Abstract
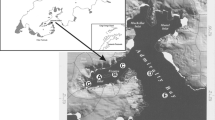
For 2 years, a baseline investigation was carried out to collect reference information of the present environmental status in the Fehmarn Belt and adjacent area. The temporal and spatial variability of phytoplankton was monitored by a combination of monitoring buoys, pigment analysis and fast screening microscopy. The overall phytoplankton succession in the Fehmarn Belt area was found to be influenced primarily by the seasonal changes, where various diatoms dominated the spring and autumn blooms and flagellates like Chrysochromulina sp., Dictyocha speculum and various dinoflagellates were occasionally abundant in late spring and summer. The phytoplankton groups were remarkably uniform horizontally in the investigation area while large differences in both biomasses and composition of individual phytoplankton groups were seen vertically in the water column, especially in the summer periods, in which the two-layer exchange flow between the North Sea and the Baltic Sea is showing a particularly strong stratification in the Fehmarn Belt. The chlorophyll a concentrations ranged continuously from 1 to 3 μg/L at the three permanent buoy stations during the 2 years of monitoring, except for the spring and autumn blooms where chlorophyll a increased up to 18 μg/L in the spring of 2010 and up to 8 μg/L in the autumn of 2009. Recurrent blooms of filamentous cyanobacteria are common during the summer period in the Baltic Sea and adjacent areas, but excessive blooms of cyanobacteria did not occur in 2009 and 2010 in the Fehmarn Belt area. The combination of the HPLC pigment analysis method and monitoring buoys continuously measuring fluorescence at selected stations with fast screening of samples in the microscope proved advantageous for obtaining information on both the phytoplankton succession and dynamic and, at the same time, getting information on duration and intensity of the blooms as well as specific information on the dominant species present both temporally and spatially in the large Fehmarn Belt area.













Similar content being viewed by others
References
Andersen, J. H., Axe, P., Backer, H., Carstensen, J., Claussen, U., Fleming-Lehtinen, V., et al. (2011). Getting the measure of eutrophication in the Baltic Sea: towards improved assessment principles and methods. Biogeochemistry, 106, 137–156.
Barlow, R., Stuart, V., Lutz, V., Sessions, H., Sathyendranath, S., Platt, T., et al. (2007). Seasonal pigment patterns of surface phytoplankton in the subtropical southern hemisphere. Deep-Sea Research I, 54, 1687–1703.
Blasco, D. (1978). Migration of dinoflagellates off Baja California Coast. Marine Biology, 46, 41–47.
Brunet, C., & Lizon, F. (2003). Tidal and diel periodicities of size-fractionated phytoplankton pigment signatures at an offshore station in the southeastern English Channel. Estuarine, Coastal and Shelf Science, 56, 833–843.
Cloern, J. E. (1996). Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics, 34, 127–168.
Figueroa, F. L., Niell, F. X., Figueiras, F. G., & Villarino, M. L. (1998). Diel migration of phytoplankton and spectral light field in the Ría de Vigo (NW Spain). Marine Biology, 130, 491–499.
Gameiro, C., Cartaxana, P., & Brotas, V. (2007). Environmental drivers of phytoplankton distribution and composition in Tagus Estuary, Portugal. Estuarine, Coastal and Shelf Science, 75, 21–34.
Hadju, S., Höglander, H., & Larsson, U. (2007). Phytoplankton vertical distributions and composition in Baltic Sea cyanobacterial blooms. Harmful Algae, 6, 189–205.
Hansson, M., & Håkansson, B. (2007). The Baltic Algae Watch System—a remote sensing application for monitoring cyanobacterial blooms in the Baltic Sea. Journal of Applied Remote Sensing, 1(1), 011508. doi:10.1117/1.2834770.
Hansson, M., & Öberg, J. (2009). Cyanobacterial blooms in the Baltic Sea. HELCOM Baltic Sea Environment Fact Sheets. Online. 08.04.2014, http://helcom.fi/baltic-sea-trends/environment-fact-sheets.
