Abstract
In the Awash River basin (Ethiopia), massive urbanization and industrialization, driven by rapid development and human settlement, are detrimental to the environment and human health as pollutants such as heavy metals (HMs) find their way into water bodies without proper treatment. The purpose of this study was to assess the HMs content and pollution sources within the basin. In this context, a total of 205 samples were collected from 21 surface water sampling stations. Heavy metal concentrations were measured using the Perkin Elmer NexION 350 ICP-MS with inductively coupled plasma. Findings demonstrate that high levels of HMs, such as Al, Mn, Mo, As, V, Fe, and Ba were exhibited with the value of 1257 μg/L, 626.8 μg/L, 116.7 μg/L, 61.2 μg/L, 100.5 μg/L, 1082.7 μg/L, and 211.7 μg/L, respectively. Among 20 HMs analyzed, 20% of the parameters within the study area were above the WHO limit for drinking water; Al (157 μg/L), V (100.5 μg/L), Fe (1082.7 μg/L), Mn (626.8 μg/L), and Mo (103.8 μg/L) were exhibited at sites along the river system. Likewise, 57% of water samples showed high values of As at many stations down the river systems. In particular, high HM concentrations seen in the upper Awash are primarily controlled by anthropogenic activities such as untreated industrial, agricultural, and domestic discharges, while the high HM concentrations in the middle Awash samples were likely due to the influence from the Lake Beseka that has high HM concentrations due to geological process. In conclusion, securing potable water for the rapidly increasing population in Addis Ababa and in the watersheds of Awash is unsafe to sustain the environment and the human health.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Wastes released from industry, domestic effluents, and agricultural runoffs have an impact on water bodies and are a subject of overgrowing concern worldwide. Heavy metals (metalloids) such as arsenic have severely contaminated the groundwater (GW) and surface water (SW) in Africa (Shaji et al., 2021). In addition to this, growing industrialization and urbanization (Das et al., 2009; Farkas et al., 2007; Wang et al., 2012) in the Awash River watershed has severely damage the ecosystem due to the toxin discharged into water bodies. Heavy metal (HMs) pollution in the SW has become a growing concern for the environment and people’s health (Ali & Khan, 2018; Huang et al., 2019; Jafari et al., 2018; Ravenscroft et al., 2009; Tiwari et al., 2015; Tiwari & Singh, 2014; Zhang et al., 2007). Pollutants come from a number of sources, primarily anthropogenic and geogenic (Bhuiyan et al., 2011; Muhammad et al., 2011; Ntengwe, 2006; Wei & Yang, 2010). When excessive amounts of pesticides and fertilizer are washed downstream by rain, they endanger aquatic life and have even been linked to the eutrophication of water hyacinth at Koka dam. Agricultural runoff may be a cause of HM pollution (As, Cd, Cu, Pb, U, and Zn) in aquatic bodies, and industrial disposal could also lead to high HMs such as As, Cd. Cr, Hg, Ni, Zn, and Pb concentrations.
Many countries have experienced similar problem, with anthropogenic sources like industrial wastewater such as mercury from Chlor-alkali plants, mining, and smelter wastes, such as arsenic and cadmium (Muibat et al., 2016), urban run-off (Alexandra et al., 2020), particularly lead, agricultural run-off, atmospheric deposition, and leaching from solid waste dumps (He et al., 2005; Kimbrough, 2009; Paul, Clement, et al., 2012) and natural sources (i.e, weathering of rocks, leaching of soils, volcanic ash). Anthropogenic activities like urban wastewater discharge contain a variety of pollutants including salts, metals, metalloids, pathogens, residual drugs, organic materials, and active residues of personal care products. The other potential sources relating to the water chemistry in a basin are the types of geologic materials that are present and the length of time that water is in contact with those materials. Natural activities in the form of oxide ores (Al, Mn, Sb) and sulfides ores (Fe, As, Pb, Zn, Cu, and Ni) and atmospheric deposition (As, Cd, Cr, Cu, Pb, Hg, and U) have shown the impact to the water chemistry from geological processes in the basin. For instance, in the middle valleys of the Awash Basin, the movement of water between GW and SW provides a major pathway for chemical transfer between terrestrial and aquatic systems (Tesedenya, 2018), which greatly affects the lake water qualitatively as well as quantitatively (Kalbus et al., 2006; Lamontagne et al., 2005; Schmidt & Schubert, 2007).
Industrial waste discharged into nearby bodies of water, such as rivers, raises water temperature and slows organism metabolism. This increases their need for oxygen. The smell of heavily polluted rivers like the Modjo, Akaki, and Atbella Rivers is intolerable. The discharge of effluent from wastewater treatment plants (WWTPs) has major detrimental effects on the health of aquatic ecosystems (Abebe et al., 2023; Yimer et al., 2020). Despite numerous studies showing that the concentration of pollutants, such as HMs, in streams has been rising and degrading the basin's water sources. There is still debate as to how much large- and small-scale agriculture has been practiced in the region (urban and peri-urban areas). However, almost all scales of agriculture are using river water for agricultural uses. In addition, the river Awash is also serving a source of domestic water supply (for Adma town and Methara town). Hence, depending on the chemical composition of the minerals that are weathered, the relative abundance of the major inorganic chemicals dissolved in the water changes, and the water chemistry of surface waters, including lakes and rivers, has been affected by the pollutants drained to them (Lewis et al., 2021) Once polluting substances are introduced into river systems, they are transported and transformed by physical, chemical, biological, and biochemical processes. Most likely, the widest spread of geogenic contamination affects human health significantly, with impacts such as elevated concentrations of As (Smith et al., 2000; Annette et al., 2008), non-specific health effects, causing cancer (Murgo, 2001), and also a detrimental impact on a human (Kim & Kim, 2015; Zeng et al., 2019).
