Abstract
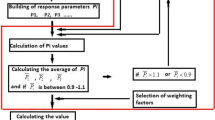
A heavy metal ion-selective electrode (ISE) with highly multiple response performances, rather than a high single response performance, is needed urgently for in situ, real-time environmental monitoring. In this study, we present an integrated measurement of six response performance variables such as the response slope, selectivity, dynamical range, detection limit, response time, and lifetime. They are selected and used as the indicators of the quality assessment for Pb2+-ISEs. The measurement, named as electrode comprehensive quality index (IECQ), is a single number for a given ISE. The comprehensive qualities of 114 Pb2+-ISEs reported in the literature were evaluated through the index method. Twenty-one Pb2+-ISEs-based polymer membrane with top 3 IECQ values for seven different properties have been recommended by evaluating and screening of the electrodes. Five Pb2+-ISEs-based polymer membrane with the best single response performance were also provided. The recommended Pb2+-ISEs, along with the corresponding Pb2+-ISEs with the miniaturized configurations, will provide helpful guideline for the application of Pb2+-ISE with highly multiple response performances in real-time environmental monitoring.


Similar content being viewed by others
References
Abu-Shawish, H. M. (2009). A mercury(II) selective sensor based on N,N-bis(salicylaldehyde)-phenylenediamine as neutral carrier for potentiometric analysis in water samples. Journal of Hazardous Materials, 167, 602–608.
Bakker, E., & Pretsch, E. (2001). Potentiometry at trace levels. Trends in Analytical Chemistry, 20, 11–19.
Barzegar, M., Mousavi, M. F., Khajehsharifi, H., Shamsipur, M., & Sharghi, H. (2005). Application of some recently synthesized 9, 10-anthraquinone derivatives as new class of ionophores responsive to lead (II) ion. IEEE Sensors Journal, 5, 392–397.
Braungardt, C. B., Achterberg, E. P., Axelsson, B., Buffle, J., Graziottin, F., Howell, K. A., Illuminati, S., Scarponi, G., Tappin, A. D., Tercier-Waeber, M.-L., & Turner, D. (2009). Analysis of dissolved metal fractions in coastal waters: an inter-comparison of five voltammetric in situ profiling (VIP) systems. Marine Chemistry, 114, 47–55.
Buffle, J., Altmann, R. S., Filella, M., & Tessier, A. (1990). Complexation by natural heterogeneous compounds: Site occupation distribution functions, a normalized description of metal complexation. Geochimica et Cosmochimica Acta, 54, 1535–1553.
Buhlmann, P., & Umezawa, Y. (2000). Lifetime of ion-selective electrodes based on charged ionophores. Analytical Chemistry, 72, 1843–1845.
Ceresa, A., Bakker, E., Hattendorf, B., Gunther, D., & Pretsch, E. (2001). Potentiometric polymeric membrane electrodes for measurement of environmental samples at trace levels: new requirements for selectivities and measuring protocols, and comparison with ICPMS. Analytical Chemistry, 73, 343–351.
Cude, C. G. (2001). Oregon water quality index a tool for evaluating water quality management effectiveness. Journal of the American Water Resources Association, 37, 125–137.
De Marco, R., Clarke, G., & Pejcic, B. (2007). Ion-selective electrode potentiometry in environmental analysis. Electroanalysis, 19, 1987–2001.
Dobbie, M. J., & Dail, D. (2013). Robustness and sensitivity of weighting and aggregation in constructing composite indices. Ecological Indicators, 29, 270–277.
Ellison, S. L. R., & Williams, A. (2012). EURACHEM/CITAC Guide:Quantifying uncertainty in analytical measurement (3rd ed.pp. 26–27) QUAM:2012.P1.(ISBN 978–0–948926-30-3).
Gorai, A. K., Kanchan, Upadhyay, A., Tuluri, F., Goyal, P., & Tchounwou, P. B. (2015). An innovative approach for determination of air quality health index. Science of the Total Environment, 533, 495–505.