Hansson, M., & Öberg, J. (2010). Cyanobacterial blooms in the Baltic Sea. HELCOM Baltic Sea Environment Fact Sheets. Online. 08.04.2014, http://helcom.fi/baltic-sea-trends/environment-fact-sheets.
Henriksen, P. (2009). Long-term changes in phytoplankton in the Kattegat, the Belt Sea, the Sound and the western Baltic Sea. Journal of Sea Research, 61, 114–123.
Higgins, W. H., Wright, S. W., & Schlüter, L. (2011). Quantitative interpretation of chemotaxonomic pigment data. In S. Roy, C. A. Llewellyn, E. S. Egeland, & G. Johnsen (Eds.), Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography (pp. 257–313). Cambridge: Cambridge University.
Hooker, S. B., van Heukelem, L., Thomas, C. S., Claustre, H., Ras, J., Schlüter, L., et al. (2005). The Second Sea-WiFS HPLC Analysis Round-Robin Experiment (SeaHARRE-2). NASA Techical Memorandum. 2005–212785, NASA Goddard Space Flight Center, Greenbelt, Maryland.
Jakobsen, F., Hansen, I. S., Hansen, N. O., & Østrup-Rasmussen, F. (2010). Flow resistance in the Great Belt, the biggest strait between the North Sea and the Baltic Sea. Estuarine, Coastal and Shelf Science, 87, 325–332.
Jochem, F., & Babenerd, B. (1989). Naked Dictyocha speculum—a new type of phytoplankton bloom in the Western Baltic. Marine Biology, 103, 373–379.
Jung, T., Vitart, F., Ferranti, L., & Morcrette, J. J. (2011). Origin and predictability of the extreme negative NAO winter of 2009/10. Geophysical Research Letters, 38, L07701. doi:10.1029/2011GL046786.
Kahru, M., Horstmann, U., & Rud, O. (1994). Satellite detection of increased cyanobacterial blooms in the Baltic Sea: natural fluctuation or ecosystem change? Ambio, 23, 469–472.
Kinkade, C. S., Marra, J., Dickey, T. D., Langdon, C., Sigurdson, D. E., & Welle, R. (1999). Diel bio-optical variability observed from moored sensors in the Arabian Sea. Deep-Sea Research, 46, 1813–1831.
Kononen, K. (1992). Dynamic of the toxic cyanobacterial blooms in the Baltic Sea. Finnish Marine Research, 261, 3–36.
Korpinen, S., Meski, L., Andersen, J. H., & Laamanen, M. (2012). Human pressures and their potential impact on the Baltic Sea ecosystem. Ecological Indicators, 15, 105–114.
Lemaire, E., Abril, G., De Wit, R., & Etcheber, H. (2002). Distribution of phytoplankton pigments in nine European estuaries and implications for an estuarine typology. Biogeochemistry, 59, 5–23.
Mackey, M. D., Mackey, D. J., Higgins, H. W., & Wright, S. W. (1996). CHEMTAX—a program for estimating class abundance from chemical markers: application to HPLC measurements of phytoplankton. Marine Ecology Progress Series, 144, 265–283.
Mouritsen, L. T., & Richardson, K. (2003). Vertical microscale patchiness in nano- and microplankton distributions in a stratified estuary. Journal of Plankton Research, 25(7), 783–797.
Rzymski, P., Poniedziałek, B., & Karczewski, J. (2011). Gastroenteritis and liver carcinogenesis induced by cyanobacterial toxins. Gastroenterologia Polska, 18(4), 159–162.
Sackmann, B. S., Perry, M. J., & Eriksen, C. C. (2008). Seaglider observations of variability in daytime fluorescence quenching of chlorophyll-a in Northeastern Pacific coastal waters. Biogeosciences Discussions, 5, 2839–2865.
Sarmento, H., & Descy, J. P. (2008). Use of marker pigments and functional groups for assessing the status of phytoplankton assemblages in lakes. Journal of Applied Phycology, 20, 1001–1011.
Schlüter, L., & Møhlenberg, F. (2003). Detecting presence of phytoplankton groups with non-specific pigment signatures. Journal of Applied Phycology, 15, 465–476.
