Abstract
Water, as an indispensable constituent of life, serves as the primary source of sustenance for all living things on Earth. The contamination of surface water with heavy metals poses a significant global health risk to humans, animals, and plants. Sharkiya Governorate, situated in the East Nile Delta region of Egypt, is particularly susceptible to surface water pollution due to various industrial, agricultural, and urban activities. The Bahr Mouse Stream, crucial for providing potable water and supporting irrigation activities in Sharkiya Governorate, caters to a population of approximately 7.7 million inhabitants. Unfortunately, this vital water source is exposed to many illegal encroachments that may cause pollution and deteriorate the water resource quality. In a comprehensive study conducted over two consecutive seasons (2019–2020), a total of 38 surface water samples were taken to assess the quantity of heavy metals in surface water destined for human consumption and other applications, supported by indices and statistics. The assessment utilized flame atomic absorption spectrophotometry to determine the concentration of key heavy metals including iron (Fe), manganese (Mn), cadmium (Cd), copper (Cu), lead (Pb), zinc (Zn), nickel (Ni), cobalt (Co), and chromium (Cr). The calculated mean value of the Water Quality Index (WQI) was found to be 39.1 during the winter season and 28.05 during the summer season. This value suggests that the surface water maintains good quality and is suitable for drinking purposes. Furthermore, the analysis indicated that the concentrations of heavy metals in the study area were below the recommended limits set by the World Health Organization and fell within the safe threshold prescribed by Egyptian legislation. Despite the identification of localized instances of illegal activities in certain areas, such as unauthorized discharges, the findings affirm that the Bahr Mouse stream is devoid of heavy metal pollution. This underscores the importance of continued vigilance and regulatory enforcement to preserve the integrity of these vital water resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is a priceless natural resource that is required for both human survival and the health of ecosystems. Pollution occurs when an aquatic ecosystem is badly damaged by the addition of too many things, rendering the water unfit for its intended usage (Olatunji et al., 2017; Nour et al., 2022a). Among the most pervasive contaminants in aquatic ecosystems are heavy metals, known for their persistence and toxicity, with concentration further exacerbated by biomagnification processes (Dervash & Mushtaq, 2019; Nour & Nouh, 2020). Notably, heavy metals, such as lead, cadmium, and mercury, accumulate in water reservoirs, penetrating the food chain and posing significant ecological risks (Nour, 2015; Pandiyan et al., 2021). Moreover, their consumption has been linked to severe health implications, including cancer and neurological impairment in humans (Alharbi et al., 2023; Al-Kahtany et al., 2023a; Nour et al., 2022b). Anthropogenic activities, such as industrial discharge, domestic sewage, non-point source runoff, and atmospheric precipitation, stand as principal contributors to the infiltration of toxic heavy metals into aquatic systems (Akhtar et al., 2021; Nour et al., 2021; Ramadan et al., 2021). This further exacerbates the global concern over heavy metal contamination in aquatic environments (Al-Kahtany et al., 2023b; Sobhanardakani et al., 2018).
Egypt, a densely populated developing country with a highly arid climate, faces substantial challenges in managing its water resources, given its requirement of 114 BCM annually. With the goals outlined in Egypt Vision 2030 focusing on the preservation of natural water resources, the significance of monitoring heavy metal concentration in surface water becomes paramount amidst challenges posed by climate change, water scarcity, rapid population growth, and infrastructure developments such as the Grand Ethiopian Renaissance Dam construction.
The East Delta region, encompassing Sharkiya, Dakhalia, and Damietta governorates, is characterized by a network of canals, drains, and lakes that traverse and delineate its territory. These water bodies are categorized into freshwater systems, such as the Damietta Branch of the Nile River, and irrigation canals including Baḩr Mouse, El Raiah El Tawfeiki, Bahr Abu Akhder, and Ismailia Canal, as well as saline water bodies comprising the Suez Canal, El Temsah Lake, and El Manzala Lake.
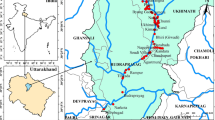
Focusing on the Sharkiya Governorate, our target area covers 4922 km2 (1169285 faddan; Fig. 1) and lies within the coordinates of latitude 30° 30′ and 31° 00′ N and longitudinal 31° 15′ and 31° 50′ E, with an elevation of 10 m above the sea level. Notably, the population of Sharkiya governorate is projected to exceed 7.7 million individuals by 2021, as reported by the Central Agency for Public Mobilization and Statistics.
Baḩr Mouse, a crucial freshwater canal originating from the Tawffiky Diversion directly linked to the River Nile near Banha city in the north of Sharkiya Governorate, Egypt (Fig. 1), serves as the main source of drinking water, irrigation, and fishing activities within the region. However, field observations reveal concerning levels of pollution in the Bahr Mouse stream, marked by the presence of various illegal contaminants including garbage, demolition waste, sewage discharge, and industrial effluent from clay brick factories. In addition, it is constantly polluted with amounts of components such as fertilizer residues and human wastes, further deteriorating its quality. Despite the evident pollution burden, systematic research analyzing heavy metal contents and its impact on human health in this area remains scarce. Hence, the primary aim of this study is threefold: (a) to estimate the concentrations of heavy metals in the surface water of the Baḩr Mouse stream, (b) to investigate the physicochemical parameters of the stream water, and (c) to calculate the ecological risk indices of water samples collected from the study area. Through this research, we aim to provide valuable insights into the current state of water quality in the investigated area, shedding light on potential implications for human health and the environment. We emphasize the importance of addressing the adverse effects of recent economic development and rapid population growth on heavy metal levels, urging decision-makers to prioritize measures aimed at safeguarding water resources and public health.
