Abstract
Until the 1960s, Lake Maruit was one of Egypt’s most productive coastal brackish lakes. Continuous polluted discharge from Alexandria city resulted in long-term deterioration. The Egyptian government started a lake restoration program in 2010. Biological linkages between pelagic and benthic communities were assessed in November 2012 using parasitism and predation. This study examined ectoparasites infesting tilapia fish from 300 samples. The platyhelminth ectoparasite, Monogenea, and parasitic-copepod Ergasilus lizae were detected. Platyhelminthes parasitized Oreochromis niloticus and Oreochromis aureus, whereas the crustacean parasitized Coptodon zillii. The parasitic prevalence was low for Cichlidogyrus sp. and Ergasilus lizae. Benthic biotas were similar across basins. Fish abundance does not respond directly to benthic biotic components. Phytoplankton and benthic microalgae were not the main fish diet. Data on Halacaridae and fish clustered, indicating that either Halacaridae responds to their environment like fish or fish prey upon them because of their size. Linear correlations between pelagic, benthic biota, and parasite-infected fish indicate parasites may control their hosts. Some bioindicators indicate that stressed ecosystems differ from unstressed ecosystems. Fish species and biota abundances were low. Inconsistency in the food web and an absence of direct interactions between prey and predators are bioindicators of disturbed ecosystems. The low prevalence of ectoparasites and lack of heterogenous distribution of the various examined biota are bioindicators of habitat rehabilitation. Ongoing biomonitoring to better understand habitat rehabilitation is suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal lakes are productive water bodies (Kjerfve, 1994) that suffer from natural disturbances and anthropogenic stressors that cause significant deterioration of their habitats worldwide (Kennish & Paerl, 2010). Restoration of physical, chemical, and biological components of deteriorated aquatic ecosystems is the aim to improve water quality and reduce eutrophication (Søndergaard, 2007) or to control allochthonous inputs or restore the natural hydrology (Janssen et al., 2019). In contrast to natural recovery, some restoration methods, such as hydrological, mechanical, and dredging, may harm biota or have other negative effects on the environment (Alhamarna & Tandyrak, 2021; Amico et al., 2004; Wilcox' & Whillans, 1999).
Pelagic and benthic habitats are essential components of lakes (Schindler & Scheuerell, 2002), and each includes essential flora and fauna that differ in mode of life and dispersion. The pelagic–benthic linkages are complicated because of many direct and indirect intermediate relationships (Palmer et al., 2000). Predation is one direct influence of the pelagic biota that affects benthic species, which act as intermediate hosts for many parasitic species and are responsible for parasitic transmission to the food web via vertical migration to the hyper-benthic zone or the water column (Amundsen et al., 2013; Marcogliese, 2002). In shallow lakes, the ecological interaction strengths of the benthic–pelagic linkages increase as the lake depth and surface area decrease (Schindler & Scheuerell, 2002), and their study is mandatory to understand some environmental issues in aquatic habitats (Lamberti et al., 2010). The high fish production in a shallow lake is partly related to benthic organisms that represent a food source. Several fish and benthos species are directly linked with parasites as their host, whereas the latter relies on host species for feeding (Hechinger et al., 2006). Fish parasites were used to assess environmental changes in coastal waters as early warning indicators (Kleinertz & Palm, 2013; Palm, 2011). Some studies applied the parasite index in an association with health assessment index as an indicator for water quality (Crafford & Avenant-Oldewage, 2009). The highest ectoparasite infestation in some marine ecosystems is found in the most polluted areas (Vidal, 2008), the ectoparasite index is a quick and simple way to assess the health of a fish community (Sara et al., 2013), and parasite effects on fish health are recommended for monitoring in stream management programs (Nedić et al., 2018).
Most lake ecosystems have a high amount of allochthonous organic matter exceeding the autochthonous amounts, and bacterial activity can mobilize external organic matter into the food web (Kufel et al., 1997; Wetzel, 1992). Allochthonous organic matter can have a relatively high contribution (60%) to fish biomass production and most of their potential prey (Karlsson et al., 2015). But, allochthonous matter can reduce the penetration of light, which can suppress food web productivity (Craig et al., 2015). However, the source of organic matter (e.g., terrestrial or detritus-based) can affect direct and indirect use by different trophic levels and different feeding modes of invertebrates (Riera, 1998).
Lake Maruit is a brackish ecosystem located south of Alexandria, Egypt, has a small surface area and is separated from the sea by high carbonate ridges formed during the Pleistocene age (El- Masry & Friedman, 2000). It has an artificial connection with the sea via the El-Mex pump station that maintains the water depth at ~ 2.8 m to 3 m below sea level. Since the 1960s, it was a sink for different pollutants and agricultural effluents discharged from the Qalaa and El-Umum drains (El-Rayis et al., 2019). Lake Maruit’s surface area had gradually reduced to 63 km2 (Ahmed & Barale, 2014), its physical and biological conditions changed, and its water quality was effected by discharges and land reclamation projects (El Kafrawy et al., 2017). It was designated in 1992 as the most polluted lake in Egypt (EEAA, 2015).
Many studies have documented the long-term deterioration of water quality and heavy metal impacts on different biota prior to the restoration program (1969–2010, Table 1) (Abd-Allah & Ali, 1994; Abdallah, 2011; Adham et al., 2008; Amr et al., 2005; Arafa & Ali, 2008; El-Rayis et al., 2019; El Nabawi et al., 1987; Elghobashy et al., 2001; Hassaan et al., 2009; Hassan & El-Rayis, 2018; Hussein & Gharib, 2012; Khalil, 1998; Khalil & Koussa, 2013a, b; Oczkowski & Nixon, 2008; Prenner et al., 2006; Saad, 1973). Adverse effects are caused by hydrocarbon pollution and pesticides (Barakat et al., 2002, 2010, 2012; Khairy, 2013). However, studies after lake restoration (2011–2020) reported inconsistent results concerning water quality, eutrophication, and effects on biota (i.e., plankton, benthos, and fish) (Table 1). Few studies focused on the socioeconomic impacts (Abdrabo & Hassaan, 2010), and many studies proposed additional lake restoration projects (El-Hattab, 2015; El-Rayis et al., 2019; Selim & El-Raey, 2018). The restoration program started when the government dug channels in 2010 diverting the El-Qalaa drain’s discharge directly into the El-Umum drain without passing through the Main Basin. In 2012, another channel was dug to divert the Western wastewater plant (WWWPT) effluent into the El-Mex pump station via the El-Umum drain (Shaaban, 2022; Shreadah et al., 2020).
By comparing stations within lake basins, the present study assessed Lake Maruit after two phases of the restoration program. Parasitism infections are directly related to pollution and could indicate ecosystem water quality (Jiménez-García et al., 2020). So, the first objective was to assess Lake Maruit’s health by investigating the prevalence of fish ectoparasites; second, to compare biotas’ variability (fish numbers, meiofaunal groups, benthic microalgae, microbes, and phytoplankton) among stations within basins; and third, to discover trophic links in the food web and associate organic matter with pelagic and benthic biota. Disturbance can affect community structure trophic links in the grazing or detritus food chains (Vinogradov et al., 2018).
Materials and methods
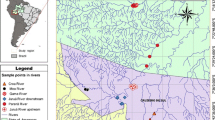
Lake Maruit extends about 25 km south of Alexandria, with a maximum width of 10 km and an average depth of one meter (Abdel-Moneim et al., 2012; Barakat et al., 2010). The lake is divided into five basins: Main Basin, the Southeast Basin, the Northwest Basin, the Southwest Basin, and the Fish Farm Basin. Three water sources feed the lake daily: (1) the main agricultural drain, El-Umum, which is connected to the M on its west side via many channels; (2) the El Qalaa drain that feeds the lake with a mixture of agricultural and sewage effluents from the East Wastewater Plant (EWWTP) (Shreadah et al., 2020); and (3) the West Wastewater Plant (WWWTP) on the southwest side of the M. The different discharge sources mix with freshwater discharge from the El Noubaryia canal, and industrial oil effluents enter from the N from the oil company at Main basin, which are transported into the Mediterranean Sea via the El-Mex pump station (Fig. 1). Sources of dumped pollution into the S are limited, and this basin is bounded by El-Umum agricultural drain and El Noubaryia freshwater canal.