Guilbault, G. G., Durst, R. A., Frant, M. S., et al. (1976). Recommendations for nomenclature of ion-selective electrodes. Pure and Applied Chemistry, 48, 127–132.
Gumpu, M. B., Sethuraman, S., Krishnan, U. M., & Rayappan, J. B. B. (2015). A review on detection of heavy metal ions in water – an electrochemical approach. Sensors and Actuators B: Chemical, 213, 515–533.
Gupta, K. C., & D’Arc, M. J. (2001). Lead (II) ion selective electrodes based on diphenylmethyl- N-phenylhydroxamic acid ionophore in cyanocopolymer matrix. IEEE Sensors Journal, 1, 275–282.
Gupta, V. K., Ganjali, M. R., Norouzi, P., Khani, H., Nayak, A., & Agarwal, S. (2011). Electrochemical analysis of some toxic metals by ion-selective electrodes. Critical Reviews in Analytical Chemistry, 41, 282–313.
Guzinski, M., Lisak, G., Kupis, J., Jasinski, A., & Bochenska, M. (2013). Lead(II)-selective ionophores for ion-selective electrodes: a review. AnalyticaChimica Acta, 791, 1–12.
Hanrahan, G., Pati, D. G., & Wang, J. (2004). Electrochemical sensors for environmental monitoring: design, development and applications. Journal of Environmental Monitoring, 6, 657–664.
Inamuddin, & Alam, M. M. (2008). Studies on the preparation and analytical applications of various metal ion-selective membrane electrodes based on polymeric, inorganic and composite materials—a review. Journal Macromolecular Science, Part A, 45, 1084–1101.
Inamuddin, Rangreez, T. A., Naushad, M., & Al-Ahmad, A. (2015). Synthesis and characterisation of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) Zr(IV) monothiophosphate composite cation exchanger: analytical application as lead ion selective membrane electrode. International Journal of Environmental Analytical Chemistry, 95, 312–323
Karami, H., Mousavi, M. F., & Shamsipur, M. (2003). Flow injection potentiometry by a new coated graphite ion-selective electrode for the determination of Pb2+. Talanta, 60, 775–786.
Khan, A. A., & Baig, U. (2012). Electrically conductive membrane of polyaniline–titanium(IV) phosphate cation exchange nanocomposite: applicable for detection of Pb(II) using its ion-selective electrode. Journal of Industrial and Engineering Chemistry, 18, 1937–1944.
Khan, A. A., Khan, M. Q., & Shaheen, S. (2016). Synthesis, characterization, and electroanalytical studies of Pb2+-selective polypyrrole-Zr(IV) phosphate ion exchange membrane. Journal of Solid State Electrochemistry, 20, 2079–2091.
Lindner, E., Toth, K., & Pungor, E. (1984). Lead-selective neutral carrier based liquid membrane electrode. Analytical Chemistry, 56, 1127–1131.
Mary-Lou, T.-W., & Martial, T. (2008). Remote in situ voltammetric techniques to characterize the biogeochemical cycling of trace metals in aquatic systems. Journal of Environmental Monitoring, 10, 30–54.
Namour, P., Lepot, M., & Jaffrezic-Renault, N. (2010). Recent trends in monitoring of European water framework directive priority substances using micro-sensors: a 2007–2009 review. Sensors, 10, 7947–7978.
Oesch, U., & Simmon, W. (1980). Lifetime of neutral carrier based ion-selective liquid-membrane electrodes. Analytical Chemistry, 52, 692–700.
Parra, E. J., Blondeau, P., Crespo, G. A., & Xavier Rius, F. (2011). An effective nanostructured assembly for ion-selective electrodes. An ionophore covalently linked to carbon nanotubes for Pb2+ determination. Chemical Communications, 47, 2438–2440.
Sanchez, J., & del Velle, M. (2001). A new potentiometric photocurable membrane selective to anionic surfactants. Electroanalysis, 13, 471–476.