Materials and methods
Study region
Baḩr Mouse, a freshwater stream spanning approximately 84 km2 with widths ranging from 10 to 20 m, traverses Sharkiya Governorate in Egypt, bounded by latitudes 30° 29′ 16″ N, 31° 12′ 56.8″ N and longitudes 30° 55′ 53.3″ E, 31° 42′ 23.1″ E (Fig. 1). The water depth fluctuates between 1.5 m and a comparatively uncommon 4 m. Notably, Baḩr Mouse meanders through several urban centers including Zagazig, Abu Kibir, Hehia, Al Ibrahimiya, Kafr Saqr, Awlad Saqr, and Minya Al Qamh cities. Surrounding the research area are prominent geographical features: the west is bordered by the Nile River (Damietta Branch), the east by the Suez Canal, the north by Manzala Lake, and the south by the Ismailia Canal (Fig. 1).
The research area’s climate is characterized by a warm winter punctuated by intermittent rainfall, followed by a hot arid summer with moderate humidity, and medium-speed wind. The region experiences an average annual temperature of 20.3 °C, with the highest temperature of 36.7 °C in July and the lowest temperature of 6.4 °C in January. Geological studies by El-Fayoumy (1968) have identified dominant sedimentary formations in the eastern Nile Delta, comprising Tertiary and Quaternary sedimentary sequences. Tertiary rocks include Eocene, Oligocene, Miocene, and Pliocene formations, while Quaternary deposits, predominantly Nile silts (Holocene) interspersed with Pre-Nile deposits, are widely distributed throughout the study area.
Sample collection and heavy metal analysis
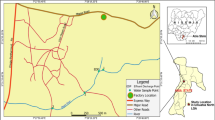
Thirty-eight water samples were taken from nineteen localities in Sharkiya Governorate, covering seasonal periods from December 2018 to August 2019 (Fig. 2). The sampling process involved precise geographical delineation utilizing Geographic Position System (GPS) instruments, and the map of the study area was drawn using Arc GIS (10.1). Each sample, collected from approximately 1 m below the water surface to minimize potential surface contamination, was carefully preserved in sterile 1-l plastic containers. At each location, about 1 l of water was collected. The properties of water samples were measured in situ using a portable calibrated multi-parameter instrument. More specifically, measurements were conducted using a Mercury-in-glass thermometer for temperature (T) and a Horiba multi-parameter water quality checker U-51 for pH, electrical conductivity (EC), and total dissolved solids (TDS) analyses. After in situ measurements, the samples were treated with 1 mm of HNO3 in a one-liter sample to arrest microbial activities (APHA, 1995). They were stored in an icebox (at 4 °C,) for transport to the laboratory. Then, 0.45-m membrane filters were used to filter the samples (white rim, Whatman no. 42, Germany). Generally, samples were prepared and analyzed according to the procedures described by Oregioni and Astone (1984) for heavy metals. The Fe, Mn, Cd, Cu, Pb, Zn, Ni, Co, and Cr contents were determined by the flame atomic absorption spectrophotometry (Perkin Elmer Model 2830). The analysis was carried out in the Central Laboratory at the Faculty of Veterinary Medicine, Zagazig University.
Photographs showing the polution phenomenons in the study area: a and b the surface and sides of the stream is totally covered with garbage, plastics materials, and dead animals, c the waste of materials of Red brick factory on the blank of the stream, d the waste water pump of the factory discharge into the stream, the color of water displays highly polluted of the stream
Mixed standards were prepared containing Fe, Cu, Zn, Pb, and Cd with concentrations of 0.5 ppm 1.0 ppm 2 ppm, 4 ppm, and 10 ppm. All detection limits are given in micrograms per liter and were determined using elemental standards in dilute aqueous solution. Appropriate drift blank was taken before the analysis of samples. The analytical data quality was guaranteed through the implementation of laboratory quality assurance and quality control methods, including the use of standard operating procedures, calibration with standards, analysis of reagent blanks, recovery of known additions, and analysis of replicates. This is to verify that decontamination procedures and laboratory protocols were adequate (Oregioni and Astone, 1984). Triplicate measurements were made for each sample, with differences between replicates not exceeding 3%, to uphold stringent accuracy standards. The average values of three replicates were taken for each determination. Quantitative estimation of metal concentration was estimated under standardized conditions as described in the instrument manual. Detection limits, established at a 98% confidence level, were determined as follows:1.5 μg/l for Zn, 5 μg/l for Pb, 9 μg/l for Cu, 1.5 μg/l for Mn, and 0.8 μg/l for Cd.
Water quality indices
Five indices (WQI, HEI, Cd, HMPI, and PI) were applied to evaluate the water quality and pollution status of heavy metals in the surface water of Bahr Mouse stream. Water Quality Index (WQI) is a mathematical concept that calculates the combined effect of each standard parameter on the water’s overall quality (APHA, 2012). It can serve as a sign of water pollution due to natural inputs and anthropogenic activity (Muyen et al., 2016). The Weighted Arithmetic Water Quality Index method (WAWQI) developed by Brown et al. (1972) was used in our study to calculate the WQI of surface water by the following equations:
where N is the number of parameters, \({\text{W}}i\) is the relative weight of each parameter, \({\text{Q}}i\) is the water quality rating, w \(i\) is the weight of each parameter, ∑w \(i\) is the sum of weight of all 10 parameters, \(K\) is the proportionality constant, S \(i\) is the WHO maximum allowable limits for each parameter in mg/L, M \(i\) is the measured concentration for each parameter in each water sample, and \({\text{V}}o\) is the ideal value for each parameter, (0 for all) except pH = 7 (Tripaty & Sahu, 2005). WQI were classified into five categories: excellent, (WQI < 25); good, (26–50); poor, (51–75); very poor, (76–100), and > 100 unfit for drinking water consumption. Additionally, the pollution evaluation index provides a good grading system for water quality (Biswas et al., 2017).
Heavy metal Evaluation Index (HEI) is a method used to assess the overall impact of each parameter on the quality of the water (Saleem et al., 2019). Typically, HEI is utilized to determine the pollution level caused by HMs (Dippong et al., 2020). It is calculated by the equation:
where M \(i\) is the measured concentration and MAC \(i\) is the maximum allowable concentration for each parameter. Nine parameters (Fe, Mn, Cu, Cd, Pb, Zn, Ni, Co, and Cr) were used in the calculation and water samples can be divided into three groups: low (HEI < 10), medium (10 < HEI < 20), and high contamination (HEI > 20) (Ghaderpoori et al., 2018).