As a result, the surface water bodies are becoming polluted, due to potential sources combined in a river system contaminating water resources and posing a serious water security problem in the basin (Esayas & Bernd, 2009). The effects of consuming HMs from various indirect sources such as in soil (Esmaeili et al., 2021; Sun et al., 2013), vegetables (Fathabad et al., 2018), food items (Fakhri et al., 2018), and food chains (Muchuweti et al., 2006) were detected as a result of polluted water-based agriculture. Exposure to these kinds of environmental contaminants thereby raises the likelihood of environmental and human health concerns (Fei et al., 2018). The effects of consuming HMs from various indirect sources such as in soil (Esmaeili et al., 2021; Sun et al., 2013), vegetables (Fathabad et al., 2018), food items (Fakhri et al., 2018), and food chains (Muchuweti et al., 2006) were detected as a result of polluted water-based agriculture. Exposure to these kinds of environmental contaminants thereby raises the likelihood of environmental and human health concerns (Fei et al., 2018). In order to understand the water quality (WQ) dynamics of the ARB and to ensure safe drinking water, sampling has been carried out by the Awash Basin Authorities, for the analysis of physicochemical issues (Yimer & Geberekidan, 2020; Yimer & Jin, 2020), nutrient problems including eutrophication (Bussi et al., 2021), and salinity (Jin et al., 2021). However, the HMs determination and associated concerns with human health and environmental impacts and their concentrations have not been studied extensively. Thus, the purpose of this study was to evaluate the spatial and temporal variations of HMs and their potential sources in the Awash basin using combined field sampling, laboratory analysis, and statistical analysis.
Materials and methods
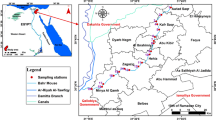
Description of the study area
The Awash Basin is the most developed, utilized, abused, impacted, and most populous (over 15% or nearly 18.6 million out of 120 million) basin in Ethiopia (Kebede et al., 2021). Awash River basin is the fourth and seventh in terms of area and volume of water, respectively. The basin covers a total area of 112,000 km2 and has an annual flow of 4.9 billion m3. More than half of the basin is covered by this study, which consists of the upper and middle valleys of the basin Awash (Fig. 1). The research area spans more than half of the basin and includes Awash 7 Kilo, Ambo, Sebeta, Bishoftu, Gelan, Adama, Modjo, and other towns, as well as the major city of the country Addis Abeba. It also contains more than 50% of the potential polluting industries. These include tanneries, paint factories, slaughterhouses, textiles, breweries, soft drink factories, sugar factories, hospitals, and pharmaceuticals. Additionally, the research area is vulnerable to water contamination, scarcity, and flooding.
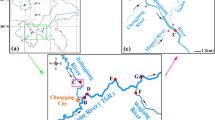
Study design Beseka
Water bodies, including tributaries, the Koka Dam, and Lake Beseka, as well as gauged and ungagged stations along the main Awash River basin, were sampled. Grab sampling methods were frequently used to take samples in the middle of the month. To reduce the risk of contamination, the plastic bottles were rinsed. Samples were collected on February 2020 and April, June, July, and October 2021. The sampling frequency was carried out twice a year for HM analysis (20 HMs) and four times a year for physicochemical analysis (some WQ parameters), depending on the spatial and temporal variability of the basin.
Sampling stations/study area
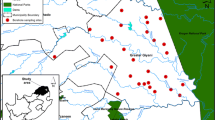
In three sub basins of the Awash basin, sampling locations were chosen based on accessibility, pollution load, the presence of unsettling influences, the availability of a stable stream bed, safety, and security. For this task, a total of 125 physicochemical samples and 80 HMs samples were collected from 21 surface water and groundwater locations for 18 months.
Sample collection procedures
The representative water sample was collected using polyethylene plastic bags and the prescribed procedures. As a result, a sample of water was obtained from 21 stations using grab sampling methods. Before collecting 1.5 l of water samples from the sample stations for the majority of physicochemical and heavy metals analyses, the sample containers were rinsed three times using distilled water. This was done to prevent any cross-contamination from previous samples. We left an airspace equivalent to about 1% of the container volume to account for thermal expansion during transport. Sample containers were sealed using self-adhesive paper that includes the sample ID, time, and date of collection, in order to detect unauthorized tampering with samples up until the time of analysis. Samples were acidified and air-freighted from Ethiopia to the Department of Earth Sciences at Oxford University, UK, and allowed to equilibrate overnight to permit re-dissolution of any precipitates or materials adsorbed to the bottles.
Data quality management and analysis
Measurements of Mo, As, Sr, Ba, Fe, Cr, Al, Mn, U, V, and Zn were conducted by inductively coupled plasma mass spectrometry (ICP-MS) using a Perkin Elmer NexION 350D instrument, which was coupled with an Elemental Scientific prepFAST M5 autosampler (Table 1). For the study, the instrument was calibrated using the method of external calibration, where the concentration for the measured sample set was extrapolated from linear regressions generated from raw counts per second data from a series of standards. All blanks, standards, and samples were diluted using 2% v/v nitric acid (HNO3−) and doped Rn, In, Ir, and Re internal standards to normalize for any instrument drift. Additionally, for quality control purposes, an external standard was diluted and measured from a custom-bought-blended QMx multi-elemental standard to verify the calibrations. The certified reference materials (CRMs) SLRS-6 (river water standard) from NRC Canada and SPS-SW2 surface water standard LGC-UK were also measured in conjunction with the samples.
Laboratory analysis
In situ analysis was made to analyze pH, temperature, electrical conductivity (EC), and total dissolved solids (TDS) using a multi-meter and a partly portable Palintest micro 800 multi-meter, while alkalinity, bicarbonate, carbonate, and total hardness (TH) were measured using a 7500 photometer. At present, different techniques have been used for the determination of HMs, including AAS, XRF, ICP-MS/OES, and AAS (Al-Saydeh et al., 2017). In the study, ICP-MS was used to determine the concentration and types of HMs present in the SWs of the ARB.
Statistical analysis
The Pearson correlation method was used to determine the correlation coefficient (r) between the variables. Analysis of SW samples was compared to WHO limits. The Turkey-Kramer test was used to compare the quality of SWs across all sites using an analysis of variance at a 5% significance level. Data analysis was made using SPSS version 23 and Minitab statistical package version, which were used for the day to identify the sources of actions and assess the commonalities; principal component analysis and factor analysis were utilized (Ashayeri et al., 2018; Yang et al., 2018).
Results and discussions
Physicochemical parameters (pH, EC, TDS, TH, and Alk) of surface water samples
The total dissolved solid (TDS) concentration proves a measure of dissolved inorganic chemicals present in the water samples. In the study, the highest TDS was observed in SW13 at 2482 mg/L in the dry season exceeding the threshold of WHO (1500 mg/L). This high value of TDS indicates high salinity and makes the SW of Lake Beseka (LB) less suitable for drinking and irrigation uses. Salinity is the cumulative sum of the cations and anions present in the water that has a significant impact on the soil salinity and degrades the palatability of the surface water. In fact, salinity does not cause serious health effects as compared to other geogenic contaminants, however, which is a potential indicator for the presence of dissolved ions in the water samples. A high TDS value was also observed in SW1. The pH ranged from 7.86 to 9.5. This high pH value (9.5) was observed at station SW13. The concentrations have good associations with GW pH, being greater under alkaline conditions, regardless of whether oxic or anoxic (Ayotte et al., 2011; Smedey & Kinniburgh, 2001). Similarly, the highest EC value (3650 μS/cm) was observed in SW13 (Table 2). It was above the maximum allowable limits of WHO. While the lowest EC value of 172 μS/cm was observed at SW6, in the wet season.