Sampling sites and study design
Three stations were sampled in three basins: M, N, and S (Fig. 1). Three grab deployments were collected at each station, and three subsamples were taken from each grab deployment. Due to the shallowness and high contamination levels of Lake Maruit, stations were sampled randomly to cover most of the area in each basin. At all basins, station 1 was near the freshwater canal. Stations M2 and M3 were near WWWTPs and El-Umum drains. Stations N2 and N3 are located next to El-Umum agricultural drain and oil companies, whereas stations S2 and S3 are situated between El-Umum drain and El Noubaryia canal. The study design is hierarchical, where the basin is a fixed factor, stations are nested within basins, and deployments are nested within stations. It is a fully nested design where the correct F-test for the basin is the mean square (MS) for the basin divided by the MS for the station, and for the station, its MS is divided by the MS for deployment. In this study, 81 samples were collected for 3 basins multiplied by 3 stations, 3 deployments, and 3 replications.
Field collection
All samples were collected in November 2012, after two years of the restoration program initiation. Fish were caught with trammel nets using watershed police boat (zodiac). Sediment samples were collected using small Van Veen grab size (9 kg). Five sediment samples were taken from each grab. For meiofaunal analysis, three subsamples were taken using an 11-cm hand-held core with a 4.9-cm2 surface area. For benthic microalgae (BMA) and total organic matter (TOM) analyses, two samples were withdrawn by a small plastic vial (a surface area of 2 cm2). Sediments for microbial analysis were collected using a 1-ml sterilized syringe and stored in sterilized bags. Sediment samples were kept in an icebox for laboratory analysis within 3 to 48 h after collection. Three liters of water samples were collected by Niskin bottle for phytoplankton analysis, and a neutral 4% formalin solution with a few drops of acidified Lugol’s solution was used for fixation (Montagnes et al., 1994).
Laboratory analysis
Fish samples were sorted and identified to species level, total lengths were measured to the nearest (cm), and fish were grouped into size classes. The gills of each fish were examined for ectoparasites; and parasites were collected from the infected gill filaments, preserved in 70% ethyl alcohol, inspected, and identified on an Olympus compound microscope (Kabata, 1970).
The Huys et al. (1996) decantation method was used to extract meiofauna from sediment samples. The sample was vigorously agitated in water in a 1-l stoppered container, left to stand for a few seconds allowing sediment particles to settle, and the supernatant water was poured through a 63-μm mesh sieve. The procedure was repeated several times to remove all organisms. Organisms were sorted and counted, and abundances were extrapolated to individuals per 10 cm2 (Mitwally, 2015).
Phytoplankton samples were left for settlement for 36 to 48 h, and then, the supernatant was siphoned, the remaining volume adjusted to a 100 ml, cells were counted using an inverted microscope, and abundances were expressed as cells/l. Benthic microalgae were extracted, counted according to Schwinghamer (1983), and reported as cell/cm3 of sediment.
For microbial analysis, sediment samples were diluted in sterile water (aged and filtered through a 0.45-μm filter), spread plated onto nutrient agar medium (beef extract 3 g/l, peptone 5 g/l, and agar 15 g/l) (Guerra-Floresa et al., 1997), sonicated for 5 min, vigorously shaken, and allowed to settle for 5 s, and the collected supernatant water was incubated at 30 °C for a period of 24 to 48 h before counting colony-forming units (CFU/ ml) (Chelossi et al., 2003).
The TOM was analyzed according to El Wakeel and Riley (1957). A dried-ground sediment sample was treated with chromic acid and titrated with ferrous ammonium sulfate solution, using the ferrous phenanthroline indicator. The percentage of TOM was calculated using organic carbon values in the Olausson (1975) equation.
Data analysis
The biota and TOM data were square-root transformed. The Durbin–Watson test (Durbin & Watson, 1971) was used to check for autocorrelation between the four response variables (number of total fish, non-infected fish, infected fish with ectoparasites, and the ratio of infected to total fish number). The multicollinearity among ten biological variables and TOM was examined using Draftsman correlations (Anderson et al., 2008). The fully nested ANOVA was run to test for differences in the mean data of each response (fish) and predictors (10 different biotas and TOM) among basins and stations. In many cases, the basin factor was not significant, but the station factor was significant. This required the experimental design to devolve to a 1-way, simple main effects, model for basin–station differences, and grab deployments within each station are a nested from replication. Nested ANOVA and simple main effects ANOVA were run using SAS software. The Tukey post hoc test was applied after a significant F ratio to test for pairwise comparisons among level means, whereas the t-test was used to determine if the length of infected fish differed from the non-infected individuals using Minitab software. Multiple linear regression analysis (GLMM) was applied four times to predict linear relationships between each response variable of fish data and the biotic predictors as a proposed fish diet at the significant – level ≤ 0.05.
All biota community structures were examined with multivariate analyses using PRIMER 7 software. The first step was constructing a Bray–Curtis similarity index of square-root transformed data. Data was plotted using nonmetric multidimensional scaling (nMDS). The PERMANOVA procedure (Anderson et al., 2008) was run to test for differences in community structure among basins, stations nested in basins, and deployments nested in basins and stations (i.e., a fully nested model). The hierarchical agglomerative cluster analysis based on the association index (Clarke & Gorley, 2015) was used to examine links between pelagic biota, benthos, TOM, and their linkage to fish. The test statistic (Pi) was used to identify the deviation of the observed from the predicted permuted profiles (Clarke et al., 2008) at the significant α level of ≤ 0.05. Coupling between the individual clusters of the same trophic level was classified as a tie, whereas a “linkage” was classified as different trophic level couplings. The nMDS analysis was conducted twice: to visualize the distances between pelagic and sediment biota and to discriminate among the lake basins. The test was based on the Bray–Curtis similarity matrix and the same cluster analysis. The similarity percentage analysis (SIMPER) is using the same Bray–Curtis matrix at a lower cut-off index of 70% (Clarke & Gorley, 2015) to test for the percentage of similarity among the lake basins and station groups and to identify organisms that had contributions across lake basins and stations. Stations were classified into three groups: Gr. 1 includes stations 1 across all basins, and Gr.2 and Gr. 3 each include all stations 2 and 3, respectively.
Results
There were three tilapia fish species, Oreochromis niloticus (Linnaeus, 1758), O. aureus (Steindachner, 1864), and Coptodon zillii (Gervais, 1848), but O. niloticus was the dominant species. Each fish species grouped into three size classes (Fig. 2). O. niloticus was the longest (10–20 cm), and the smallest was C. zillii (6–17 cm). Among parasite-infected fish, O. niloticus was the longest (17–20 cm) and C. zillii was the shortest (5–10 cm). The parasitic infestation was distributed evenly among size categories of O. aureus. There were no statistically significant differences in fish size between the infected and non-infected fish (t-test, P = 0.85). The ectoparasites comprised the parasitic Monogenea (Cichlidogyrus sp.) and parasitic copepods (Ergasilus lizae). Cichlidogyrus sp. infected the two Oreochromis species, and Ergasilus lizae parasitized C. zillii. The prevalence of infections of Monogenea and parasitic copepods was 15% and 3%, respectively.
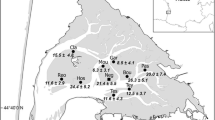
The biota predictors were tabulated by basin in Table 2. The highest and the lowest abundances (individuals 10 cm−2) of total meiofauna and its taxa were detected at the N and S basins, respectively, with few exceptions (e.g., Halacaridae). The M basin contained the highest abundances of phytoplankton (78.02 × 103 ± 32.83 × 103 cell/l), BMA (32.70 × 103 ± 27.47 × 103 cell/l), and microbes (228.1 ± 119.7 CFU/ml). The lowest phytoplankton and microbe abundances were detected at S basin (3.19 × 103 ± 1.7 103 cell/l, 167.4 ± 60.9 CFU/ml, respectively), whereas the lowest density of BMA was recorded at N basin. The percentage of TOM ranged from 8.5% at M to 4.8% at S basins.
The Durbin–Watson statistics revealed positive autocorrelations among the fish variables and ranged from 1.80 (ratio of the infected fish number to the total fish numbers) to 1.01 (the non-infected fish number). The correlation coefficients were less than the cut-off value (0.95) for all the biotic metrics and TOM, so the results are not reported here.
The hierarchical ANOVA revealed non-significant differences among basins in the mean numbers of four fish variables. However, the simple main effects ANOVA on stations nested in basins were significant for fish groups, except for the ratio between the infected fish to the total fish numbers (Table 3). The variations in the total fish and the non-infected fish counts were obvious in stations 1, 2, and 3 at the M and S stations (Fig. 3A, B), and the highest counts were detected within station 2 (M). Similar counts of infected fish were recorded within the M and S stations, except for St.3 (M), which had a higher standard deviation (Fig. 3C). The highest ratios were found at station 2 in the M and S (Fig. 3D). However, a simple main effect ANOVA revealed no variation among stations for the ratio data.