Degree of contamination (Cd) summarizes the combined effects of a number of quality parameters considered harmful to domestic water (Backman et al., 1998). It is calculated by:
where N is the number of parameters, \({\text{Cf}}i\) is the contamination factor of ith component, \({\text{M}}i\) is the analytical value of ith component, and \({\text{MAC}}i\) is the maximum permissible concentration of ith component. According to Al-Ani et al. (1987), the Cd values were classified as, low (Cd < 1), medium (Cd = 1–3), and high contamination (Cd > 3).
The Heavy Metal Pollution Index (HMPI) considers the combined effect of each heavy metal on the overall water quality (Vasant et al., 2016). Based on the significant parameters affecting water chemistry, HMPI is utilized to assess the impact of both anthropogenic and natural activities (Ghaderpoori et al., 2018). According to Mohan (1996), it is calculated by:
where N is the number of parameters, \({\text{W}}i\) is the relative weight of each parameter, \({\text{Q}}i\) is the water quality rating, w \(i\) is the weight of each parameter, ∑w \(i\) is the sum of weight of all 10 parameters, \(K\) is the proportionality constant, S \(i\) is the WHO maximum allowable limits for each parameter in mg/L, M \(i\) is the measured concentration for each parameter, and \({\text{I}}i\) is the ideal value for each parameter in each water sample which were taken as zero for all of metals in this study like some research (Dede, 2016; Reza & Singh, 2010). The critical value for HMPI is 100 (Prasad & Bose, 2001).
Pollution Index (PI) is used to evaluate the degree of heavy metal contamination in water samples (Odukoya & Abimbola, 2010). PI is based on individual metal calculations with the equation:
where Mi is the measured concentration for each parameter, S \(i\) is the WHO maximum allowable limits and Nm is the number of heavy metals. According to Caerio et al. (2005), PI values were classified into six classes: (1) no effect (PI < 1), (2) slightly affected (1–2), (3) moderately affected (2–3), (4) strongly affected (3–5), and (5) seriously affected (˃5).
Results and discussion
Physiochemical parameter analyses
The properties of the surface water samples were assessed in situ, encompassing water temperature (℃), pH, electrical conductivity (EC) (μs/cm), and total dissolved solids (TDS) (mg/L). Temperature, recognized as a pivotal factor affecting aquatic ecology (Huet, 1986; Ali et al., 2021), exhibits seasonal variations. The measured temperature values were between 18.7–20.4 and 29.6–34.5 °C in winter and summer, respectively (Fig. 3; Table 1).
The lowest value (18.7 °C) was recorded at station 6 (Near Izbat Al Bakakrah, Al Ibrahimiya) in winter, while the notable highest recorded temperature (34.5 °C) at station 14 (Tall Hawein, Zagazig) during summer exceeded the permissible limit of WHO (2022) standards. Elevated temperatures speed up chemical reactions which significantly affect the growth and biological activity of aquatic organisms while constraining the diversity of species inhabiting freshwater environments (Magaña, 2020).
Hydrogen ion concentration (pH) regulates the solubility of metals in aquatic ecosystems, with most species thriving within a pH range of 6.0 to 9.0, and exhibiting heightened activity around pH 7 (El-Sayed et al., 2020). pH values of the Bahr Mouse stream varied between 8.3–9 and 8.2–8.8 (alkaline) during winter and summer, respectively (Fig. 3; Table 1). WHO (2022), USEPA (2018), and EWQS (2007) reported that the pH of drinking water should be in the pH range of 6.5–8.5, while the FAO (1994) gives an upper limit of 8.5 for pH in irrigation water. The mean value of 8.5 fell within the permissible range stipulated by international standards. Electrical conductivity (EC) is a sensitive indicator of water contamination, with deviations from normal ranges often indicative of pollution. Naturally, water has a low conductivity, but if liquid effluents are discharged into water bodies, contamination will cause it to rise. The EC of water samples collected during summer ranged from 352 to 391 μs/cm, averaging 375.26 μs/cm. These values fell within the permissible limits of EWQS (2007) and FAO (1994) standards. Total dissolved solids (TDS) concentrations varied between 336–506 and 177–195 mg/L during the winter and summer seasons, respectively (Fig. 3; Table 1). Notably, TDS levels showed a noticeable increase during low water levels in the winter with most values falling below the permissible limit (500 mg/L) set by WHO (2022), USEPA (2018), and EWQS (2007) for drinking water and FAO (1994) for irrigation water.
Heavy metal analyses
The seasonal variation in concentrations of water heavy metals is presented in Table 2 and 3, accompanied by a graphical representation in Fig. 4. The hierarchical order of concentrations observed across seasons was as follows: Cr > Fe > Pb > Ni > Co > Mn > Zn > Cu > Cd. Remarkably, the highest recorded iron (Fe) concentration was 0.0074 ppm during winter at station 19 (Minya Al Qamh), whereas it peaked at 0.1049 ppm during the summer at station 6 (Al Ibrahimiya). Conversely, the lowest Fe concentration was noted at 0.0017 ppm during winter at station 2 (Kafr Saqr) and 0.006 ppm during summer at station 15 (Zagazig), with an average concentration of 0.0275 ppm (Fig. 4; Table 2 and 3). The range of Fe concentration (0.0040 to 0.0542 ppm) fell within the safety threshold established by WHO (2022), USEPA (2018), and EWQS (2007) for drinking water, as well as FAO (1994) guidelines for irrigation water. Notably, these concentrations are relatively lower when compared with findings from other local and international studies (Table 4 and 5).