Physicochemical parameters (pH, EC, TDS, F, Cl, and Alk) of groundwater samples
Out of sixteen GW samples collected from the middle valley of Awash, seven samples were particularly selected to compute the quality of the GW and the SW interactions in the LB catchment. Accordingly, the findings show that high concentrations were exhibited with the mean and range values pH 8.74 (10.15 to 8.21), EC 3152 μS/cm (15645 μS/cm to 517 μS/cm), TDS 2099 mg/L (10826 mg/L to 268 mg/L), fluoride 7.85 mg/L (18.2 mg/L to 1.97 mg/L, chloride 244.54 mg/l (412.5 mg/L to 117.5mg/L), and alkalinity 885 mg/L (1600 mg/L to 225 mg/L) all are above the limits of WHO. For instance, high values of fluoride were seen in stations GW1 (18.2 mg/L) and GW2 (12.45 mg/L) and also in GW8 (13.5 mg/L), GW11 (15.55 mg/L), GW12 (12.15 mg/L), and GW13 (15.35 mg/L) were exhibited near LB (deep wells) and in Amibera irrigation sites (piezo stations), respectively. Previous studies indicate that the lake water chemistry has been mainly affected by the GW interaction or recharge due to GW to SW flow because; GW flux is the major component of the lake’s water input Dinka, 2020).
Spatial and temporal variability of heavy metal concentration
The spatial and temporal distribution of HMs and metalloids in the SWs was evaluated (Table 3). The range of these HMs (metalloids) in SWs was distributed evenly and found highly significant variability. For instance, the mean concentration of HMs increased as going from upstream catchments starting at station SW1 into downstream stations like SW21. The concentrations of these HMs (metalloids) (Ti, Cr, Co, Ni, Cu, Zn, Ge, Rb, Sr, Ba, Sb, Pb, and U) were within the WHO limits (Fig. 2), while the concentration of HMs (metalloids) like Mn, Mo, As, Al, V, and Fe in the sampled waters exceeded the WHO standards (WHO, 2011).
Heavy metal concentration
Table 4 contrasts the findings from the same watersheds and those from the Awash River basin. Samples from the study showed higher average concentrations for most compared heavy metals, with the exception of some elements like Fe and Zn, according to a comparison between other basin-wide studies in the Awash River basin. The concentration of Cr, As, Ni, and Mo in this study is higher than the mean values in reports from previous studies, according to a comparison with data from previous studies.
Aluminum (Al), iron (Fe), and manganese (Mn)
Naturally, the concentration of iron (Fe) in SW bodies is high. However, exceptionally the greatest portion of Fe above the recommended guideline values was exhibited in stations SW1, SW13, SW14, SW15, SW11, and SW9 with high values of 1082.7 μg/L (> threefolds compared to the allowable limit 10 μg/L), 631.3 μg/L, 598.7 μg/L, 459.4 μg/L, 379.6 μg/L, and 343.9 μg/L, respectively. Correspondingly, high concentrations of Mn varied from 626.8 μg/L to 4.41 μg/L were recorded. Manganese (Mn) exceeded the respective threshold values (100 μg/L) in SW3 (at Modjo River after industrial/tanneries wastes) 626.8 μg/L. Similarly, high values of Mn in stations SW11 (at Methara sugar factory mill waste) and SW12 (at Merti camp sewage discharge) exhibited 173.5 μg/L and 132.8 μg/L, respectively. The sources of pollution are mainly associated with industrial waste (SW3), sugar factories’ mill wastes (SW11), and sewage discharge (SW12) (Fig. 3).
In the study, the most common pollutants, namely, Zn, Cr, Co, Hg, As, Cu, Ni, Pb, and others like Fe, Mn, V, U, Sr, Sn, and Al, were analyzed. For instance, the highest concentrations of Al above the WHO limits were exhibited at stations SW1 (> sixfolds), SW13 (> fourfolds), SW14 (> fourfolds), SW9 (> threefolds), SW15 (> two and a half folds), SW20 (> two and a half folds), SW19 (> twofolds), SW11 (> twofolds), SW6 (> twofolds), SW8, and SW21 with the values of 1257 μg/L, 816 μg/L, 758.8 μg/L, 630.5 μg/L, 556.2 μg/L, 549.2 μg/L, 450.7 μg/L, 449.4 μg/L, 402.2 μg/L, 297.7 μg/L, and 273.9 μg/L, respectively. It implies that over 50% of water samples showed above the WHO limits (WHO, 2011). Perhaps, this spatial variability in stations SW1, SW6, SW8, SW9, and SW11 might come from industrial sources, in stations SW13, SW14, SW15, SW18, and SW19 natural sources (or leaching of soils, geochemistry), whereas stations SW18, SW19, SW20, and SW21 might be associated with the flux discharge come from industrial sources from LB.
Due to natural process, localized enrichments of some elements, namely, Al, Mn, Cu, Zn, Sr, and Fe, were found with a value of 449.4 μg/L, 173.5 μg/L, 119 μg/L, 100 μg/L, 382 μg/L, and 343.95 μg/L, respectively. In the study, samples that impacted HMs pollution were mainly from industrial wastes derived (upstream Koka) and rock weathering (GW-SW) interactions. Titanium (Ti) ranged from 50.57 to 0.86 μg/L with mean values of 11.8 μg/L. A high concentration of Ni was observed in SW1 and SW17 with a value of 13.38 μg/L and 0.81 μg/L, respectively, with a mean value of 2.81 μg/L. Cobalt (Co) varied 5.03 μg/L and 0.12 μg/L in stations SW1 and SW17 respectively. The assessment of Ba exhibited high concentration in SW1, ranging from 211.7 to 10.56 μg/L (SW17) with a mean value of 53.24 μg/L. The concentration of HMs, namely, Ti, Co, Ni, Ge, Rb, Sr, Ba, Sn, Sb, and Pb, exhibited below the limits. The concentration of Ti was high in stations SW13, SW14, SW15, SW16, and SW17 due to natural activities. It varied from 50.57 μg/L (SW13) to 0.86 μg/L (SW7) with a mean value of 11.79 μg/L (Fig. 4). The amount of Ge ranged from 1.52 μg/L (SW17) and 0.12 μg/L (SW2) with a mean value of 0.50 μg/L. A high Rb concentration was exhibited in station SW17 (31.77 μg/L) and a low concentration in SW7 (3.26 μg/L) with a mean value of 12.2 μg/L.