Sardohan-Koseoglu, T., Kir, E., & Dede, B. (2015). Preparation and analytical application of the novel Hg(II)-selective membrane electrodes based on oxime compounds. Journal of Colloid and Interface Science, 444, 17–23.
Singh, A., Sharma, R. K., Agrawal, M., & Marsha, F. M. (2010). Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food and Chemical Toxicology, 48, 611–619.
Slaveykova, V. I., Wilkinson, K. J., Ceresa, A., & Pretsch, E. (2003). Role of fulvic acid on lead bioaccumulation by Chlorella kesslerii. Environmental Science & Technology, 37, 1114–1121.
Sun, X. (2003). A new evaluation on comprehensive quality of ion-selective electrode and its application in development of doxycycline-selective PVC membrane electrode. Journal of Instrumental Analysis, 22, 1–4 (in Chinese).
Sun, X. X., Xu, M. H., Sun, C. J., & Aboul-Enein, H. Y. (2007). Weighting factor in calculation of comprehensive quality index and optimization of PVC membrane composition for ion selective electrode. Instrumentation Science and Technology, 35, 469–479.
Sun, L., Sun, C., & Sun, X. (2016). Screening highly selective ionophores for heavy metal ion-selective electrodes and potentiometric sensors. Electrochimica Acta, 220, 690–698.
Sunda, W., & Guillard, R. R. L. (1976). The relationship between cupric ion activity and the toxicity of copper to phytoplankton. Journal of Marine Research, 34, 511–529.
Tang, X., Wang, P. Y., & Buchter, G. (2018). Ion-selective electrodes for detection of lead (II) in drinking water: a mini-review. Environments, 5, 95.
Tutulea-Anastasiu, M. D., Wilson, D., del Valle, M., Schreiner, C. M., & Cretescu, I. (2013). A solid-contact ion selective electrode for copper(II) using a succinimide derivative as ionophore. Sensors, 13, 4367–4377.
Tyagi, S., Agarwal, H., & Ikram, S. (2009). Application of calixarene ionophores in PVC-based ion selective electrodes for heavy metal detection. The IUP Journal of Chemistry, II, 68.
Umezawa, Y. K., Umezawa, K., & Sato, H. (1995). Selectivity coefficients for ion-selective electrodes: recommendation methods for reporting KA, B POT values. Pure and Applied Chemistry, 67, 507–518.
Xie, L., Qin, Y., & Chen, H.-Y. (2013). Preparation of solid contact potentiometric sensors with self-plasticizing triblock polymer and ionic liquid-polymer composites. Sensors and Actuators B, 186, 321–326.
Yu, P., Liu, S., Zhang, L., Li, Q., & Zhou, D. (2018). Selecting the minimum data set and quantitative soil quality indexing of alkaline soils under different land uses in northeastern China. Science of the Total Environment, 616, 564–571.
Zachara, J. E., Toczyłowska, R., Pokrop, R., Zagórska, M., Dybko, A., & Wróblewski, W. (2004). Miniaturised all-solid-state potentiometric ion sensors based on PVC-membranes containing conducting polymers. Sensors and Actuators B, 101, 207–212.
Zahran, E. M., New, A., Gavalas, V., & Bachas, L. G. (2014). Polymeric plasticizer extends the lifetime of PVC-membrane ion-selective electrodes. Analyst, 139, 757–763.
Zareh, M. M., Ghoneim, A. K., & Abd El-Aziz, M. H. (2001). Effect of presence of 18-crown-6 on the response of 1-pyrrolidine dicarbodithioate-based lead selective electrode. Talanta, 54, 1049–1057.
Funding
This work was partially supported by the Summit of the Six Top Talents Program in Jiangsu Province (2012-SWYY-030 and 2017-SWYY-081) and the foundation of Jiangsu Province educational committee (16KJB180031) (L.Sun).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L., Sun, C. & Sun, X. An integrated measurement of six response performance indicators for lead ion-selective electrodes and application. Environ Monit Assess 191, 744 (2019). https://doi.org/10.1007/s10661-019-7908-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7908-z