the battery of toxicity tests. Scientific Reports, 10(1), 16593 https://doi.org/10.1038/s41598-020-73468-4
Kolarova, N., & Napiórkowski, P. (2021). Trace elements in aquatic environment. Origin, distribution, assessment and toxicity effect for the aquatic biota. Ecohydrology & Hydrobiology, 21(4), 655–668. https://doi.org/10.1016/j.ecohyd.2021.02.002
Kovacik, A., Arvay, J., Tusimova, E., Harangozo, L., Tvrda, E., Zbynovska, K., Cupka, P., Andrascikova, S., Tomas, J., & Massanyi, P. (2017). Seasonal variations in the blood concentration of selected heavy metals in sheep and their effects on the biochemical and hematological parameters. Chemosphere, 168, 365–371. https://doi.org/10.1016/j.chemosphere.2016.10.090
Kovacik, A., Tirpak, F., Tomka, M., Miskeje, M., Tvrda, E., Arvay, J., Andreji, J., Slanina, T., Gabor, M., Hleba, L., Fik, M., Jambor, T., Cisarova, M., & Massanyi, P. (2018). Trace elements content in semen and their interactions with sperm quality and RedOx status in freshwater fish Cyprinus carpio: A correlation study. Journal of Trace Elements in Medicine and Biology, 50, 399–407. https://doi.org/10.1016/j.jtemb.2018.08.005
Kovacik, A., Tvrda, E., Miskeje, M., Arvay, J., Tomka, M., Zbynovska, K., Andreji, J., Hleba, L., Kovacikova, E., Fik, M., Cupka, P., Nahacky, J., & Massanyi, P. (2019). Trace Metals in the Freshwater Fish Cyprinus carpio: Effect to Serum Biochemistry and Oxidative Status Markers. Biological Trace Element Research, 188(2), 494–507. https://doi.org/10.1007/s12011-018-1415-x
Kročková, J., Massányi, P., Sirotkin, A. V., Lukáč, N., & Kováčik, A. (2013). Nickel-Induced Structural and Functional Alterations in Porcine Granulosa Cells In Vitro. Biological Trace Element Research, 154(2), 190–195. https://doi.org/10.1007/s12011-013-9733-5
Lakra, K. C., Mistri, A., Banerjee, T. K., & Lal, B. (2022). Analyses of the health status, risk assessment and recovery response of the nutritionally important catfish Clarias batrachus reared in coal mine effluent-fed pond water: A biochemical, haematological and histopathological investigation. Environmental Science and Pollution Research, 29(31), 47462–47487. https://doi.org/10.1007/s11356-022-18971-z
Lee, J. W., Shin, Y.-J., Kim, H., Kim, H., Kim, J., Min, S.-A., Kim, P., Yu, S. D., & Park, K. (2019). Metformin-induced endocrine disruption and oxidative stress of Oryzias latipes on two-generational condition. Journal of Hazardous Materials, 367, 171–181. https://doi.org/10.1016/j.jhazmat.2018.12.084
Lushchak, V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology, 101(1), 13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Lushchak, V. I. (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biological Interactions, 224, 164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Lushchak, V. I. (2016). Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiology and Biochemistry, 42(2), 711–747. https://doi.org/10.1007/s10695-015-0171-5
Machado, A. A. D. S., Hoff, M. L. M., Klein, R. D., Cardozo, J. G., Giacomin, M. M., Pinho, G. L. L., & Bianchini, A. (2013). Biomarkers of waterborne copper exposure in the guppy Poecilia vivipara acclimated to salt water. Aquatic Toxicology, 138–139, 60–69. https://doi.org/10.1016/j.aquatox.2013.04.009
Malik, N., Biswas, A. K., Qureshi, T. A., Borana, K., & Virha, R. (2010). Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environmental Monitoring and Assessment, 160(1–4), 267–276. https://doi.org/10.1007/s10661-008-0693-8
Massanyi, P., Stawarz, R., Halo, M., Formicki, G., Lukac, N., Cupka, P., Schwarcz, P., Kovacik, A., Tusimova, E., & Kovacik, J. (2014). Blood concentration of copper, cadmium, zinc and lead in horses and its relation to hematological and biochemical parameters. Journal of Environmental Science and Health, Part A, 49(8), 973–979. https://doi.org/10.1080/10934529.2014.894322
Monferrán, M. V., Garnero, P., De Los Angeles Bistoni, M., Anbar, A. A., Gordon, G. W., & Wunderlin, D. A. (2016). From water to edible fish. Transfer of metals and metalloids in the San Roque Reservoir (Córdoba, Argentina). Implications associated with fish consumption. Ecological Indicators, 63, 48–60. https://doi.org/10.1016/j.ecolind.2015.11.048
Muller, C. H., Lee, T. K. Y., & Montaño, M. A. (2013). Improved Chemiluminescence Assay for Measuring Antioxidant Capacity of Seminal Plasma. In D. T. Carrell & K. I. Aston (Eds.), Spermatogenesis (Vol. 927, pp. 363–376). Humana Press. https://doi.org/10.1007/978-1-62703-038-0_31
Nunes, B., Caldeira, C., Pereira, J. L., Gonçalves, F., & Correia, A. T. (2015). Perturbations in ROS-related processes of the fish Gambusia holbrooki after acute and chronic exposures to the metals copper and cadmium. Environmental Science and Pollution Research, 22(5), 3756–3765. https://doi.org/10.1007/s11356-014-3580-6