Total meiofaunal abundance and its taxa groups were significantly different among stations nested in basins (Table 4). The deployment effect was not significant except for Ostracoda (P ≤ 0.0001). The other predictors (phytoplankton, BMA, microbial counts, and TOM) varied significantly among basins for phytoplankton and TOM.
PERMANOVA (Table 5) revealed significant differences for biota predictors and TOM among basins (Pperm = 0.0332), stations nested in basins (Pperm ≤ 0.0001), and deployments nested within basins and stations (Pperm = 0.0073). The pair-wise comparisons revealed noticeable differences for biota within stations nested at the M (Pperm ≤ 0.0001). S stations were significantly different, as P(MC) revealed. At the N stations, significant variations were detected between station 1 compared to stations 2 and 3 (P(MC) = 0.0002 and 0.0036, respectively, for station 1 vs. station 2 and station 3).
The four GLMM models revealed a significant contribution of nine predictor variables for the infected fish model, but only a minimum of two predictor variables for the non-infected/infected ratio model (Table 6). However, the influence of biotic taxa had negative coefficients (− 0.12– −0.25), except for phytoplankton, and BMA, which were near zero (0.01). The TOM had the highest contribution to the fish models and ranged from −1.95 to −0.96, respectively, for the total fish and the infected fish with ectoparasites models. The regression R2 values ranged from 49 to 52%, except for the ratio model where R2 was only 8%. Meiofauna had the highest significant positive contribution to the infected fish (0.37), followed by a negligible direct contribution of phytoplankton and BMA in the same model.
Dendrograms of the hierarchical cluster analysis (Fig. 4) showed 11 individual groups, forming four clusters, 16 significant ties (0.01), and their similarities decreased as the Pi statistic values increased, except for tie 19 (for phytoplankton similarity to TOM and microbes). The cluster on the right comprised phytoplankton, BMA, microbes, and TOM, comprising three pairs of ties, 16, 19, and 20, and are all primary producers. The similarities decreased from 86 to 71%, respectively, between ties 16 and 20. The clusters in the center represented sediment fauna that contained five ties; 13, 14, 15, 17, and 18, and their similarities percentage ranged from 95% with a tie between nematodes and meiofauna (13) to 80% between turbellaria and ostracods tie (18). The intermediate faunal cluster formed a significant linkage with the primary producers (> 67% similarity). The third cluster consisted of total fish numbers and linked significantly to linkage 21, forming a new one (22) with similarity and Pi values equal 61% and 50, respectively. The fourth cluster of Halacaridae (sea mites), a linkage 23, formed a link with the fish linkage 22 with the lowest similarity at 54%.
The hierarchical cluster analysis based on taxa levels and linkages at a significant level = 0.01. Similarity values in parentheses. Abbreviations: BMA, benthic microalgae; TOM%, percentage of total organic matter. All taxa were listed in Table 5
According to the results of the nMDS study (Fig. 5A), phytoplankton and BMA cluster close together on the left-hand side. The total number of fish is located at the bottom of the right-hand side, at equal distances from Halacaridae and TOM. Total meiofaunal, nematode, and microbial data were close to one another on the mid-top of the plot. Nematodes and TOM were arranged in a crescent shape among the other biota taxa. There were six distinct groupings among the basins (Fig. 5B). The M grouping was made up of four dispersed subgroupings evenly spaced between stations M1 and M3; all are on the right side of the plot. However, there was an overlap among stations’ replicates at the M. The S had three distinct subgroupings: S1, S2, and S3, which were distributed on the left, in the center, and somewhat to the right. In the middle, two prominent N groups formed. There is no overlap between replicated S and N stations. However, stations at each basin are clustering close to one another and in some cases; the deployments within stations are clustering.
A The non-metric multidimensional scaling (nMDS) analysis for pelagic and benthic biota. Abbreviations: BMA, benthic microalgae; TOM%, percentage of total organic matter. B The nMDS analysis across the lake for multiple data groupings at each basin. Abbreviation: M, Main Basin; N, Northwestern Basin; S, Southwestern Basin. The numbers in the data labels indicate station and replicate
Results of SIMPER analysis (Table 7) revealed similarities across each basin ranging from 84.60 (N) to 66.86% (S), and the range across the station groups (Gr.) varied between 61.26% (Gr.1) and 71.79% (Gr.3). The phytoplankton and BMA had the highest percentage contribution to the similarity across the M and N, and total meiofauna contributed 8.04% to the similarity at MB. The average dissimilarities between M vs. S, M vs. N, and S vs. N were 52.54%, 35.15%, and 39.60%, respectively, whereas values versus station groups fluctuated between ~ 41%, 40%, and 33%, respectively, for Gr.1 vs. Gr.2, Gr.1 vs. Gr.3, and Gr.2 vs. Gr.3. The contribution of the phytoplankton and BMA was responsible for the discrimination between basins (Table 6). The similarities among stations’ groups were higher than the dissimilarity values. The phytoplankton and BMA were essential contributors to similarities and dissimilarities among stations' groups, and meiofaunal contributed ~ 8.50% to the similarities within Gr.1 (stations 1) across all basins.
Discussion
The present study assessed the biological condition of the Lake Maruit ecosystem after restoration. Fish catch across the lake retained low number of species, consisting of three tilapia species: Oreochromis niloticus, O. aureus, and Coptodon zillii, and their distributions were similar among basins. Ectoparasites on fish had relatively low prevalence rates (3 to 15%). The helminthic Monogenea infested the two Oreochromis species, whereas the crustacean ectoparasites infected C. zillii. The total fish and the non-infected fish were correlated negatively with TOM, ostracods, and microbes. Surprisingly, the regression coefficients between the infected fish and most biota predictor data differed in sign and magnitude. The TOM concentration was related to fish abundance, but not phytoplankton and benthic microalgae, and there were no consistent pelagic–benthic linkages. The phytoplankton and benthic microalgae drove the similarity and the dissimilarity indices across the lake basins and stations, and meiofauna contributed to the similarity within the lake’s basins and nested stations. Comparisons with earlier studies prior to the restoration program (Table 1) revealed some evidence of restoration, such as low ectoparasites prevalence, similar fish and biota distribution, and a lack of eutrophication. The low number of catch species and abundance of fish and biota, the lack of correlations between fish and biota, and the inconsistent linkages between food web components suggest that the restoration is incomplete.n the linkageFisheries in Egyptian coastal lakes have suffered from overfishing, illegal fishing, and the deterioration of the water quality for many years, and this is likely a cause of decreased fish diversity (Mehanna, 2020). However, the species composition found in the present study (i.e., 3 species) was low relative to the 25 and 11 species found in Lake El Borollus and Qarun Lake, respectively (El-Serafy et al., 2014; Younis, 2018). Our findings might be attributed to the synergistic effects of the restoration and an accidental oil leak from the Al-Amerya and Pertoject petroleum companies. Mechanical and hydraulic dredging of the aquatic ecosystem increases fish mortality, impacts physiology, and changes fish behavior (Wenger et al., 2017). The polycyclic aromatic hydrocarbon concentrations were one to two orders of magnitude higher at Lake Maruit than in other estuaries worldwide (Barakat et al., 2010). Moreover, according to the General Authority for Fish Resources Development (GAFRD), the total fish production from Egypt’s natural lakes has dramatically declined from 43% by the end of the 1990s to 19% in 2018 (Mehanna, 2022).
The ectoparasites infection rate in the current study was 3% (parasitic-copepod) and 15% (Monogenea), which is a relatively low infestation rate compared to the 57% rate at Qarun Lake (Mehanna, 2020). El-Rashidy (unpublished data) examined tilapia fish for parasitic copepods from Lake Maruit in 2000 before lake restoration and detected a 15.5% prevalence of E. lizae on tilapia species. Nofal and Abdel-Latif (2017) recorded an 76% infection rate of Monogenea in Lake Manzala, accompanied by massive fish death due to deterioration of water quality. The infection rate found in Lake Maruit lies between the frequent and irregular Monogenea infection rate according to Koniyo et al. (2020) and a low copepod prevalence (Klimpel et al., 2006). Mitwally (2015) and Shreadah et al. (2020) documented noticeable improvements in Lake Maruit environmental conditions, except for phosphorous loading, which could be responsible for the infestation rate decline. Low ectoparasites prevalence is a good sign of lake rehabilitation after the restoration process.