Furthermore, Pearson’s correlation coefficient analysis illustrated a moderately significant positive correlation between Fe content and Cr (r = 0.523), along with a significant negative correlation coefficient with Cu (r = − 0.548) at the 0.01 level (Table 6). This elucidates the interplay between these metals and their dynamics within aquatic environments. Manganese (Mn), a naturally occurring metal vital for human and animal health (Hem, 1985; Kacmaz, 2020), exhibited concentrations ranging from 0.0011 to 0.0239 ppm during winter and non-detectable to 0.0024 ppm during summer. These concentrations remained below the established limit of 0.05 mg/L for Secondary Drinking Water Regulation (SDWR), which is not specifically regulated by the USEPA. However, they are notably comparable to previous studies by Abdel-Hamed et al. (2018) on Bahr Mouse water (0.03 ppm). Correlation analysis indicated a moderately significant positive relationship between Mn and Cu (r = 0.655) at the 0.01 level, as well as a low significant negative correlation coefficient with Cd (r = − 0.443) at the 0.05 level (Table 6).
Copper (Cu) concentration in the studied water samples showed that the highest recorded values of 0.0033 and 0.0034 ppm were observed at station 9 (Hehia) during winter and summer, respectively. Conversely, the lowest Cu values (0.0003 ppm) during winter were noted at station 16 (Kafr Ar Rubaemayah, Minya Al Qamh) and non-detectable during summer at station 6 (Al Ibrahimiya) and station 18 (Near Kafr El Saedy, Minya Al Qamh). The average Cu concentration across all samples was 0.0013 ppm (Fig. 4). As such, Cu concentrations remain within the safety limits outlined by EWQS (2007) and below the recommended thresholds set by WHO (2022), USEPA (2018), and FAO (1994) and they are low when compared with other local and international studies (Table 5).
The concentration of cadmium (Cd) remained below the detection limit in most of the water samples, and lower than the recommended limits outlined by Egyptian Law, as well as guidelines established by WHO (2022), USEPA (2018), and FAO (1994) (Table 4). The highest measured Cd concentration was 0.0003 ppm during winter at station 18 (Near Kafr El Saedy, Minya Al Qamh) and 0.0009 ppm during summer at station 17 (Kafr Badawi, Minya Al Qamh). These values were notably low in comparison with other pertinent local and international studies (Table 5). Lead (Pb) concentration showed seasonal fluctuations, with the highest value (0.0108 ppm) during winter at station 15 (Zagazig) and 0.0560 ppm during summer at station 4 (Kafr Saqr). Conversely, the lowest Pb concentration fell below the detection limit during winter at station 9 (Hehia) and in most collected water samples during summer (Table 3). The average Pb concentration of 0.0054 ppm (Fig. 4) remained below the safety limits stipulated by USEPA (2018) and WHO (2011) as shown in Table 4. Additionally, these concentrations were lower when juxtaposed with data from various local and international studies (Table 5).
Zinc (Zn), crucial for numerous biological processes (Kacmaz, 2020), exhibited concentrations ranging from 0.0011 to 0.0083 ppm during winter, and non-detectable to 0.0019 ppm during summer. These concentrations are below the established thresholds (Table 4) and are lower than those observed in comparative studies (Table 5). Zn content has a low significant positive correlation with Co (r = 0.374) at the 0.05 level (Table 6). Industrial effluents and wastewater are potential sources of zinc oxide, used as an oxidizing agent to create the fatty acids utilized in soap production (El-Sayed et al., 2011).
Nickel (Ni) concentration varied with the highest values recorded at stations 10 (Hehia) and 8 (Kofour Negm Village, Al Ibrahimiya) of 0.0110 and 0.0109 ppm, respectively, while reaching 0.0193 ppm during summer at station 2 (Hanout, Kafr Saqr). Conversely, Ni contents were non-detectable at various stations during both winter and summer. The average Ni concentration was 0.0089 ppm (Fig. 4), all within established safe limits (Table 4) and comparatively lower than data from local and international studies (Table 5).
Cobalt (Co), essential as a trace mineral for various organisms including humans and animals, displayed variable concentrations across seasons. Co contents ranged from 0.0026 ppm at station 1 (Awlad Saqr) to 0.0001 ppm in station 9 (Hehia) during winter, and from 0.0543 ppm at station 17 (Minya Al Qamh) to 0.0023 ppm at station 5 (Kafr Saqr) during summer. These concentrations remained within the safety limits outlined by EWQS (2007) and below the recommended limits of WHO (2022), USEPA (2018), and FAO (1994) (Table 4). Additionally, Co levels in most samples were lower than the mean concentrations reported in pertinent studies (Abdel-Hamed et al., 2018; El-Sayed et al., 2011) (Table 5).
The distribution of heavy metals in the studied samples from the Bahr Mouse stream confirms the results by Abd El-Wahed (2007), who indicated that Fe, Mn, Zn, and Pb concentrations in surface water were below the standard permissible limits. On the other hand, El-Sayed (2011) reported that heavy metals have high concentrations, especially zinc and chromium. This may be attributed to wastes of industrial activities and wastewater. The levels of metals exhibited seasonal fluctuations, where all heavy metal concentrations in water samples (except Cu) showed significant variations between seasons (P-values < 0.05). Summer has the highest concentrations of Fe, Cu, Cd, Pb, Ni, and Co (0.1049, 0.0034, 0.0009, 0.056, 0.0193, and 0.0543 ppm), respectively. In contrast, the highest concentrations of Mn, Zn, and Cr were recorded in winter (0.0239, 0.0083, and 0.4518 ppm) respectively. Abd El-Aal (2020) ascribed the rise in metal concentration in water during hot seasons to the release of heavy metals from sediment to the water under the influence of both high temperature and fermentation process which is caused by the organic materials breakdown. Numerous research studies (i.e., El-Safy & Al-Ghannam, 1996; Hamed, 1998) carried out on the seasonal changes of heavy metal contents. They suggested that these seasonal variations could be a result of industrial activity and wastewater.