Vanadium (V) and uranium (U)
The uneven distribution of HMs such as V and U tends to increase slightly downstream of LB. The concentrations of V and U varied from 100.5 to 3.24 μg/L and 10.88 μg/L and 0.49 μg/L, respectively. High concentrations of V and U were exhibited in station SW1 due to industrial and urban wastes from Addis Ababa. Both V and U were high in LB, due to the features of the lake catchment, the aquifer, and volcanic ash. In the study, particularly in these two areas, high levels of HMs have been identified. Most of the heavily concentrated area is LB due to rock weathering.
Tin (Sn), antimony (Sb), lead (Pb), copper (Cu), nickel (Ni), and barium (Ba)
All samples of Sn, Sb, and Pb did not exceed the WHO. The concentration of Sn ranges from 0.99 μg/L to not detected (in SW1, SW8, SW9, SW10, and SW11) and with a mean value of 0.39 μg/L. The concentration of antimony (Sb) varies from 6.76 μg/L to not detected significant value in station SW5 and had a mean value of 1.78 μg/L. The concentrations of Pb in many of the samples were lower than the WHO permitted limit, ranged 2.42 μg/L (SW1) and 0.32 μg/L (SW21) with a mean value of 1.23 μg/L. The sources for the high concentration of Pb, Sn, Co, Ni, and Ba in stations SW1, SW2, SW1, SW1, and SW1, respectively, are anthropogenic sources, including industrial wastes, domestic wastes, sewage, and agricultural runoff. As shown in Fig. 5i, ii, contour plots were used to explore the relationships between variables.
Arsenic (As) and molybdenum (Mo)
In natural waters, As is mostly found in its inorganic form as oxyanions of arsenite As(III) or arsenate As(V). However, both forms are highly toxic inorganic species (Fendorf et al., 2010). In the study, the findings in Table 3 exhibited that the highest values of As above the limit of WHO (10 μg/L) were recorded in 57 % of water samples collected from stations SW1 (23.01 μg/L), SW3 (10.9 μg/L), SW11 (10.18 μg/L), SW12 (23.75 μg/L), SW13 (57.22 μg/L), SW14 (43.37 μg/L), SW15 (35.12 μg/L), SW16 (46.62 μg/L), SW17 (61.23 μg/L), SW18 (17.67 μg/L), SW19 (11.98 μg/L), and SW21 (10.89 μg/L). This heterogeneous spatial distribution of As in SW of ARB mainly derives from natural activities in the middle valley of Awash. The geogenic or volcanic ash contamination may be the cause for the elevated concentration of As in LB (Alcaine et al., 2020; Frascoli & Hudson-Edwards, 2018; Smedey & Kinniburgh, 2001).
The sources might also be the leaching of soil, volcanic ash, and rock weathering in the lake catchment and river waters, where As(V) is the dominant species. Therefore, the main sources for the presence of a high concentration of As in the SW of the ARB might be anthropogenic in SW1, SW3, and SW11. While the sources of As in stations SW12, SW13, SW14, SW15, SW16, and SW17 were predominantly geogenic; tectonic, clay, volcanic ash, and sand weathering phenomenon. Shockingly, high concentrations of As in stations SW18, SW19, and SW21 in the LB area were associated with the lake-river interaction and the discharge of the lake water flux into the ARB. Rhyolitic and volcanic ash in the Ethiopian Rift Valley (WHO, 2008), being a possible natural contaminant of GW sources (ARSLAND, 2006), elevate the concentration of As(V), which is predominantly accompanied by high V, Mo, and U concentrations (Alcaine et al., 2020; Smedley & David, 2017). Arsenic (As) is considered by some researchers to have more serious health effects than any other environmental contaminant (Smith & Steinmaus, 2009).
Molybdenum (Mo) occurs naturally in minerals, rocks, and soils as well as in aqueous form (with oxidation states of IV and VI) (Paullin et al., 2001). This study found that SW had a high concentration of Mo. Some, though not all, have intermixed felsic volcanic ash, which potentially contributes to an enriched and labile Mo source in Ethiopia. The highest amounts of Mo were recorded in six different stations; except SW1, the other five stations are from LB including SW13, SW14, SW15, SW16, and SW17 with the content of 103 μg/L, 88.9 μg/L, 90.98 μg/L, 116.7 μg/L, 111.7 μg/L, and 91.95 μg/L, respectively. The potential source of Mo for SW1 might be anthropogenic and industrial waste. While the remaining five stations are mainly associated with the geogenic natured of the studied water or/and the relative enrichment. Its source of pollution is totally geogenic, weathering, leaching, and volcanic eruptions have been reported (Paul, Clements, et al., 2012).
The lake waters have correspondingly high concentrations of U and V, F, and Mo 90 (Klemperer & Cash, 2007; Smedley et al., 2017). A large range of Mo concentrations was seen in lake waters, depending on ambient redox, pH, and salinity variations (Reimann et al., 2002). The range of Mo was found to be from 1.30 to 108.69 μg/L, which can be toxic in high doses. The highest values of Mo 116.7 μg/L (>70 μg/L) and As 61.2 μg/L (sixfolds greater than the allowable limits of WHO (>10 μg/L), were obtained in LB. Therefore, the measured highest concentrations of Mo and As at LBs stations might be due to the inflow of water (interaction of surface and subsurface). Surprisingly, unexpected levels of Mo, As, V, and U were observed in the same sites (SW1, SW13, SW14, SW15, SW16, and SW17). Of these six stations, five of them are from LB. Earlier, the highest values were exhibited by studies, with the mean value of Mo (246 μg/L) and As (41.2 μg/L) (Klemperer & Cash, 2007), Mo 250 μg/L, and As 67.3 μg/L were recorded in LB (Smedley et al., 2017). Thus, this situation boosts the mobility of Mo and which has some notable overlap with that of a number of other anions/oxyanions (Smedey & Kinniburgh, 2001; Smedley & Kinniburgh, 2002). Notably, Mo, U, V, and As revealed similar spatial and temporal homogeneity. The feasibility of Mo, and correlated with U, V, and As, with a significant Pearson correlation of 93% (r = 0.929), 93% (r =0.93), and 74% (r =0.741) respectively.