Heterogeneous distributions of benthic biota are common in disturbed versus undisturbed environments (Pan et al., 2000; Solar et al., 2015). So, the lack of significant variations among basins found here could be a good indicator of the restoration success. All the biota responded to the environmental conditions in a similar way, and environmental conditions were suitable for pelagic and benthic biota to survive. Most physicochemical and sedimentological parameters were improved after the restoration program (Mitwally, 2015; Shreadah et al., 2020). However, the causes of the relatively low diversity of benthic biota in Lake Maruit are probably related to long-term exposure to disturbance, and these biotas require longer periods to recover. It can take a long time before biota composition can recover from disturbance in ecosystems according to Janse et al. (2015). High variability at the smaller station-scale (Tables 3, 4, and 5) agreed with findings by Mitwally and Abada (2008) indicating that small-scale heterogeneous distributions are due to the local biological interaction and physical processes, which could attenuate among-basin variability.
Aquatic ecosystems contain many complex trophic links (Gallardo et al., 2016) but the multiple linear regression (Table 6) indicates that any specific taxa is potentially part of the fish diet in Lake Maruit. The lack of strong links could be related to the irregularity of fish feeding patterns and opportunistic feeding habits. Tilapia species diets include fauna and flora (El-Sayed, 2019). However, the inverse relationship between sediment–biota and fish may reflect their different responses to TOM as a proxy for allochthonous sources flowing into Lake Maruit. The TOM concentration on average (Table 2) was ~ 7 times higher than that at El-Mex Bay (1.43%), according to Salem et al. (2013). The allochthonous organic matter effects fish production and food web productivity (Craig et al., 2015), probably due to the attenuation of light penetration, as suggested by Karlsson et al. (2009). Mitwally (2015) documented a positive response between meiofauna and organic matter, whereas fish abundance had a negative linear regression with TOM in Lake Maruit (Table 6). Fish and benthic fauna respond differently to anthropogenic stressors in shallow ecosystems (Snickars et al., 2014). There is a linear regression link between the numbers of biota and the fish with ectoparasite infections (Table 6), and this is likely because parasites alter their host’s behavior and may have indirectly mediated the relationships between predators and prey (Forrester et al., 2019; Grutter et al., 2011). Johnson (2001) discovered a change in fish diet following parasite infection, although the positive association between total meiofauna and infected fish suggests that fish may switch diets as an indirect effect of parasite infestation. It is necessary to conduct more research on the feeding strategy and the impact of ectoparasites on fish behavior and community structure.
The food web in aquatic habitats is complex and has intricate connections (Woodward, 2009). However, the Lake Maruit food web consisted of four simple groups (Fig. 4), probably due to the absence of some trophic links, such as macrofauna and zooplankton. Thompson et al. (2012) concluded that some highly resolved, very comprehensive food webs still have limitations, as they ignored microbial taxa and soft-bodied meiofaunal taxa because obtaining the data is time-consuming and expensive. The presence of the four components of primary producers (i.e., phytoplankton, BMA, microbes, and organic matter) indicates that Lake Maruit is a grazing and detritus-based ecosystem. The intermediate faunal cluster (Fig. 4) highlights the importance of meiofauna as a central trophic linkage, not a dead-end, which agrees with Schmid-Araya et al. (2002). The high number of linkages (22) between fish, meiofauna, and primary producers (Fig. 4) reflects that tilapia species likely have opportunistic feeding modes and that meiofaunal organisms are likely part of the diet for fish in Lake Maruit, as Schmid-Araya et al. (2016) concluded. However, Halacaridae (Fig. 5) was the only biota that had a direct link with fish occupying the top of the food chain, which is an inconsistent finding in that it is not found in other natural ecosystems (Peel et al., 2019). The causes of this contradiction may be connected to the anthropogenic stressors impacting Lake Maruit that caused lake size shrinkage (from 200 to 63 km2) and the engineered restoration, as the natural disruption and human-induced changes modified the food web (Tunney et al., 2012). However, it is also possible that the fish and Halacaridae are simply correlated without causation or have similar diets. Further biomass investigations beyond the abundance data are recommended in future studies for a better understanding of food web connectivity.
Phytoplankton and BMA comprised a bulk of the O. niloticus diet (Dempster et al., 1993), detritus represented ~ 45% of O. aureus (Al-Wan & Mohamed, 2019), and plant tissue was dominant in C. zillii stomach (Shalloof et al., 2020). Our findings of the correlation between TOM, sediment biota, and fish, and between fish, phytoplankton, and BMA (Fig. 5A) could indicate that Lake Maruit is a detritus-driven ecosystem. The high TOM concentration drives the relationship between pelagic and benthic habitats, as it reduced light, diminishing fish vision, and decreased the accessibility of sediment fauna as fish prey. The Halacaridae’s size ranged from 0.75 to 0.90 mm (Proctor, 2004), and this may allow the fish’s vision to hunt them easily. It is known that the larger the prey size, the greater the fish’s ability to locate and eat them (Wetzel, 2001). Many studies documented fish feeding on Halacaridae (Luxton, 1990; Mcmahon et al., 2005), meiofaunal taxa (Ptatscheck et al., 2020), and the intensive feeding of O. niloticus in the River Nile, Egypt, on nematodes (Abada et al., 2017). The intermediate trophic link of zooplankton and the low coefficients (Table 6) between phytoplankton, BMA, and fish indicates a lack of a strong trophic link among these groups.
The multiple subset groups in each basin (Fig. 5B) are likely the result of the different modes of biota lifestyle and dispersion. Four subgroupings at the M basin revealed the active movement of pelagic fish, horizontal phytoplankton passive movement, and sediment-associated biota with limited vertical passive migrations of meiofauna. However, two subgroups at N and S suggested the unsuitability of these basins to the active fish movement, probably due to smaller surface area and shallow depths. The dynamic dispersal modes enable the fish to move freely and select the most suitable environmental conditions, whereas the passive phytoplankton dispersers are limited by wind-induced currents (Lansac-Toha et al., 2019). The different dispersal modes of biota drove the compositional dissimilarity within a lentic ecosystem (Oikonomou & Stefanidis, 2020). The similarity within MB data suggested obvious linkages between pelagic and benthic biota, and the overlapping indicated the high basins similarity.
The relatively high values of similarity percentages among basins compared to those within station groups in the ANOVAs (Tables 3 and 4) and the nMDS (Fig. 5B) indicate that heterogeneity is higher in small spatial scales than larger spatial scales. This is probably due to the variable local environmental factors at each site. Hawkins et al. (2000) attributed the significant differences in fish and fauna within small distances to local environmental habitat features of each location and commented that similarities among sites declined as a function of the distances between them.
Phytoplankton and BMA were responsible for the high similarity within the MB and NW (Table 7), probably due to passive dispersion being the driver of phytoplankton distribution (Beisner et al., 2006). However, the higher average abundance of BMA abundance compared to phytoplankton abundance at the S could be the reason for the increase in total meiofauna contribution to the similarity (Table 7), as BMA is a favorable meiofaunal diet (Mitwally et al., 2004; Montagna, 1984). The synergetic factors within each basin at Lake Maruit were responsible for the meiofauna response to the environmental conditions (Mitwally, 2015).
Comparisons with earlier studies before restoration (Abdallah, 2011; Khalil, 1998) revealed some temporary signs of rehabilitation, as eutrophication was not indicated by the decline in phytoplankton abundance, which was three times (Table 2) lower than that recorded in autumn 2004 (Hussein & Gharib, 2012). Microbial counts were low relative to those found by Hassan and El-Rayis (2018), which is an indicator of sewage discharge reduction. The high phosphorous concentration (Mitwally, 2015; Shreadah et al., 2020) probably was the factor that masked the long-term restoration effectiveness, as an increase in chlorophyll concentration and bacteria was detected in 2016 (Abd El-alkhoris et al., 2020; Abdelfattah et al., 2017; El Zokm et al., 2018) (Table 1). Meiofauna abundance at Lake Maruit was slightly lower (Mitwally, 2015) than that at the least polluted El Borollus Lake (Mitwally & Abada, 2008), indicating meiofaunal tolerance to disturbance due to its broad range of groups (Schratzberger & Warwick, 1999), rapid re-colonization, and ability to migrate down the sediment (Schratzberger et al., 2000). The macrofaunal size fraction was dominated by broken shells and fragmented bodies of three groups after restoration (Mitwally, 2015) compared to 11–12 species before restoration (Khalil & Koussa, 2013a; Khalil et al., 2016), indicating macrofaunal sensitivity to the mechanical restoration processes. However, we excluded macrofauna and zooplankton from the current study because of the prevalence of dead macrofauna bodies and the relatively empty zooplankton samples after restoration versus Khalil and Koussa (2013b) before restoration. A low ectoparasite infestation rate was an indicator of restoration effectiveness. However, the drop in pelagic and benthic biota abundances, lack of trophic correlations, and inconsistent linkages among trophic groups is likely due to mechanical effects of restoration construction, such as dredging impacts aquatic life (Chen et al., 2021). Further monitoring over time and seasons is needed to assess biota recovery after restoration and to evaluate long-term change.