Correlation analysis revealed a moderately significant positive correlation between temperature values and Ni (r = 0.5) at 0.01 level. Additionally, TDS exhibited a low significant positive correlation with Cr (r = 0.444) at 0.05 level (Table 6). Moreover, EC values have a low significant positive correlation with Mn, Cu, and Zn (r = 0.304, 0.344, and 0.333, respectively) at the 0.05 level, while demonstrating a low significant negative correlation with Cd and Ni (r = − 0.427 and 0. − 334, respectively) at the same level (Table 6).
Heavy metal contamination and water quality assessment
The assessment of Bahr Mouse surface water quality was conducted utilizing the Weighted Arithmetic Water Quality Index (WAWQI). This method amalgamates various physicochemical parameters through mathematical equations to generate a single value representative of the overall quality status. The selection of ten parameters for calculating the Water Quality Index (WQI) for potable water encompasses pH, total dissolved solids (TDS), iron (Fe), manganese (Mn), copper (Cu), cadmium (Cd), lead (Pb), zinc (Zn), nickel (Ni), and chromium (Cr). The determination of WQI values adhered to the water quality standards outlined by the World Health Organization and the Egyptian Ministry of Health. The WQI values ranged from 144.28 (indicating an unfit condition) at Station 3 in Kafr Saqr City to 2.92 (representing an excellent condition) at Station 9 in Hehia City during winter (Fig. 5a).
Similarly, during summer, the WQI values spanned from 125.3 (indicative of an unfit condition) at Station 4 in Kafr Saqr City to 5.39 (demonstrating an excellent condition) at Station 7 in Al-Ibrahimiya City (Fig. 5b). Analysis indicated that approximately 52.6% of water stations had WQI values below 25, signifying excellent water quality, while 31.6% of stations registered WQI values ranging between 25 and 50, indicating good water quality for drinking during winter (Table 7). However, three samples (3, 5, and 13) with elevated WQI (> 100) denoted unsuitable water quality for both drinking and fish culture (Fig. 5a). Of these, samples 3 and 5 were sourced from Kafr Saqr City, characterized by agricultural activities, urban development, and significant household wastes discharged into water bodies. Sample 13 originated from Zagazig City, where urban activities and industrial operations, such as the red brick factory, contributed to water pollution.
During summer, approximately 58% of water stations had WQI values below 25, indicating excellent water quality for drinking, while 31.6% of stations recorded WQI values ranging between 25 and 50, representing good water quality except for two samples (4 and 14) with elevated WQI values, signifying poor and unfit water quality for drinking, respectively (Fig. 5b; Table 7). The former sample was sourced from Kafr Saqr City, where agricultural activities and household waste discharge were prominent, while the latter was from Zagazig City, characterized by urban activities and industrial effluents, including those from adjacent red brick factories.
The mean WQI values across different stations depicted in Fig. 5c and Table 7 showed that 73.7% of water stations had WQI values below 25, representing excellent water quality, while 21.1% had WQI values ranging between 25 and 50, indicating good water quality (Fig. 5c). Notably, only station 4 (located in Kafr Saqr City) had WQI value between 50 and 75, revealing poor water quality which is unsuitable for drinking, attributed to the discharge of household wastes into the water stream by local inhabitants. The overall mean WQI values for all stations during different seasons were 39.1 and 28.05 (during winter and summer, respectively), indicating a good condition (Table 7). On average, the water quality of Bahr Mouse was deemed good (20.94) and suitable for drinking. The observed ranges of WQI values greater than 100 as previously mentioned, underscores the spatial and temporal variability in water quality across the studied region.
The Fe content exhibits a moderately significant positive correlation with Cr, as well as Mn with Cu, implying similar geochemical behavior of these metals (Gu et al., 2016). In addition, Zn content has a low significant positive correlation with Co, indicating potential leaching from anthropogenic sources such as agricultural and domestic waste (Nour & Nouh, 2020; Prasanna et al., 2012).
Analysis presented in Table 7, Fig. 6a, and Fig. 6b reveals higher values of the Heavy Metal Pollution Index (HEI) during winter compared to summer, suggesting rainfall acted as a driver of water pollution. Station 3 (9.38) during winter and station 4 (5.88) during summer exhibited the maximum HEI levels in Bahr Mouse surface water (Table 7). The average HEI for winter and summer samples was 3.19 and 1.18 respectively. Mean HEI values across different stations ranged from 0.189 to 5.065 (Fig. 6c), indicating low-contamination levels (< 10), and suitability of the water for drinking purposes.
Regarding contamination degree (Cd) during winter, all stations except station 3 (2.37) in Kafr Saqr City were classified as low-contaminated (Fig. 6a; Table 7). In summer, all stations remained low-contaminated (Fig. 6b). The average contamination degree average for all stations during different seasons was − 4.34 in winter and − 4.31 in summer, classified as low-contaminated (Table 7).
During winter, most stations had low Heavy Metal Pollution Index (HMPI) values below the critical value of 100, except stations 3, 5 (Kafr Saqr City), and 13 (Zagazig City) which had 144.32, 137.76, and 109.9 respectively (Fig. 7a; Table 7). In summer, all stations had low HMPI values except station 4 in Saqr City, which exceeded the critical value (125.31) (Fig. 7b). Mean HMPI values across stations Fig. 7c and Table 7, showed 100% of water stations with HMPI < 100, denoting safe water for drinking. The overall mean HMPI values for all stations during different seasons were 38.95 in winter and 28.88 in summer (Table 7), suggesting Bahr Mouse water was generally safe (35.56) and suitable for drinking.
Similarly, Pollution Index (PI) values during both winter and summer seasons, classified all stations as unaffected (< 1) by heavy metals except station 3 (Kafr Saqr City) in winter and station 4 (Kafr Saqr City) in summer which were slightly affected (Figs. 8a and b). Average PI values confirmed no significant impact of heavy metal across sites and their water was suitable for drinking (Fig. 8c). Collectively, graphical representations of the five water quality indices (WQI, HEI, Cd, HMPI, and PI) demonstrate consistency in results, affirming Bahr Mouse water’s suitability for drinking and irrigation purposes.