Principal component and factor analysis
As seen in Fig. 6, there was no point above the reference line, so this implies that the data analyzed did not significantly affect the analysis. The rotated factor loading analysis in factor one shows that high loading values of U, Mo, V, As, Ti, and Rb, had high influence values of 0.98, 0.97, 0.95, 0.918, 0.896, 0.549, and 0.525, and how large positive loading factor exhibited in factor one respectively. So, this factor indicates similar sources, which might be the natural activities (weathering of sedimentary rocks) and might leathering soils. In factor two, Ti had a small positive loading value of 0.364 and had a weak influence on the variables. Factor four, Cr, Mn, and Ba showed positively large loading factors with 0.93, 0.73, and 0.344 respectively. In sum, the loading results of the factors, and together all factors explained 0.804 or 80.4% of the variation of data.
Source apportionment of heavy metals
Sources of heavy metal pollution before Lake Beseka mix (BLBM)
In the study, among 20 HMs analyzed, 20% of the parameters within the catchments (study area) were above the WHO limit, i.e., Al (1257 μg/L), V (100.5 μg/L), Fe (1082.7 μg/L), Mn (626.8 μg/L) and Mo (103.8 μg/L) at station SW1. Likewise, the highest values (even under the limit) of Cr (23.22μg/L), Co (5.03 μg/L), Ni (13.4 μg/L), Cu (310.99 μg/L), and Ba (211.7 μg/L) were recorded in stations SW3, SW1, SW1, and SW6, respectively (Fig. 7). Arguably, the main sources of pollution in the upper Awash River basin come from industrial and urban wastes, agricultural runoff (pesticides, fertilizers), and sewage discharge. The untreated urban and industrial wastes and agricultural runoff potentially contribute as primary sources and are also responsible for the incidence of HMs in the upstream Koka. Therefore, human activities and poor wastewater management contributed to the increasing concentrations of pollutants and resulted in the deterioration of the receiver water.
Sources of heavy metal pollution at Lake Beseka (LB)
Pollution sources in Lake Beseka water are mainly related to the geological properties of lake water as a fundamental source. The geology, geochemistry, and rift features of the lake basin may relate primarily to volcanic ash, a potential source of HMs in groundwater. As shown in Fig. 6, the water samples analyzed show high concentrations of HMs (metalloids). The values displayed on these stations exceeded WHO limits such as As, V, Mo, and U. Seasonal variations in HMs in lake water are directly related to variations in GW concentrations. It may be present due to the subsurface and groundwater interaction with the lake water; hereby, the sources of HMs concentration mainly come from the catchment geology and volcano cases. Accordingly, high concentrations of HMs such as V, As, and Mo exhibited in stations SW13, SW14, SW15, SW16, and SW17 with a concentration of 69.87 μg/L, 57.2 μg/L, and 62.03 μg/L; 72.78 μg/L, 43.4 μg/L, and 90.98 μg/L; 71.72 μg/L, 35.1 μg/L, and 116.66 μg/L; 61.97 μg/L, 45.6 μg/L, and 111.7 μg/L; and 94.19 μg/L, 61.2 μg/L, and 91.95 μg/L, respectively.
Correlation and regression between heavy metals
As shown in Table 3, ANOVA was performed, by using SPSS (Version 23) at a 95% confidence interval to determine the differences among the samples. The correlational analysis including Pearson’s correlation is an important basis for determining the sources of HMs. So, it was used to evaluate the correlation matrix between HMs (metalloids) in the SW within the basin. Table 3 illustrates a strong positive statistical correlation between Ge vs V, Ge vs Rb, Rb vs U, Ba vs Ni, and, Fe vs Al, with correlation coefficients of 0.934 (93.4%), 0.923 (92.3%), 0.913 (91.3%), 0.928 (92.8%), and 0.92 (92.0%), respectively.
Similarly, the significant spatial differences (ρ < 0.05) between Ge and U, As and Rb, As and Ge, Ba and Co, As and U, Ti and Fe, and As and V were positively correlated with 80.6%, 81.8%, 78.7%, 78 %, 77%, 72.4%, and 70.7%, respectively. The proportion of variation was computed using R squared (R2) (Fig. 4). The proportion of variation was also computed using R squared (R2) (Fig. 8a–h). The figures prove that Al and Fe, As and Mo, As and V, Mo and V, Mo and Rb, Mo and U, Co and Ni, Ni and Ba, and account for 84.7%, 73.8%, 70.5%, 89%, 92.6%, 89.5%, 93.6%, and 90.3% of the variation respectively. Therefore, a plot of HMs (Fig. 8a–h) showed a strong linear fit, and the data obtained (R2) coefficients between the aforementioned parameters have a common origin (Yuanan et al., 2013).
Sources of heavy metal pollution after Lake Beseka mix (ALBM)
In the potential pollution sources of HMs (E.g As, Mo, V, and U) after Lake Beseka mix (ALBM), in stations SW18, SW19, SW20, and SW21, the source of pollution comes from the discharge of LB and the wastes discharged from sugar factory mills and Merti camp’s sewage discharge. Even though, the SW11 and SW12 and also the upstream discharge have an effect on it. Perhaps, the geogenic activities surrounding LB pollute the river water downstream of Methara. Already many years ago, the lake connected with the river water through an artificially constructed channel and enabled a dynamic exchange of underground materials (Furi, 2011). Degradation of LB SW chemistry due to GW inflow is becoming an urgent issue. This could be a possible source of high HM concentrations at stations SW13, SW1, SW15, SW16, and SW17. Although sources such as municipal waste (SW11), sugar factory waste (SW12), geogenic sources (SW13-SW17), and anthropogenic discharges can pollute the river at stations SW1, SW2, SW3, SW4, SW5, and SW6, in sum, due to daily activities, untreated sewage from industry, households, and municipalities has degraded water resources. Interactions between groundwater and surface lakes in Ethiopia’s Main Rift Valley and possible HM contamination in lake water samples showed the possibility of HMs increasing in lake water. However, due to the sparse data in Ethiopia’s Awash Basin HMs levels were not well studied on LB. Therefore, this study was conducted to evaluate the interaction between GW-SW and HM occurrence via groundwater interaction across the LB and AR.