In the current study, pelagic and benthic biota interactions could be used as bioindicators in monitoring and assessment programs for aquatic habitats. Some evidence indicates stressed vs. unstressed ecosystems. The stressed ecosystem suffered from low fish species and other biota abundances compared to its earlier history or surrounding areas. The inconsistency in the food web and the lack of direct relationships between prey and predators are other bioindicators of disturbed ecosystems. Bioindicators of the rehabilitated habitat’s unstressed condition are a low prevalence of ectoparasites on fish and the absence of heterogeneous distribution of different investigated biota.
Conclusion
The current study assessed the Lake Maruit, Egypt, after the restoration of long-term deterioration. The low prevalence of ectoparasites on fish and the high similarity of pelagic–benthic biota distribution across lake basins are positive indicators for lake rehabilitation after the restoration. However, the inconsistent links in the food web and the high organic matter that correlate with different biota could be due to the anthropogenic stressor. The large size of Halacaridae enables fish to locate and prey up on them and could be the cause of their positive correlation. Our results concluded that there is some evidence of the effectiveness of the restoration program at Lake Maruit. However, others could indicate that Lake Maruit suffered from degradation in 2012.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Abada, A. E. A., Ghanim, N. F., Sherif, A. H., & Salama, N. A. (2017). Benthic freshwater nematode community dynamics under conditions of tilapia aquaculture in Egypt. African Journal of Aquatic Science, 42(4), 381–387. https://doi.org/10.2989/16085914.2017.1410464
Abd-Allah, A. M. A., & Ali, H. A. (1994). Residue levels of chlorinated hydrocarbons compounds in fish from El Max Bay and Maryut Lake, Alexandria Egypt. Toxicological & Environmental Chemistry, 42(1–2), 107–114. https://doi.org/10.1080/02772249409357992
Abd El-alkhoris, Y. S., Abbas, M. S., Sharaky, A. M., & Fahmy, M. A. (2020). Assessment of water and sediments pollution in Maruit Lake Egypt. Egyptian Journal of Aquatic Biology & Fisheries, 24(1), 145–160.
Abdallah, M. A. (2011). Potential for internal loading by phosphorus based on sequential extraction of surficial sediment in a shallow Egyptian Lake. Environmental Monitoring and Assessment, 178(1–4), 203–212. https://doi.org/10.1007/s10661-010-1682-2
Abdel-hamid, O. M., & Galal, T. A. (2019). Effect of pollution type on the phytoplankton community structure in Lake Mariut, Egypt. Egyptian Journal of Botany, 59(1), 39–52.
Abdel-Moneim, A. M., El-Saad, A. M. A., Hussein, H. K., & Dekinesh, S. I. (2012). Gill oxidative stress and histopathological biomarkers of pollution impacts in Nile Tilapia from Lake Mariut and Lake Edku Egypt. Journal of Aquatic Animal Health, 24(3), 148–160. https://doi.org/10.1080/08997659.2012.675924
Abdelfattah, S. S., Massoud, M. A., Amer, R. A., & Ghorab, M. A. (2017). Assessment of the physico-chemical characteristics and water quality analysis of Mariout Lake, Southern of Alexandria Egypt. Journal of Environmental & Analytical Toxicology, 7(1), 1–19. https://doi.org/10.4172/2161-0525.1000421
Abdrabo, M. A., & Hassaan, M. A. (2010). Wetland socioeconomy: The case of Lake Maryut (Egypt). In F. Scapini & G. Ciampi (Eds.), Coastal water bodies (pp. 21–43). Dordrecht: Springer. https://doi.org/10.1007/978-90-481-8854-3_2
Adham, K., Khairalla, A., Abu-Shabana, M., Abdel-Maguid, N., & Moneim, A. A. (2008). Environmental stress in Lake Maryut and physiological response of Tilapia zilli Gerv. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, 32(9–10), 2585–2598. https://doi.org/10.1080/10934529709376705
Ahmed, M., & Barale, V. (2014). Satellite surveys of lagoon and coastal waters in the southeastern Mediterranean area. In V. Barale & M. Gade (Eds.), Remote sensing of the African seas (pp. 379–401). Dordrecht: Springer. https://doi.org/10.1007/978-94-017-8008-7_19
Al-Wan, S. M., & Mohamed, A.-R. M. (2019). Analysis of the biological features of the blue tilapia, Oreochromis aureus in the Garmat Ali River, Basrah, Iraq. Asian Journal of Applied Sciences, 7(06), 776–787. https://pdfs.semanticscholar.org/ad88/cb40f5c1f72c5ad0b205a137c22fedc3318e.pdf
Alhamarna, M. Z., & Tandyrak, R. (2021). Lakes Restoration Approaches. Limnological Review, 21(2), 105–118. https://doi.org/10.2478/limre-2021-0010
Amico, F. D., Darblade, S., Avignon, S., Blanc-Manel, S., & Ormerod, S. J. (2004). Odonates as indicators of shallow lake restoration by liming: Comparing adult and larval responses. Restoration Ecology, 12(3), 439–446.
Amr, H. M., EL-Tawila, M. M., & Ramadan, H. M. M. (2005). Assessment of pollution levels in fish and water of Main Basin, Lake Maruit. The Journal of Egyptain Public Health Association, 80(1,2), 51–76.
Amundsen, P.-A., Lafferty, K. D., Knudsen, R., Primicerio, R., Kristoffersen, R., Klemetsen, A., & Kuris, A. M. (2013). New parasites and predators follow the introduction of two fish species to a subarctic lake: Implications for food-web structure and functioning. Oecologia, 171, 993–1002. https://doi.org/10.1007/s00442-012-2461-2)
Anderson, M. J., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVA+for PRIMER: Guide to software and statistical methods. PRIMER-e (Quest Research Limited): Plymouth, Uk, 1–214.
Arafa, M. M., & Ali, A. T. (2008). Effect of some heavy metals pollution in Lake Mariout on Oreochromis niloticus fish. Egyptian Journal of Comparative Pathology and Clinical Pathology, 21(3), 191–201.
Barakat, A. O., Kim, M., Qian, Y., & Wade, T. L. (2002). Organochlorine pesticides and PCB residues in sediments of Alexandria Harbour Egypt. Marine Pollution Bulletin, 44, 1421–1434.
Barakat, A. O., Mostafa, A., Wade, T. L., Sweet, S. T., & El Sayed, N. B. (2010). Spatial distribution and temporal trends of polycyclic aromatic hydrocarbons (PAHs) in sediments from Lake Maryut, Alexandria Egypt. Water, Air, & Soil Pollution, 218(1–4), 63–80. https://doi.org/10.1007/s11270-010-0624-5
Barakat, A. O., Mostafa, A., Wade, T. L., Sweet, S. T., & El Sayed, N. B. (2012). Spatial distribution and temporal trends of persistent organochlorine pollutants in sediments from Lake Maryut, Alexandria Egypt. Marine Pollution Bulletin, 64(2), 395–404. https://doi.org/10.1016/j.marpolbul.2011.12.019
Beisner, B. E., Peres-Neto, P. R., Lindstrom, E. S., Barnett, A., & Longhi, M. L. (2006). The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology, 87(12), 2985–2991.
Chelossi, E., Vezzulli, L., Milano, A., Branzoni, M., Fabiano, M., Riccardi, G., & Banat, I. M. (2003). Antibiotic resistance of benthic bacteria in fish-farm and control sediments of the Western Mediterranean. Aquaculture, 219(1–4), 83–97. https://doi.org/10.1016/s0044-8486(03)00016-4
Chen, X., Wang, Y., Sun, T., Huang, Y., Chen, Y., Zhang, M., & Ye, C. (2021). Effects of sediment dredging on nutrient release and eutrophication in the gate-controlled estuary of northern Taihu Lake. Journal of Chemistry, 2021, 1–13. https://doi.org/10.1155/2021/7451832
Clarke, K. R., & Gorley, R. N. (2015). Getting started with PRIMER 7. PRIMER-E Ltd, Plymouth, 1st edition, 1–18.
Clarke, K. R., Somerfield, P. J., & Gorley, R. N. (2008). Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology, 366(1–2), 56–69. https://doi.org/10.1016/j.jembe.2008.07.009
Crafford, D., & Avenant-Oldewage, A. (2009). Application of a fish health assessment index and associated parasite index to Clarias gariepinus (Teleostei: Clariidae) in the Vaal River system, South Africa. African Journal of Aquatic Science, 34(3), 261–272. https://doi.org/10.2989/AJAS.2009.34.3.8.984
Craig, N., Jones, S. E., Weidel, B. C., & Solomon, C. T. (2015). Habitat, not resource availability, limits consumer production in lake ecosystems. Limnology and Oceanography, 60(6), 2079–2089. https://doi.org/10.1002/lno.10153
Dempster, P. W., Beveridge, M. C. M., & Baird, D. J. (1993). Herbivory in the tilapia Oreochromis nilotics: A comparisons of feeding rates on phytoplankton and periphyton. Journal of Fish Biology, 43, 385–392.