Conclusions
Based on the comprehensive analysis of heavy metal contamination and environmental risk assessment conducted in the Bahr Mouse stream, East Nile Delta, Egypt, several key findings emerge as follows:
-
Physiochemical parameter analysis revealed seasonal variations in water temperature, pH, electrical conductivity (EC), and total dissolved solids (TDS), with temperatures exceeding permissible limits during summer, while pH, EC, and TDS remained within acceptable ranges outlined by international standards. These variations underscore the importance of monitoring these parameters for assessing water quality and potential environmental risks.
-
Heavy metal analyses demonstrated the presence of various metals in the surface water samples, with concentrations fluctuating across seasons. Notably, most heavy metal concentrations remained within safe limits established for drinking water, although seasonal variations and spatial distribution patterns were observed. Correlation analyses provided insights into the interplay between different heavy metal species and their environmental dynamics.
-
Water quality assessment utilizing the Weighted Arithmetic Water Quality Index (WAWQI) highlighted overall good water quality in the Bahr Mouse stream, with some localized instances of poor water quality attributed to anthropogenic activities such as agricultural runoff, urban development, and industrial effluents. The assessment also revealed seasonal variations in water quality, with slightly high contamination levels observed during winter months.
-
Additionally, the Heavy Metal Pollution Index (HEI), contamination degree (Cd), Heavy Metal Pollution Index (HMPI), and Pollution Index (PI) methodologies corroborated the findings of the WQI assessment, indicating consistency in evaluating water quality across different indices. These indices collectively affirmed the suitability of Bahr Mouse water for drinking and irrigation purposes, despite localized contamination hotspots.
In summary, the study provides valuable insights into the heavy metal contamination and environmental risks associated with surface water in the Bahr Mouse stream, offering essential information for policy makers, environmental regulators, and stakeholders to develop effective mitigation strategies and safeguard water resources in the East Nile Delta region of Egypt. Ongoing monitoring and management efforts are crucial to maintaining water quality standards and ensuring the sustainability of freshwater ecosystems in the area.
References
Abd El-Aal, R. F., El Sayed, S. S. M., Attia, M. S., Donia, N. S., & Goher, M. E. (2020). Pollution indices and distribution pattern of heavy metals in Qarun Lake water, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 24(1), 593–607.
Abdel-Hamed, E. M., Soliman, K. G., & Nasr-Alla, A. E. (2018). Quality of surface water in San El-Hagar Region, Sharkia Governorate, Egypt. Zagazig Journal of Agricultural Research, 45(5), 1683–1700.
Abd El-Wahed, S. A. (2007). Impact of use of treated wastewater on some eco-agricultural characteristics in Bahr El-Bakar area. Unpublished Master of Science, Department of Soil Science, Faculty of Agriculture, University of Ain Shams, Egypt, 184p.
Akhtar, N., Syakir Ishak, M. I., Bhawani, S. A., & Umar, K. (2021). Various natural and anthropogenic factors responsible for water quality degradation: A review. Water, 13(19), 2660.
Alharbi, T., Nour, H. E., Al-Kahtany, Kh., Giacobbe, S., & El-Sorogy, A. (2023). Sediment’s quality and health risk assessment of heavy metals in the Al-Khafji area of the Arabian Gulf, Saudi Arabia. Environ Earth Sci, 82, 471. https://doi.org/10.1007/s12665-023-11171-z
Al-Ani, M. Y., Al-Nakib, S. M., Ritha, N. M., & Nouri, A. H. (1987). Water quality index applied to the classification and zoning of Al-Jaysh canal, Baghdad-Iraq. Journal of Environmental Science & Health Part A, 22(4), 305–319.
Ali, M. M., Ali, M. L., Rakib, M. R. J., Islam, M. S., Habib, A., Hossen, S., & Phoungthong, K. (2021). Contamination and ecological risk assessment of heavy metals in water and sediment from hubs of fish resource river in a developing country. Toxin Reviews, 41(4), 1253–1268. https://doi.org/10.1080/15569543.2021.2001829
Al-Kahtany, Kh., El-Sorogy, A., Alharbi, T., Giacobbe, S., & Nour, H. E. (2023a). Health risk assessment and contamination of potentially toxic elements in southwest of the Red Sea coastal sediment. Regional Studies in Marine Science, 65, 103103. https://doi.org/10.1016/j.rsma.2023.103103
Al-Kahtany, Kh., Nour, H. E., El-Sorogy, A., & Alharbi, T. (2023b). Ecological and health risk of heavy metals contamination in mangrove sediments Red Sea coast. Marine Pollution Bulletin, 192, 115000. https://doi.org/10.1016/j.marpolbul.2023.115000
American Public Health Association (APHA)/ AWWA, (2012). Standard Methods for the examination of water and waste water, 22nd edition. American Water Works Association, Denver, WPCF Washington DC, USA, 1360 pp, ISBN 978-087553-013-0.
APHA. (1995). Standard methods for the examination of water and wastewater. In L. S. Clesceri, A. E. Greenberg, A.D. Eaton (Eds.). American Public Health Association, (19th edn., 1467). Washington, D. C.
Backman, B., Bodiš, D., Lahermo, P., Rapant, S., & Tarvainen, T. (1998). Application of a groundwater contamination index in Finland and Slovakia. Environmental Geology, 36, 55–64. https://doi.org/10.1007/s002540050320
Biswas, P. K., Uddin, N. A. S., Sultana, S., & Ahmed, T. (2017). Evaluation of heavy metal pollution indices in irrigation and drinking water systems of Barapukuria coal mine area, Bangladesh. American Journal of Water Resources, 5(5), 146–151.
Brown, R. M., McClelland, N. I., Deininger, R. A., & O’Connor, M. F. (1972). A water quality index – Crossing the psychological barrier. Indicators of Environmental Quality: Proceedings of a symposium held during the AAAS meeting in Philadelphia, Pennsylvania, December 26–31, 1971, 173–182. Springer US.