Schlüter, L., Møhlenberg, F., Havskum, H., & Larsen, S. (2000). The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas; testing the influence of light and nutrients on pigment/chlorophyll a-ratios. Marine Ecology Progress Series, 192, 49–63.
Schlüter, L., Garde, K., & Kaas, H. (2004). 4-keto-myxoxanthophyll-like pigment is a diagnostic pigment for the toxic cyanobacteria Nodularia spumigena in the Baltic Sea. Marine Ecology Progress Series, 275, 69–78.
Schlüter, L., Lutnæs, B. F., Liaaen-Jensen, S., Garde, K., Kaas, H., Jameson, I., et al. (2008). Correlation of the content of hepatotoxin nodularin and glycosidic carotenoids, 4-ketomyxol-20-fucoside and novel 10-O-methyl-4-ketomyxol-20-fucoside, in 20 strains of the cyanobacterium Nodularia spumigena. Biochemical Systematics and Ecology, 36, 749–757.
Schlüter, L., Henriksen, P., Nielsen, T. G., & Jakobsen, H. H. (2011). Phytoplankton composition and biomass across the southern Indian Ocean. Deep-Sea Research I, 58, 546–556.
Van Heukelem, L., & Thomas, C. (2001). Computer assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. Journal of Chromatography, A, 910, 31–49.
Villarino, M. L., Figueiras, F. G., Jones, K. J., Alvarez-Salgado, X. A., Richard, J., & Edwards, A. (1995). Evidence of in situ diel vertical migration of a red-tide microplankton species in Ria de Vigo (NW Spain). Marine Biology, 123, 607–617.
Wasmund, N. (1997). Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Internationale Revue der gesamten Hydrobiologie, 82, 169–184.
Wasmund, N., & Uhlig, S. (2003). Phytoplankton trends in the Baltic Sea. ICES Journal of Marine Science, 60, 177–186.
Wasmund, N., Nausch, G., & Matthäus, W. (1998). Phytoplankton spring blooms in the southern Baltic Sea–Spatio-temporal development and long-term trends. Journal of Plankton Research, 20, 1099–1117.
Wasmund, N., Göbel, J., & Von Bodungen, B. (2008). 100-years-changes in the phytoplankton community of Kiel Bight (Baltic Sea). Journal of Marine Systems, 73, 300–322.
Wilhelm, C., Rudolph, I., & Renner, W. (1991). A quantitative method based on HPLC-aided pigment analysis to monitor structure and dynamics of the phytoplankton assemblage—a study from Lake Meerfelder (Eifel, Germany). Archiv für Hydrobiologie, 123, 21–35.
Wright, S. W., van den Enden, R. L., Pearce, I., Davidson, A. T., Scott, F. J., & Westwood, K. J. (2010). Phytoplankton community structure and stocks in the Southern Ocean (30–80°E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep-Sea Research II, 57, 758–778.
Zapata, M., Jeffrey, S. W., Wright, S. W., Rodríguez, F., Garrido, J. L., & Clementson, L. (2004). Photosynthetic pigments in 37 species (65 strains) of Haptophyta, implications for oceanography and chemotaxonomy. Marine Ecology Progress Series, 270, 83–102.
Zapata, Z., Fraga, S., Rodríguez, F., & Garrido, J. L. (2012). Pigment-based chloroplast types in dinoflagellates. Marine Ecology Progress Series, 465, 33–52.
Acknowledgments
This work was carried out under contract to Femern A/S, and their consent to publish the results is gratefully acknowledged. We are grateful to Simon Wright, Australian Antarctic Division, CSIRO, for the CHEMTAX program ver. 1.95. M. Allerup is thanked for skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlüter, L., Møhlenberg, F. & Kaas, H. Temporal and spatial variability of phytoplankton monitored by a combination of monitoring buoys, pigment analysis and fast screening microscopy in the Fehmarn Belt Estuary. Environ Monit Assess 186, 5167–5184 (2014). https://doi.org/10.1007/s10661-014-3767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3767-9