Durbin, J., & Watson, G. S. (1971). Testing for serial correlation in least squares regression. III. Biometrika, 58(1), 1–19.
EEAA. (2015). Alexandria Coastal Zone Management Project (ACZMP). Ministry of Environmental Affairs & Egyptian Environmental Affairs Agency.SFG1484V, 2(6), 1–15.
Elghobashy, H. A., Zaghloul, K. H., & Metwally, M. A. (2001). Effect of some water pollutants on the Nile tilapia, Oreochromisniloticus collected from the River Nile and some Egyptian lakes. Egyptian Journal of Aquatic Biology and Fisheries, 5(4), 251–219.
El-Hattab, M. M. (2015). Change detection and restoration alternatives for the Egyptian Lake Maryut. The Egyptian Journal of Remote Sensing and Space Science, 18(1), 9–16. https://doi.org/10.1016/j.ejrs.2014.12.001
El Kafrawy, S. B., Noha, S. D., & Mohamed, A. M. (2017). Monitoring the environmental changes of Mariout Lake during the last four decades using remote sensing and GIS techniques. MOJ Ecology & Environmental Sciences, 2(5), 00037. https://doi.org/10.15406/mojes.2017.02.00037
El- Masry, K., & Friedman, G. M. (2000). Metal pollution in carbonate sediments of Main Basin of Mariute Lake, Alexandria. Egypt. Carbonates and Evaporites, 15(2), 169–194.
El Nabawi, A., Heinzow, B., & Kruse, H. (1987). As, Cd, Cu, Pb, Hg, and Zn in fish from the Alexandria Region. Egypt. Bulletin of Environmental Contamination and Toxicology, 39, 889–897.
El-Rayis, O. A., Hemeda, E. I., & Shaaban, N. A. (2019). Steps for rehabilitation of a Lake suffering from intensive pollution; Lake Mariut as a case study. Egyptian Journal of Aquatic Biology & Fisheries, 32(2), 331–345.
El-Sayed, A.-F.M. (2019). Tilapia culture (p. 275). CABI Publishing.
El-Serafy, S. S., El-Haweet, A. E. A., El-Ganiny, A. A., & El-Far, A. M. (2014). Qarun Lake fisheries; fishing gears, species composition and catch per unit effort. Egyptian Journal of Aquatic Biology and Fisheries, 18(2), 39–49.
El Wakeel, S., & Riley, J. (1957). The determination of organic carbon in marine muds. ICES Journal of Marine Science, 22(2), 180–183.
El Zokm, G. M., Tadros, H. R. Z., Okbah, A. M., & Ibrahim, G. H. (2018). Eutrophication assessment using TRIX and Carlson’s indices in Lake Mariout Water. Egypt. Egyptian Journal of Aquatic Biology & Fisheries, 22(5), 321–339.
Forrester, G. E., Chille, E., Nickles, K., & Reed, K. (2019). Behavioural mechanisms underlying parasite-mediated competition for refuges in a coral reef fish. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-52005-y
Gallardo, B., Clavero, M., Sánchez, M. I., & Vilà, M. (2016). Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology, 22(1), 151–163. https://doi.org/10.1111/gcb.13004
Grutter, A. S., Crean, A. J., Curtis, L. M., Kuris, A. M., Warner, R. R., & McCormick, M. I. (2011). Indirect effects of an ectoparasite reduce successful establishment of a damselfish at settlement. Functional Ecology, 25(3), 586–594. https://doi.org/10.1111/j.1365-2435.2010.01798.x
Guerra-Floresa, A. L., Abreu-Groboisb, A., & Gomez-Gil, B. (1997). A comparison between total viable count by spread plating and AquaPlakB for enumeration of bacteria in water from a shrimp farm. Journal of Microbiological Methods, 30, 217–220.
Hassaan, M. A., Serrano, L., Serrano, O., Mateo, M. A., & Abdrabo, M. (2009). Suitability analysis of water quality in Lake Maryuit for economic activities using GIS techniques. Sustainable Management Of Mediterranean Coastal Water Bodies, Firenze University Press, 237–253. https://library.oapen.org/bitstream/handle/20.500.12657/54894/1/9788864530154.pdf#page=248
Hassan, M. A., & El-Rayis, O. A. (2018). Calculations and solutions for heavy metals pollution load from Umum and Qalaa drains to Lake Maruit Egypt. Indian Journal of Geo Marine Science, 47(7), 140–1467.
Hassaan, M. A., El-Rayis, O. A., & Hemada, E. I. (2017). Estimation of the redox potential of Lake Mariut drainage system (Qalaa and Umum drains). Hydrology, 5(6), 82. https://doi.org/10.11648/j.hyd.20170506.11
Hawkins, C. P., Norris, R. H., Gerritsen, J., Hughes, R. M., Jackson, S. K., Johnson, R. K., & Stevenson, R. J. (2000). Evaluation of the use of landscape classifications for the prediction of freshwater biota: Synthesis and recommendations. Journal of the North American Benthological Society, 19(3), 541–556.
Hechinger, R. F., Lafferty, K. D., Huspeni, T. C., Brooks, A. J., & Kuris, A. M. (2006). Can parasites be indicators of free-living diversity? Relationships between species richness and the abundance of larval trematodes and of local benthos and fishes. Oecologia, 151(1), 82–92. https://doi.org/10.1007/s00442-006-0568-z
Helal, A. M., Attia, A. M., & Mustafa, M. M. (2017). Water conservation and management of fish farm in Lake Mariout. Life Science Journal, 14(11), 44–51. https://doi.org/10.7537/marslsj141117.08
Hussein, N. R., & Gharib, S. M. (2012). Studies on spatio-temporal dynamics of phytoplankton in El-Umum drain in west of Alexandria Egypt. Journal of Environmental Biology, 33, 101–105.
Huys, R., Gee, J. M., Moore, C., & Hamond, R. (1996). Marine and brackish water harpacticoid copepods Part I. In: Synopses of the British fauna (new series), Kermack, D. M., Barnes R. S. K and Crothers, J. H. (eds) London (2ndedition), 1–352.
Janse, J. H., Kuiper, J. J., Weijters, M. J., Westerbeek, E. P., Jeuken, M. H. J. L., Bakkenes, M., Alkemade, R., Mooij, W. M., & Verhoeven, J. T. A. (2015). GLOBIO-Aquatic, a global model of human impact on the biodiversity of inland aquatic ecosystems. Environmental Science & Policy, 48, 99–114. https://doi.org/10.1016/j.envsci.2014.12.007
Janssen, A. B. G., van Wijk, D., van Gerven, L. P. A., Bakker, E. S., Brederveld, R. J., DeAngelis, D. L., Janse, J. H., & Mooij, W. M. (2019). Success of lake restoration depends on spatial aspects of nutrient loading and hydrology. Science of the Total Environment, 679, 248–259. https://doi.org/10.1016/j.scitotenv.2019.04.443
Jiménez-García, I., Rojas-García, C. R., & Mendoza-Franco, E. F. (2020). Ecto-parasitic infection in Nile tilapia (Oreochromis niloticus) fry during male reversal in Veracruz, México. International Aquatic Research, 12, 197–207. https://doi.org/10.22034/IAR.2020.1898558.1046
Johnson, M. W. (2001). Indicators (parasites and stable isotopes) of trophic status of yellow Perch (Percha flavesscens Mitchill) in nutrient poor Canadian shield lakes. Master thesis, University of Manitoba, Canada 266.
Kabata, Z. (1970). Diseases of fishes: Crustacea as enemies of fishes. TFH Publications Neptune City.
Karlsson, J., Bergstro, A.-K., Bystro, P., Gudasz, C., & Rodri´Guez, P., & Hein, C. (2015). Terrestrial organic matter input suppresses biomass production in lake ecosystems. Ecology, 96(11), 2870–2876.
Karlsson, J., Byström, P., Ask, J., Ask, P., Persson, L., & Jansson, M. (2009). Light limitation of nutrient-poor lake ecosystems. Nature, 460(7254), 506–509. https://doi.org/10.1038/nature08179
Kennish, M. J., & Paerl, H. W. (2010). Coastal lagoons: Critical habitats of environmental change. CRC Press.