Caeiro, S., Costa, M. H., Ramos, T. B., Fernandes, F., Silveira, N., Coimbra, A., & Painho, M. (2005). Assessing heavy metal contamination in Sado Estuary sediment: An index analysis approach. Ecological Indicators, 5(2), 151–169.
CCME (Canadian Council of Ministers of the Environment) (2017). Canadian water quality guidelines for the protection of aquatic life. Water Quality Index 2.0, User’s Manual 2017 Update. Scientific Criteria Document. Canadian Council of Ministers of the Environment, Winnipeg.
Dede, Ö. T. (2016). Application of the heavy metal pollution index for surface waters: A case study for Çamlıdere. Hacettepe Journal of Biology and Chemistry, 44(4), 499–504.
Dervash, M. A., & Mushtaq, N. (2019). Chapter nine macrophytes, The natural cleaners of toxic heavy metal (THM) pollution. In H.F. Alharby, Y. Anwar , & K. Hakeem (Ed.), Environmental Contamination and Remediation (p. 255) Cambridge Scholars Publishing.
Dippong, T., Hoaghia, M. A., Mihali, C., Cical, E., & Calugaru, M. (2020). Human health risk assessment of some bottled waters from Romania. Environmental Pollution, 267, 115409. https://doi.org/10.1016/j.envpol.2020.115409
El-Bady, M. S. M. (2019). Evaluation of water quality of the surface water of the Damietta Nile Branch, Damietta Governorate, Egypt. International Journal of Environmental and Ecological Engineering, 13(11), 665–674.
El-Fayoumy, I. F. (1968). Geology of groundwater supplies in the region of the Nile Delta. PhD Dissertation. Faculty of Science, Cairo University, Egypt, 263p.
El-Safy, M. K., & Al-Ghannam, M. L. (1996). Studies on some heavy metal pollutants in fish of El-Manzala Lake. In Proceedings of the Conference on Food Borne Contamination and Egyptians Health, (pp. 151–180) Mansoura.
El-Sayed, E. S., Khater, Z., El-Ayyat, M., & Nasr, E. S. (2011). Assessment of heavy metals in water, sediment and fish tissues, from, Sharkia province, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 15(2), 125–144.
El Sayed, S. M., Hegab, M. H., Mola, H. R., Ahmed, N. M., & Goher, M. E. (2020). An integrated water quality assessment of Damietta and Rosetta branches (Nile River, Egypt) using chemical and biological indices. Environmental Monitoring and Assessment, 192, 1–16.
EWQS (Egyptian drinking water quality standards) (2007). Ministry of Health, Population Decision number 458.
FAO (Food and Agriculture Organization) (1994). Water quality for agriculture. FAO Irrigation and Drainage Paper 29. Revision, Rome, Italy.
Ghaderpoori, M., Kamarehie, B., Jafari, A., Ghaderpoury, A., & Karami, M. (2018). Heavy metals analysis and quality assessment in drinking water–Khorramabad City, Iran. Data in Brief, 16, 685–692. https://doi.org/10.1016/j.dib.2017.11.078
Githaiga, K. B., Njuguna, S. M., Gituru, R. W., & Yan, X. (2021). Water quality assessment, multivariate analysis and human health risks of heavy metals in eight major lakes in Kenya. Journal of Environmental Management, 297, 113410.
Gu, Y. G., Gao, Y. P., & Lin, Q. (2016). Contamination, bioaccessibility and human health risk of heavy metals in exposed-lawn soils from 28 urban parks in southern China’s largest city, Guangzhou. Applied Geochemistry, 67, 52–58.
Hamed, M. A. (1998). Distribution of trace metals in the River Nile Ecosystem Damietta branch between Mansoura city and Damiette province. Journal-Egyptian German Society of Zoology, 27, 399–416.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water. Department of the Interior, U. S. Geological Survey, Water-Supply, 3rd edition, Vol. 2254, University of Virginia, Charlottesville 263.
Huet, M., & Timmermans, J. A. (1986). Textbook of fish culture. Breeding and cultivation of fish (2nd Ed). Fishing News Books Ltd., England.
Kacmaz, H. (2020). Assessment of heavy metal contamination in natural waters of Dereli, Giresun: An area containing mineral deposits in northeastern Turkey. Environmental Monitoring and Assessment, 192(2), 1–2.
Magaña, J. D. (2020). Water Quality Assessment in the lower reaches of the Belize River: A baseline chemical assessment. BIOL4992 – Independent Research, Natural Resource Management Repository, 1(1), Faculty of Science and Technology, University of Belize, p. 38.
Mohan, S. V., Nithila, P., & Reddy, S. J. (1996). Estimation of heavy metals in drinking water and development of heavy metal pollution index. Journal of Environmental Science & Health Part A, 31(2), 283–289.
Muyen, Z., Rasheudujjaman, M., & Rahman, M. S. (2016). Assessment of water quality index: A case study in Old Brahmaputra river of Mymensingh District in Bangladesh. Progressive Agriculture, 27(3), 355–361.
Nour, H. E. (2015). Distribution of hydrocarbons and heavy metals pollutants in groundwater and sediments from northwestern Libya. Indian Journal of Geo-Marine Sciences, 7(44), 993–999.