Conclusion
Spatial variability and an uneven distribution of HMs were observed in the Awash River basin, particularly in the upstream catchment (SW1, SW2, SW3, and SW4) due to industrial wastewater discharge while stations like SW13, SW14, SW15, SW16, and SW17 were highly polluted by trace metals discharged from industries and municipal waste. In the Lake Beseka region, the Ethiopian Rift Valley may have contributed to elevated HMs concentration including Mo, V, U, and As and surface–groundwater interactions brings high level of Fe, Mn, Cr, Sr, Al, Ba, and Zn from the groundwater to the lake water and river water in the central basin of the valley. In general, the WQ of the basin depends on many factors, including the proportion of surface runoff (industrial and domestic runoff, agricultural runoff, and sewage), rock erosion, and solid waste runoff from inland water bodies processes and mixing of incoming water of different quality and entry of pollutants and unwanted substances.
-
High HM concentrations, such as Mo and As contamination in the central Awash Basin, were observed mainly due to geogenic activity, while Cr, Sr, Al, Ba, Fe, Mn, and Zn concentrations were particularly high in correlation at Akaki, runoff from anthropogenic sources and mostly lead in the upper reaches of the Koka.
-
At the same time, high concentrations of As, Mo, U, and V were observed in LB (geogenic source) and also in upper Koka (due to anthropogenic and/or geogenic sources). Due to the increased risk of certain trace metals, relevant authorities in the study area, such as the Ministry of Water and Energy (MoWE), environmental protection authority (EPA), and Awash Basin Administration Office (AwBAO), must regularly monitor and investigate certain risk areas (LB and after-lake mixing).
-
To reduce metal toxicity in AwRB, the Ethiopian government should improve wastewater management nationally. In addition, there is a need to ensure and protect the WQ and sustainability of waterways, and more regional studies on WQ in the basin are needed.
Overall, the study highlighted some new areas of water quality challenges. Both anthropogenic and geogenic activities contribute to the WQ degradation. Some of the new observations, however, need to be further investigated to arrive at the root causes of the problem and means to prevent further contamination of the river course.
Data availability
The data used and analyzed during this study are available if needed for further review
References
Abebe, Y., Alamirew, T., Whitehead, P., Charles, K., & Alemayehu, E. (2023). Spatio-temporal variability and potential health risks assessment of heavy metals in the surface water of Awash basin, Ethiopia. Helyon, 9(5). https://doi.org/10.1016/j.heliyon.2023.e15832
Alcaine, A. A., Schulz, C., Bundschuh, J., Jacks, G., Thunvik, R., Gustafsson, J.-P., Mörth, C.-M., Sracek, O., Ahmad, A., & Bhattacharya, P. (2020). Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Science of the Total Environment, 715, 136671. https://doi.org/10.1016/j.scitotenv.2020.136671
Alexandra, M., Helene, O., Jiri, M., & Maria, V. (2020). The pollution conveyed by urban runoff: A review sources. Science of the Environment, 709. https://doi.org/10.1016/j.scitotenv
Ali, H., & Khan, E. (2018). What are heavy metals? The long-standing controversy over the scientific use of the term ‘heavy metals’-proposal of a comprehensive definition. Toxicological & Environmental Chemistry, 100, 6–19.
Al-Saydeh, S. A., El-Naas, M. H., & Zaidi, S. J. (2017). Copper removal from industrial wastewater: a comprehensive review. Journal of Industrial and Engineering Chemistry, 56, 35–44. https://doi.org/10.1016/j.jiec.2017.07.026
Annette Johnson, C., Johnston, T. B., & Bretzler, A. (2008). Geogenic contaminants. Eawag News 65e/December 2008. https://www.eawag.ch/repository/wrq/geogenic-contamination-handbook-chapter1.pdf.
ARSLAND (2006) Sustainable management of arsenic contaminated water and soil in rural areas of Latin America. (INCO 015114). http://www.arsland.net/english/publications/D09
Ashayeri, N. Y., Keshavarzi, B., Moore, F., Kersten, M., Yazdi, M., & Lahijanzadeh, A. R. (2018). Presence of polycyclic aromatic hydrocarbons in sediments and surface water from Shadegan wetland-Iran: A focus on source apportionment, human and ecological risk assessment and sediment-water exchange. Ecotoxicology and Environmental Safety, 148, 1054–1066.
Ayotte, J. D., Gronberg, J. A. M., & Apodaca, L. E. (2011). Trace elements and radon in groundwater across the United States, 1992-2003. In Scientific Investigations Report No.5059. United States Geological Survey.
Bhuiyan, M. A. H., Suruvi, N. I., Dampare, S. B., Islam, M. A., Quraishi, S. B., Ganyaglo, S., & Suzuki, S. (2011). Investigation of the possible sources of heavy metal contamination in the lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environmental Monitoring and Assessment, 175, 633–649.
Bussi, G., Whitehead, P. D., Jin, L., Taye, M. T., Dayer, E., Hirpo, F. A., Yimer, Y. A., & Charles, K. J. (2021). Impacts of climate change and population growth on river nutrients loads in a data-scarce region: The upper Awash River (Ethiopia). Journal of Sustainability/MDPI/, 13, 1254. https://doi.org/10.3390/su13031254
Das, B., Rahman, M. M., Nayak, B., et al. (2009). Groundwater arsenic contamination, its health effects and approach for mitigation in West Bengal, India and Bangladesh. Water Qual Exposure Health, 1, 5–21. https://doi.org/10.1007/s12403-008-0002-3
Degefu, F., Lakew, A., Tigabu, Y., & Teshome, K. (2013). The water quality degradation of upper Awash River, Ethiopia. Ethiopian Journal of Environmental Studies and Management, 6. https://doi.org/10.4314/ejesm.v6i1.7
Esayas Alemayehu & Bernd Lennartez. (2009). Virgin volcanic rocks: Kinetics and equilibrium studies for the adsorption of cadmium from water. Journal of Hazardous Materials, 169, 395–401.
Esmaeili, A., Shamaei, E. M. A., Akhtar, Z. N., Hosseini, S. F., & Shokri, S. (2021). Health risk assessment of heavy metals in edible mushrooms and their effect on anemia: A review study. Journal of Chemical Health Risks. https://doi.org/10.22034/JCHR.2021.1937630.1380
Fakhri, Y., Bjørklund, G., Bandpei, A. M., Chirumbolo, S., Keramati, H., Pouya, R. H., & Sheikh Mohammad, A. (2018). Concentrations of arsenic and lead in rice (Oryza sativa L.) in Iran: A systematic review and carcinogenic risk assessment. Food and chemical toxicology, 113, 267–277.