Khairy, M. A. (2013). Assessment of priority phenolic compounds in sediments from an extremely polluted coastal wetland (Lake Maryut, Egypt). Environmental Monitoring and Assessment, 185(1), 441–455. https://doi.org/10.1007/s10661-012-2566-4
Khalil, M. T. (1998). Impact of pollution on productivity and fisheries of fisheries of Lake Mariut Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 2(2), 1–17.
Khalil, M. T., & Koussa, A. A. (2013). Impact of pollution on the biodiversity of bottom fauna of Lake Maruit Egypt. the Global Journal of Fisheries and Aquatic Research, 6(6), 282–294.
Khalil, M. T., & Koussa, A. A. (2013). Ecological studies on the zooplankton community of Lake Maruit Egypt. the Global Journal of Fisheries and Aquatic Research, 6(6), 295–311.
Khalil, M. T., Saad, A. A., Fishar, M. R. A., Abdel-Meguid, M. A., El-Bably, W. F., & Mohammed, K. A. (2016). Impact of pollution on macrobenthos diversity of Qalla Drainage System, Lake Maruit Egypt. International Journal of Environmental Science and Engineering, 7, 34–40.
Kjerfve, B. (1994). Chapter 1 Coastal lagoons. Coastal Lagoon Processes, 60, 1–8. https://doi.org/10.1016/s0422-9894(08)70006-0
Kleinertz, S., & Palm, H. W. (2013). Parasites of the grouper fish Epinephelus coioides (Serranidae) as potential environmental indicators in Indonesian coastal ecosystems. Journal of Helminthology, 89(1), 86–99. https://doi.org/10.1017/s0022149x1300062x
Klimpel, S., Palm, H. W., Busch, M. W., Kellermanns, E., & Rückert, S. (2006). Fish parasites in the Arctic deep-sea: Poor diversity in pelagic fish species vs. heavy parasite load in a demersal fish. Deep Sea Research Part I: Oceanographic Research Papers, 53(7), 1167–1181. https://doi.org/10.1016/j.dsr.2006.05.009
Koniyo, Y., Juliana, Pasisingi, N., & Kalalu, D. (2020). The level of parasitic infection and growth of red tilapia (Oreochromis sp.) fed with vegetable fern (Diplazium esculentum) flour. AACL Bioflux, 13(5), 2421–2430.
Kufel, L., Prejs, A., & Rybak, J. I. (1997). Shallow Lakes '95: Trophic cascades in shallow freshwater and brackish lakes. In Dumont, H. J. (ed.), Developments in Hydrobiology, 119, 416.
Lamberti, G. A., Chaloner, D. T., & Hershey, A. E. (2010). Linkages among aquatic ecosystems. Journal of the North American Benthological Society, 29(1), 245–263. https://doi.org/10.1899/08-166.1
Lansac-Toha, F. M., Heino, J., Quirino, B. A., Moresco, G. A., Pelaez, O., Meira, B. R., Rodrigues, L. C., Jati, S., Lansac-Tôha, A., & F. A., Velho, L. F. M. (2019). Differently dispersing organism groups show contrasting beta diversity patterns in a dammed subtropical river basin. Science of the Total Environment, 691, 1271–1281. https://doi.org/10.1016/j.scitotenv.2019.07.236
Luxton, M. (1990). The marine littoral mites of the New Zealand region. Journal of the Royal Society of New Zealand, 20(4), 367–418. https://doi.org/10.1080/03036758.1990.10426719
Marcogliese, d. J. (2002). Food webs and the transmission of parasites to marine fish. Parasitology, 124, 83–99. https://doi.org/10.1017/S003118200200149X
Massoud, A. H.S. and Safty, A.M. (2004). Environmental Problems in Two Egyptian Shallow Lakes Subjected to Different Levels of Pollution. Eighth International Water Technology Conference, IWTC8 2004, Alexandria, Egypt, 379-391.
McMahon, K. W., Johnson, L. B., & Ambrose, W. G. (2005). Diet and movement of the killifish, Fundulus heteroclitus, in Maine salt marsh assessed using gut contents and stable isotope analyses. Estuaries, 28(6), 966–973.
Mehanna, S. F. (2020). Isopod parasites in the Egyptian fisheries and its impact on fish production: Lake Qarun as a case study. Egyptian Journal of Aquatic Biology & Fisheries, 24(3), 181–191.
Mehanna, S. F. (2022). Egyptian marine fisheries and its sustainability. In: Sustainable fish production and processing (pp. 111–140). Elsevier.
Mitwally, H. (2015). Ecological factors affecting benthic fauna at Lake Maryut, Alexandria Egypt. Egyptian Journal of Experimental Biology (zoology), 11(2), 159–169.
Mitwally, H., & Abada, A. E. (2008). Spatial variability of meiofauna and macrofauna in a Mediterranean protected area, Burullus Lake Egypt. Meiofauna Marina, 16(1), 185–200.
Mitwally, H., Montagna, P., Halim, Y., Khalil, A., Dorgham, M., & Atta, M. (2004). Egyptian sandy beach meiofauna and benthic diatoms. Rappoport. Communications - Commission Internationale pour l’Exploration Scientifique de la Mer Mediterranee, 37, 537.
Mohammed, Kh. M., & Kondrashin, R. V. (2013). Detect risk zone of heavy metals contamination in water of the Lake Mariut, Alexandria, Egypt. Естественные науки, 43(2), 72–81.
Montagna, P. A. (1984). In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Marine Ecology - Progress Series, 18, 119–130.
Montagnes, D. J. S., Berges, J. A., Harrison, P. J., & Taylor, F. J. R. (1994). Estimating carbon, nitrogen, protein, and chlorophyll a from volume in marine phytoplankton. Limnolnology and Oceanography, 39(5), 1044–1060.
Nedić, Z., Skenderović, I., & Adrović, A. (2018). Study of some ectoparasites of fishes from the Sava River as part of water management in Bosnia and Herzegovina. TEM Journal, 7(2), 391.
Nofal, M. I., & Abdel-Latif, H. M. R. (2017). Ectoparasites and bacterial co-infections causing summer mortalities among cultured fishes at Al-Manzala with special reference to water quality parameters. Life Science Journal, 14(6), 72–83. https://doi.org/10.7537/marslsj140617.11
Oczkowski, A., & Nixon, S. (2008). Increasing nutrient concentrations and the rise and fall of a coastal fishery, a review of data from the Nile Delta Egypt. Estuarine, Coastal and Shelf Science, 77(3), 309–319. https://doi.org/10.1016/j.ecss.2007.11.028
Oikonomou, A., & Stefanidis, K. (2020). α- and β-Diversity patterns of macrophytes and freshwater fishes are driven by different factors and processes in lakes of the unexplored southern Balkan biodiversity hotspot. Water, 12(7), 1984. https://doi.org/10.3390/w12071984
Olausson, E. (1975). Methods for the chemical analysis of sediments. FAO Fisheries Technical Papers (FAO).
Palm, H. W. (2011). Fish parasites as biological indicators in a changing world: can we monitor environmental impact and climate change? In H. Mehlhorn (Ed.), Progress in parasitology. Parasitology research monographs. (Vol. 2). Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-642-21396-0_12
Palmer, M. A., Covich, A. P., Lake, S., Biro, P., Brooks, J. J., Cole, J., Dah, C., Gilbert, J., Goedkiip, W., Martens, K., Verhoeven, J., & Van De Bund, W. J. (2000). Linkages between aquatic sediment biota and life above sediments as potential drivers of biodiversity and ecological processes. BioScience, 5(12), 1063–1075.
Pan, Y., Stevenson, R. J., Hill, B. H., & Herlihy, A. T. (2000). Ecoregions and benthic diatom assemblages in Mid-Atlantic Highlands streams, USA. Journal of the North American Benthological Society, 19(3), 518–540.
Peel, R. A., Hill, J. M., Taylor, G. C., & Weyl, O. L. F. (2019). Food web structure and trophic dynamics of a fish community in an ephemeral floodplain lake. Frontiers in Environmental Science, 7. https://doi.org/10.3389/fenvs.2019.00192
Prenner, M. M., Ibrahim, H., Lewis, J. W., & Crane, M. (2006). Toxicity and trace metal concentrations of sediments from Lake Maryut, Alexandria Egypt. Bulletin of Environmental Contamination and Toxicology, 77(4), 616–623. https://doi.org/10.1007/s00128-006-1107-7
Proctor, H. C. (2004). Aquatic mites: From genes to communities. Experimental and Applied Acarology, 34(1–2), 210.