Nour, H. E., Alshehri, F., Sahour, H., & El-Sorogy, A. (2022a). Evaluation of sediment and water quality of Ismailia Canal for heavy metal contamination, Eastern Nile Delta, Egypt. Regional Studies in Marine Science, 56, 10714. https://doi.org/10.1016/j.rsma.2022.102714
Nour, H. E., Helal, S., & Abdel Wahab, M. (2022b). Contamination and health risk assessment of heavy metals in beach sediments of Red Sea and Gulf of Aqaba, Egypt. Marine Pollution Bulletin, 177(1–3), 113517. https://doi.org/10.1016/j.marpolbul.2022.113517
Nour, H. E., Ramadan, F., Aita, S., & Zahran, H. (2021). Assessment of sediment quality of the Qalubiya drain and adjoining soils, eastern Nile Delta, Egypt. Arabian Journal of Geosciences, 14(7), 535. https://doi.org/10.1007/s12517-021-06891-0·
Nour, H. E., & Nouh, E. (2020). Using coral skeletons for monitoring of heavy metals pollution in the Red Sea coast, Egypt. Arabian Journal of Geoscience, 13(10), 341. https://doi.org/10.1007/s12517-020-05308-8
Odukoya, A. M., & Abimbola, A. F. (2010). Contamination assessment of surface and groundwater within and around two dumpsites. International Journal of Environmental Science & Technology, 7, 367–376.
Olatunji, O. M., & Horsfall, I. T. (2017). Effect of open waste dump on ground water quality at Rukpokwu, Port Harcourt, Nigeria.
Oregioni, B., & Aston, S. R. (1984). Determination of selected trace metals in marine sediments by flame/flameless atomic absorption spectrophotometer. IAEA Monaco Laboratory Internal Report. Now cited in reference method in pollution studies, (38).
Pandiyan, J., Mahboob, S., Govindarajan, M., Al-Ghanim, K. A., Ahmed, Z., Al-Mulhm, N., & Krishnappa, K. (2021). An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: A perspective of tackling environmental threats for food security. Saudi Journal of Biological Sciences, 28(2), 1218–1225.
Prasad, B., & Bose, J. (2001). Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environmental Geology, 41(1–2), 183–188.
Prasanna, M. V., Praveena, S. M., Chidambaram, S., Nagarajan, R., & Elayaraja, A. (2012). Evaluation of water quality pollution indices for heavy metal contamination monitoring: A case study from Curtin Lake, Miri City, East Malaysia. Environmental Earth Sciences, 67, 1987–2001.
Ramadan, F., Nour, H. E., Aita, S., & Zahran, H. R. (2021). Evaluation of heavy metals accumulation risks in water of the Qalubiya drain in East Delta, Egypt. Arabian Journal of Geosciences, 14, 1–11.
Reza, R., & Singh, G. (2010). Heavy metal contamination and its indexing approach for river water. International Journal of Environmental Science and Technology, 7(4), 785–792.
Saleem, M., Iqbal, J., & Shah, M. H. (2019). Seasonal variations, risk assessment and multivariate analysis of trace metals in the freshwater reservoirs of Pakistan. Chemosphere, 216, 715–724. https://doi.org/10.1016/j.chemosphere.2018.10.173
Sobhanardakani, S., Tayebi, L., & Hosseini, S. V. (2018). Health risk assessment of arsenic and heavy metals (Cd, Cu Co, Pb, and Sn) through consumption of caviar of Acipenser persicus from Southern Caspian Sea. Environmental Science and Pollution Research, 25, 2664–2671. https://doi.org/10.1007/s11356-017-0705-8
Tripaty, J. K., & Sahu, K. C. (2005). Seasonal hydrochemistry of groundwater in the barrier spit system of the Chilika Lagoon, India. Journal of Environmental Hydrology, 13, 1–9.
USEPA (United States Environmental Protection Agency) (2018). Drinking water standards and health advisories tables. Office of Water. U. S. Environmental Protection Agency Washington, DC March. https://www.epa.gov/dwstandardsregulations/2018-drinking-water-standardsand-advisory-tables
Ustaoğlu, F. (2020). Evaluation of the effect of dissolved metals detected in Değirmendere Dam (Amasya, Turkey) on drinking and irrigation water quality. Turkish Journal of Agriculture-Food Science and Technology, 8(12), 2729–2737.
Vasant, W., Dipak, P., Aniket, M., Ranjitsinh, P., Shrikant, M., Nitin, D., Manesh, A., & Abhay, V. (2016). GIS and Statistical Approach to Assess the Groundwater Quality of Nanded Tehsil, (M.S.) India. In S. Satapathy, & S. Das, (eds.) Proceedings of First International Conference on Information and Communication Technology for Intelligent Systems: Volume 1. Smart Innovation, Systems and Technologies, vol 50. Springer, Cham. https://doi.org/10.1007/978-3-319-30933-0_41
WHO (World Health Organization) (2011). Guidelines for drinking water quality, Fourth edition. World Health Organization, Geneva, Switzerland, 564p. ISBN: 978-92-4-154815-1.
WHO (World Health Organization) (2022). Guidelines for drinking water quality, Guidelines for drinking-water quality: Fourth edition incorporating the first and second addenda. World Health Organization, Geneva, Switzerland, 237–482.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Fatma Ramadan, Hamdy E. Nour, Nermin Abdel Wahed, Ahmad Rakha, and Mohamed Faisal contributed to the study’s conception and design. Field investigation and sample collection were performed by Nermin Abdel Wahed, Hamdy Nour, Fatma Ramadan, and Ahmad Rakha. The laboratory analyses were performed by Nermin Abdel Wahed. The manuscript was written and revised by Nermin Abdel Wahed, Fatma Ramadan, Hamdy E. Nour, Abdulgafar K. Amuda, and Mohamed Faisal. All authors commented on previous versions of the manuscript. Fatma Ramadan, Hamdy E. Nour, Nermin Abdel Wahed, Ahmad Rakha, Abdulgafar K. Amuda, and Mohamed Faisal read and approved the final version of manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This manuscript has not been published elsewhere in part or in entirety and is not under consideration by another journal. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. We have not submitted our manuscript to a preprint server before submitting it to the Environmental Monitoring and Assessment Journal.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, F., Nour, H.E., Wahed, N.A. et al. Heavy metal contamination and environmental risk assessment: a case study of surface water in the Bahr Mouse stream, East Nile Delta, Egypt. Environ Monit Assess 196, 429 (2024). https://doi.org/10.1007/s10661-024-12541-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12541-1