Farkas, A., Erratico, C., & Vigano, L. (2007). Assessment of the environmental significance of heavy metal pollution in surficial sediments of the River Po. Chemosphere, 68, 761–768. https://doi.org/10.1016/j.chemosphere.2006.12.099
Fathabad, A. E., Moazzen, S., Nazmara, S., Fakhri, Y., Alimohammadi, M., & Khaneghah, A. M. (2018). Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: A risk assessment study. Food and Chemical Toxicology, 115, 436–446. https://doi.org/10.1016/j.fct.2018.03.044
FDRE-EPA. (2003). Guideline ambient environment standards for Ethiopia. The Environmental Protection Authority and the United Nations Industrial Development Organization. Prepared under the Ecologically Sustainable Industrial Development (ESID) Project US/ETH/99/068/ETHIOPIA (unpublished material).
Fei, X., Lou, Z., Christakos, G., Ren, Z., Liu, Q., & Lv, X. (2018). The association between heavy metal soil pollution and stomach cancer: A case study in Hangzhou City, China. Environmental Geochemistry and Health, 40(6), 2481–2490. https://doi.org/10.1007/s10653-018-0113-0
Fendorf, S., Michael, H. A., & Van Geen, A. (2010). Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science, 328(5982), 1123–1127. https://doi.org/10.1126/science.1172974
Frascoli, F., & Hudson-Edwards, K. A. (2018). Geochemistry, mineralogy and microbiology of molybdenum in mining-affected environments. Journal of Molecules, 8(2), 1–18.
Furi, W. (2011). Hydrogeological system analysis of the Middle Awash Basin, Ethiopia. Unpublished Ph.D. Thesis, University of Poitiers, France.eval.
He, Z. L., Yang, X. E., & Stoffella, P. J. (2005). Trace elements in agroecosystems and impacts on the environment. Journal of Trace elements in Medicine and Biology, 19(2–3), 125–140. https://doi.org/10.1016/j.jtemb.2005.02.010
Huang, H., Lin, C., Yu, R., Yan, Y., Gongren, H., & Li, H. (2019). Contamination assessment, source apportionment, and health risk assessment of heavy metals in paddy soils of Jiulong River Basin, South East China. RSC Advances, 9, 14736–14744. https://doi.org/10.1039/C9RA02333J
Jafari, A., Kamarehie, B., Ghaderpoori, M., Khoshnamra, N., & Birijandi, M. (2018). The concentration data of heavy metals in Iranian grown and imported rice and human health hazard assessment. Data in Brief, 16, 453–459. https://doi.org/10.1016/j.dib.2017.11.057
Jin, L., Whitehead, P. G., Bussi, G., Hirpa, F., Taye, M. T., Abebe, Y., & Carles, K. (2021). Natural and anthropogenic sources of salinity in the Awash River Basin and Lake Beseka (Ethiopia): Modeling impacts of climate change and lake river interactions. Journal of Hydrology, 36, 100865.
Kalbus, E., Reinstorf, F., & Schirmer, M. (2006). Measuring methods for groundwater-surface water interactions: A review. Hydrology and Earth System Sciences, 10(6), 873–887. https://doi.org/10.5194/hess-10-873-2006
Kebede, S., Charles, K., Godefery, S., MacDonald, A., & Taylor, R. G. (2021). Regional-scale interactions between groundwater and surface water under changing aridity: Evidence from the River Awash Basin, Ethiopia. Hydrological Science Journal, 66(3), 450–463. https://doi.org/10.1080/02626667.2021.1874613
Kim, Y.-J., & Kim, J.-M. (2015). Arsenic toxicity in male reproduction and development. Development & Reproduction, 4, 167–180. https://doi.org/10.12717/DR.2015.19.4.167
Kimbrough, D. E. (2009). Source identification of copper, lead, nickel, and zinc loading in wastewater reclamation plant influents from corrosion of brass in plumbing fixtures. Environmental Pollution, 157(4), 1310–1316. https://doi.org/10.1016/j.envpol.2008.11.043
Klemperer, S. L., & Cash, M. D. (2007). Temporal geochemical variation in Ethiopian Lakes Shala, Arenguade, Awassa, and Beseka: Possible environmental impacts from underwater and borehole detonations. Journal of African Earth Sciences, 48, 174–198. https://doi.org/10.1016/j.jafrearsci.2006.10.006
Lamontagne, S., Leaney, F. W., & Herczeg, A. L. (2005). Groundwater surface water interactions in a large semi-arid floodplain: Implications for salinity management. Hydrological Processes, 19, 3063–3080.
Lewis, A. L., Sarkar, B., Wade, P., Kemp, S. J., Hodson, M. E., Taylor, L. L., Yeong, K. L., Davies, K., Nelson, P. N., Birds, M. I., Kantola, I. B., Masters, M. D., DeLucia, E., Leake, J. R., Banwart, S. A., & Beerling, D. J. (2021). Effects of mineralogy, chemistry and physical properties of basalts on carbon capture potential and plant-nutrient element release via enhanced weathering. Applied Geochemistry, 132. https://doi.org/10.1016/j.apgeochem.2021.105023
Muchuweti, J., Birkett, E., Chinyanga, R., Zvauya, M. D. S., & Lester, J. (2006). Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agriculture, Ecosystems & Environment, 112, 41–48.
Muhammad, S., Shah, M. T., & Khan, S. (2011). Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, Northern Pakistan. Microchemical Journal, 98, 334–343.
Muibat, O. F., Veronica, M. N., & Olubukola, O. B. (2016). Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Environmental Research and Public Health, 13(11), 1047. https://doi.org/10.3390/ijerph13111047
Murgo, A. J. (2001). Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist, 6, 2–28.
Ntengwe, F. W. (2006). Pollutant loads and water quality in streams of heavily populated and industrialized towns. Physics and Chemistry of the Earth, Parts A/B/C, 31, 832–839. https://doi.org/10.1016/j.pce.2006.08.025
Paul BT, Clement GY, Patolla AK, & Dwayne JS, (2012) Heavy metals toxicity and thee Environment. EXS, v 101, 133–164. https://doi.org/10.1007/978-3-7643-8340
Paul, B. T., Clements, G. Y., Anti, K. P., & Dwayane, J. S. (2012). Heavy metals toxicity and the environment. NHS publican (PMC,), 101, 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Ravenscroft, P., Brammer, H., & Richards, K. S. (2009). Arsenic pollution: A global synthesis. Wiley-Blackwell. https://doi.org/10.1002/9781444308785
Reimann, C., Bjorvatn, K., Tekle-Haimanot, R., Melako, Z., & Siewers, U. (2002). Drinking water quality, Rift Valley, Ethiopia. Norges geologiske undersøkelse, Report, 033, 132.
Schmidt, A., & Schubert, M. (2007). Using radon-222 for tracing groundwater discharges into an open-pit lignite mining lake - A case study. Isotopes in Environmental and Health Studies, 43, 387–400.