Ptatscheck, C., Brüchner-Hüttemann, H., Kreuzinger-Janik, B., Weber, S., & Traunspurger, W. (2020). Are meiofauna a standard meal for macroinvertebrates and juvenile fish? Hydrobiologia (incorporating JAQU), 847(12), 2755–2778. https://doi.org/10.1007/s10750-020-04189-y
Riera, P. (1998). 15N of organic matter sources and benthic invertebrates along an estuarine gradient in Marennes-Oleron Bay (France): Implications for the study of trophic structure. Marine Ecology Progress Series, 166, 143–150.
Saad, M. A. (1973). Distribution of phosphates in Lake Mariut, a heavily polluted lake in Egypt. Water, Air, and Soil Pollution, 2(4), 515–522.
Salem, D. M. A., Khaled, A., & El Nemr, A. (2013). Assessment of pesticides and polychlorinated biphenyls (PCBs) in sediments of the Egyptian Mediterranean Coast. The Egyptian Journal of Aquatic Research, 39(3), 141–152.
Sara, J. R., Smit, W. J., Erasmus, L. J. C., Ramalepe, T. P., Mogashoa, M. E., Raphahlelo, M. E., Theron, J., & Luus-Powell, W. J. (2013). Ecological status of Hout River Dam, Limpopo province, South Africa, using fish condition and health assessment index protocols: A preliminary investigation. African Journal of Aquatic Science, 39(1), 35–43. https://doi.org/10.2989/16085914.2013.848181
Schindler, D. E., & Scheuerell, M. D. (2002). Habitat coupling in lake ecosystems. Oikos, 98, 177–189.
Schmid-Araya, J. M., Hildrew, A. G., Robertson, A., Schmid, E., & Winterbottom, J. (2002). The importance of meiofauna in food webs: Evidence from an acid stream. Ecology, 83(5), 1271–1285.
Schmid-Araya, J. M., Schmid, P., Tod, S. P., & Esteban, G. F. (2016). Trophic positioning of meiofauna revealed by stable isotopes and food web analyses. Ecology, 97(11), 3099–3109.
Schratzberger, M., Rees, H. L., & Boyd, S. E. (2000). Effects of simulated deposition of dredged material on structure of nematode assemblages - The role of burial. Marine Biology, 136, 519–530.
Schratzberger, M., & Warwick, R. M. (1999). Differential effects of various types of disturbances on the structure of nematode assemblages: An experimental approach. Marine Ecology Progress Series, 181, 227–236.
Schwinghamer, P. (1983). Generating ecological hypotheses from biomass spectra using causal analysis: A benthic example. Marine Ecology - Progress Series, 13, 151–166.
Selim, N., & El-Raey, M. F. (2018). EIA for Lake Maryut sustainable development alternatives using GIS, RS, and RIAM software. Assiut University Bulletin of Environmental Research, 21(1), 41–57.
Shaaban, N. A. (2022). Water quality and trophic status of Lake Mariut in Egypt and its drainage water after 8-year diversion. Environmental Monitoring and Assessment, 194(6), 392. https://doi.org/10.1007/s10661-022-10009-8
Shalloof, K. A. S., El-Far, A. M., & Aly, W. (2020). Feeding habits and trophic levels of cichlid species in tropical reservoir, Lake Nasser.Egypt. The Egyptian Journal of Aquatic Research, 46(2), 159–165. https://doi.org/10.1016/j.ejar.2020.04.001
Shreadah, M. A., El-Rayis, O. A., Shaaban, N. A., & Hamdan, A. M. (2020). Water quality assessment and phosphorus budget of a lake (Mariut, Egypt) after diversion of wastewaters effluents. Environmental Science and Pollution Research, 27(21), 26786–26799. https://doi.org/10.1007/s11356-020-08878-y
Snickars, M., Weigel, B., & Bonsdorff, E. (2014). Impact of eutrophication and climate change on fish and zoobenthos in coastal waters of the Baltic Sea. Marine Biology, 162(1), 141–151. https://doi.org/10.1007/s00227-014-2579-3
Solar, R. R., & d. C., Barlow, J., Ferreira, J., Berenguer, E., Lees, A. C., Thomson, J. R., Thomson, J.R., Louzada, J., Maués, M., Moura, N.G., Oliveira, V.H. & Chaul, J.C. (2015). How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecology Letters, 18(10), 1108–1118. https://doi.org/10.1111/ele.12494
Søndergaard, M. (2007). Nutrient dynamics in lakes with emphasis on phosphorus, sediment and lake restoration. Doctor’s dissertation (DSc), National Environmental Research Institute University of Aarhus. Denmark, 1–74.
Tadros, H. R. Z., Hamdona, S. K. H., Ghobrial, M. G., El-Naggar, M. F., & Abd El-Hamid, O. M. (2019). Wastewater treatment of mariout lake drains using combined physical, chemical, and biological methods in microcosm experiments. Aquatic Science and Technology, 7(2), 46. https://doi.org/10.5296/ast.v7i2.14651
Thompson, R. M., Dunne, J. A., & Woodward, G. U. Y. (2012). Freshwater food webs: Towards a more fundamental understanding of biodiversity and community dynamics. Freshwater Biology, 57(7), 1329–1341. https://doi.org/10.1111/j.1365-2427.2012.02808.x
Tunney, T. D., McCann, K. S., Lester, N. P., & Shuter, B. J. (2012). Food web expansion and contraction in response to changing environmental conditions. Nature Communications, 3(1). https://doi.org/10.1038/ncomms2098
Vidal, L. B. (2008). Fish as ecological indicators in Mediterranean freshwater ecosystems. Ph.D. Thesis, University of Girona, 136.
Vinogradov, A. K., Bogatova, Y. I., & Synegub, I. A. (2018). Ecology of marine ports of the Black and Azov Sea Basin,377.Springer.
Wenger, A. S., Harvey, E., Wilson, S., Rawson, C., Newman, S. J., Clarke, D., Saunders, B. J., Browne, N., Travers, M. J., Mcilwain, J. L., & Erftemeijer, P. L. (2017). A critical analysis of the direct effects of dredging on fish. Fish and Fisheries, 18(5), 967–985. https://doi.org/10.1111/faf.12218
Wetzel, R. (1992). Gradient-dominated ecosystems: Sources and regulatory functions of dissolved organic matter in freshwater ecosystems. Hydrobiologia (incorporating JAQU), 229, 181–198.
Wetzel, R. G. (2001). Limnology: lake and river ecosystems. Gulf Professional Publishing.
Wilcox, D. A., & Whillans, T. H. (1999). Techniques for restoration of disturbed coastal wetlands of the Great Lakes. Wetlands, 19(4), 835–857.
Woodward, G. U. Y. (2009). Biodiversity, ecosystem functioning and food webs in fresh waters: Assembling the jigsaw puzzle. Freshwater Biology, 54(10), 2171–2187. https://doi.org/10.1111/j.1365-2427.2008.02081.x
Younis, A. M. (2018). Environmental impacts on Egyptian Delta Lakes’ biodiversity: A case study on Lake Burullus. In M. Casin (Ed.), Egyptian coastal lakes and wetlands: Part II (pp. 107–128). Springer.
Acknowledgements
The authors are grateful to Dr. Abeer Elsmaan, a lecturer at The National Institute of Oceanography and Fisheries, for Tilapia fish identification, and Ms. Samar Farghaly, a teaching assistant at the faculty of Science Kafrelsheikh University for fish allometric measurements. This work is a part of a research project (Environment-11), funded by the Alexandria University, Egypt, a program for research enhancement (ALEX-REP), and complementary to Mitwally (2015). The first author is the PI of the Environment-11 project, grateful to all the project team members for their contribution to analyzing all biotic and organic matter data except for meiofauna. Our deepest thanks are to the manuscript’s anonymous reviewers for their insightful suggestions and comments that helped to improve the manuscript’s quality.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work is a part of a research project (Environment-11), funded by Alexandria University, Egypt, a program for research enhancement (ALEX-REP), which was closed in 2015.
Author information
Authors and Affiliations
Contributions
Hanan Mitwally: Data conceptualization, curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, visualization, writing - original draft, writing, review, and editing. Hoda El Rashidy: Data curation, investigation, methodology, visualization, writing - original draft, writing, review, and editing. Paul Montagna: Formal analysis of data, visualization, writing, review, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that there are no ethical conflicts or required approvals to perform or publish the research. No surveys or human subjects were studied during this work. Therefore, the authors declare that neither informed consent nor institutional review board permissions were required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitwally, H., Rashidy, H.E. & Montagna, P. Linkages between pelagic and benthic biota in a deteriorated coastal lake after restoration, Maruit, Egypt. Environ Monit Assess 195, 919 (2023). https://doi.org/10.1007/s10661-023-11525-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11525-x