Öner, M., Atli, G., & Canli, M. (2008). Changes in serum biochemical parameters of freshwater fish oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environmental Toxicology and Chemistry, 27(2), 360. https://doi.org/10.1897/07-281R.1
Özgür, M. E., Maraş, Z., & Erdoğan, S. (2019). The relationship between semen seminal plasma ions and sperm cell velocities of wild-caught longspine scraper, <i>Capoeta trutta</i>. Archives Animal Breeding, 62(2), 557–564. https://doi.org/10.5194/aab-62-557-2019
Phoonaploy, U., Tengjaroenkul, B., & Neeratanaphan, L. (2019). Effects of electronic waste on cytogenetic and physiological changes in snakehead fish (Channa striata). Environmental Monitoring and Assessment, 191(6), 363. https://doi.org/10.1007/s10661-019-7509-x
Rajeshkumar, S., Liu, Y., Zhang, X., Ravikumar, B., Bai, G., & Li, X. (2018). Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere, 191, 626–638. https://doi.org/10.1016/j.chemosphere.2017.10.078
Ruas, C. B. G., Carvalho, C. D. S., De Araújo, H. S. S., Espíndola, E. L. G., & Fernandes, M. N. (2008). Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicology and Environmental Safety, 71(1), 86–93. https://doi.org/10.1016/j.ecoenv.2007.08.018
Shahjahan, M., Islam, M. J., Hossain, M. T., Mishu, M. A., Hasan, J., & Brown, C. (2022a). Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Science of the Total Environment, 843, 156910. https://doi.org/10.1016/j.scitotenv.2022.156910
Shahjahan, M., Taslima, K., Rahman, M. S., Al-Emran, M., Alam, S. I., & Faggio, C. (2022b). Effects of heavy metals on fish physiology – A review. Chemosphere, 300, 134519. https://doi.org/10.1016/j.chemosphere.2022.134519
Tlenshieva, A. M., Witeska, M., & Shalakhmetova, T. M. (2022). Genotoxic and histopathological effects of the Ili River (Kazakhstan) water pollution on the grass carp Ctenopharyngodon idella. Environmental Pollutants and Bioavailability, 34(1), 297–307. https://doi.org/10.1080/26395940.2022.2101544
Tvrda, E., Mackovich, A., Greifova, H., Hashim, F., & Lukac, N. (2017). Antioxidant effects of lycopene on bovine sperm survival and oxidative profile following cryopreservation. Veterinární Medicína, 62(8), 429–436. https://doi.org/10.17221/86/2017-VETMED
Tvrdá, E., Tušimová, E., Kováčik, A., Paál, D., Greifová, H., Abdramanov, A., & Lukáč, N. (2016a). Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Animal Reproduction Science, 172, 10–20. https://doi.org/10.1016/j.anireprosci.2016.06.008
Tvrdá, E., Tušimová, E., Kováčik, A., Paál, D., Libová, Ľ, & Lukáč, N. (2016b). Protective Effects of Quercetin on Selected Oxidative Biomarkers in Bovine Spermatozoa Subjected to Ferrous Ascorbate. Reproduction in Domestic Animals, 51(4), 524–537. https://doi.org/10.1111/rda.12714
Varol, M., Kaçar, E., Sünbül, M. R., & Md. Towfiqul Islam, A. R. (2022). Levels of metals and elements in tissues of fish species in the Kızılırmak River (Turkey) and assessment of health risks and nutritional benefits. Environmental Research, 214, 113791. https://doi.org/10.1016/j.envres.2022.113791
Weber, D., Davies, M. J., & Grune, T. (2015). Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biology, 5, 367–380. https://doi.org/10.1016/j.redox.2015.06.005
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This work was supported by the Slovak Research and Development Agency under the contract No. APVV-21–0168. This publication was also supported by The Ministry of Education, Science, Research and Sport of the Slovak Republic under the project VEGA 1/0571/23.
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by Anton Kovacik, Martin Fik, Julius Arvay, Jaroslav Andreji, and Peter Massanyi. Material preparation and laboratory analysis were performed by Anton Kovacik, Eva Tvrda, Marian Tomka, Norbert Revesz, Julius Arvay, Martin Fik, Lubos Harangozo, and Eva Kovacikova. Data collection and statistical analysis were performed by Anton Kovacik, Lukas Hleba, Tomas Jambor, and Miroslava Hlebova. The funding of the experiments was provided by Anton Kovacik and Peter Massanyi grants. The first draft of the manuscript was written by Anton Kovacik and Eva Kovacikova. Final manuscript was reviewed and edited by Peter Massanyi, Jaroslav Andreji, Eva Tvrda, and Tomas Jambor. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethics approval
All authors have read and understood and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that, with minor exceptions, no changes can be made to authorship once the paper is submitted.
Consent to participate
All authors confirm to participate in this study.
Conflict of interest
The authors declare that they have no relevant financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kovacik, A., Tvrda, E., Tomka, M. et al. Seasonal assessment of selected trace elements in grass carp (Ctenopharyngodon idella) blood and their effects on the biochemistry and oxidative stress markers. Environ Monit Assess 195, 1522 (2023). https://doi.org/10.1007/s10661-023-12152-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-12152-2