Shaji, E., Santosh, M., Sarath, K. V., Pranav Prakash, V., Deepchand, & Divya, B. V. (2021). Arsenic contamination of groundwater: A global Synopsis with focus on the Indian Peninsula. Geoscience Frontiers, 12, 101079.
Smedey, P. L., & Kinniburgh, D. G. (2001). Chapter one: Sources and behavior of arsenic in natural waters. British Geological Survey https://www.researchgate.net/publication/267403765. Accessed 13 Dec 2022
Smedley, P. L., Bearcock, J. M., Fordyce, F. M., Everett, P. A., Chenery, S., & Ellen, R. (2017). Stream water geochemistry atlas of the Clyde Basin (No. OR/16/015). British Geological Survey.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behavior, and distribution of arsenic in natural waters. Appl. Geochem, 17, 517–568. https://doi.org/10.1016/S0883-2927(02)00018-5
Pauline L. Smedley & David G, Kinniburgh (2017) Molybdenum in natural waters: A review of occurrence, distribution, and controls. Applied Geochemistry, vol 84, 387-432. https://doi.org/10.1016/j.apgeochem.2017.05.008
Smith, A. H., Lingas, E. O., & Rahman, M. (2000). Contamination of drinking water by arsenic in Bangladesh: A public health emergency. Bulletin of the World Health Organization, 9, 1093–1103.
Smith, A. H., & Steinmaus, C. H. (2009). Health effects of arsenic and chromium in drinking water: Recent human findings. Annual Review of Public Health, 30, 107–122. https://doi.org/10.1146/annurev.publhealth.031308.100143
Sun, J., Liu, Y., Wang, L. S., & Yu, H. (2013). Multivariate and geostatistical analyses of the spatial distribution and sources of heavy metals in agricultural soil in Dehui, Northeast China. Chemosphere, 92, 517–523.
Tesedenya, A. T. (2018). Groundwater surface water Interaction along the main course of Awash River; Integrated approach, Unpublished. MSc Thesis University of Addis Ababa University, Addis Ababa.
Tiwari, A. K., Maio, M. D., Singh, P. K., & Mahato, M. K. (2015). Evaluation of surface water quality by using GIS and a heavy metal pollution index (HPI) model in a coal mining area, India. Bulletin of Environmental Contamination and Toxicology, 95, 304–310. https://doi.org/10.1007/s00128-015-1558-9
Tiwari, A. K., & Singh, A. K. (2014). Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. Journal of the Geological Society of India, 83, 329–343. https://doi.org/10.1007/s12594-014-0045-y
Wang, R., Xu, Q., Zhang, X., et al. (2012). Health risk assessment of heavy metals in typical township water sources in Dongjiang River Basin. Huanjingkexue D Huanjingkexue, 33, 3083–3088.
Wei, B., & Yang, L. (2010). A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchemical Journal, 94, 99–107. https://doi.org/10.1016/j.microc.2009.09.014
WHO. (2008). Guidelines for drinking-water quality. Switzerland, World Health Organization.
WHO. (2011). Molybdenum in drinking water. Background document for development of WHO guidelines for drinking water quality. World Health Organization.
Yang, J. Q., Wan, Y., Li, J. J., Zou, D. W., Leng, X., & An, S. Q. (2018). Spatial distribution characteristics and source identification of heavy metals in river waters of the Huaihe River Basin, China. Marine and Freshwater Research, 69, 840–850. https://doi.org/10.1071/MF17375
Yimer, Y. A., & Geberekidan, A. (2020). The pollution status of Awash River Basin (Ethiopia) using descriptive statistical techniques. American Journal of Water Resources, 8(2), 56–68. https://doi.org/10.12691/ajwr-8-2-2
Yimer, Y. A., Geberekidan, A., & Abraha, M. (2020). Assessment on the performance of wastewater treatment plants and their impact on the surface water quality of Awash River, Ethiopia. Ethiopian Journal of Environmental Studies & Management, 13(3), 376–392.
Yimer, Y. A., & Jin, L. (2020). Impact of Lake Beseka on the water quality of Awash River, Ethiopia. American Journal of Water Resources, 8(1), 21–30. https://doi.org/10.12691/ajwr-8-1-3
Yuanan, H., Xueping, L., Jinmei, B., Kaimin, S., Zeng, E. Y., & Hefa, C. (2013). Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environmental Science and Pollution Research, 20, 6150–6159. https://doi.org/10.1007/s11356-013-1668-z
Zeng, Q., Yi, H., Huang, L., An, Q., & Wang, H. (2019). Long-term arsenic exposure induces testicular toxicity by redox imbalance G2/Mcell arrest and apoptosis inmice. Toxicology, 411, 122–132. https://doi.org/10.1016/j.tox.2018.09.010
Zhang, L., Ye, X., Feng, H., et al. (2007). Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Marine Pollution Bulletin, 54, 974–982. https://doi.org/10.1016/j.marpolbul.2007.02.010
Zinabu, G. M., & Pearce, N. J. G. (2003). Concentrations of heavy metals and related trace elements in some Ethiopian rift-valley lakes and their in-flows. Hydrobiologia, 429, 171–178. https://doi.org/10.1023/A:1024856207478
Acknowledgements
The corresponding author is highly thankful to my well-qualified and proficient professors and doctors working in REACH Program from the UK for providing the state-of-the-art scientific information to make this paper a reality.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However, this work is supported by the REACH program funded by UK Aid from the UK Foreign, Commonwealth, and Development Office for the benefit of developing countries (Program Code 201880).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yosef, Paul, and Tena; data curation: Yosef and Paul; formal analysis: Yosef; funding acquisition: Paul and Tena; investigation: Yosef, Tena, and Esayas; methodology: Yosef and Tena; writing, original draft preparation: Yosef; writing, review, and editing: Yosef, Tena, Paul, Esayas, and Li; supervision: Esayas, Li, and Paul. All authors have read and agreed to the publishable version of the manuscript.
Corresponding author
Ethics declarations
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The views expressed and information contained in it are not necessarily those of or endorsed by FCDO, which can accept no responsibility for such views or information or for any reliance placed on them.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abebe, Y., Whitehead, P., Alamirew, T. et al. Evaluating the effects of geochemical and anthropogenic factors on the concentration and treatability of heavy metals in Awash River and Lake Beseka, Ethiopia: arsenic and molybdenum issues. Environ Monit Assess 195, 1188 (2023). https://doi.org/10.1007/s10661-023-11674-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11674-z