Abstract
Microbial plant growth promoters (MPGP) are known to improve crop nutrition and root health. Here we examined the effects of individual and combined inoculation of chili pepper (variety Camino Real) with the known plant growth promoters Rhizophagus irregularis (isolate BEG87) and a commercial product of Azospirillum brasilense on chili pepper growth and biocontrol against the oomycete root pathogen Phytophthora capsici (isolate CH11). In a greenhouse pot experiment, unfertilized ten-week-old chili pepper plants with individual and combined inoculation of R. irregularis and A. brasilense were confronted with P. capsici, and four weeks later plants were harvested and scored for plant growth and disease severity. Surprisingly, both MPGP not only decreased plant growth, but also promoted root rot caused by P. capsici. This increased disease severity with P. capsici root rot in mycorrhizal chili pepper was further corroborated in a second greenhouse pot experiment with inert growth substrate and mineral fertilization. In conclusion, individual and combined inoculation with R. irregularis and A. brasilense may not only cause plant growth suppression in chili pepper plants, but also increase root rot caused by P. capsici.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chili pepper (Capsicum annuum L.) is one of the main horticultural crops grown around the world and Phytophthora root rot caused by the oomycete Phytopthora capsici Leon is among the most important limiting factors for chili pepper production (Reyes-Tena et al., 2021; Saltos et al., 2022). Since commercial chili pepper varieties with resistance to Phytophthora root rot are only partially successful, and chemical control alone does not work against this disease, biological control seems to be an important alternative disease control method.
Several microbial biocontrol agents have been reported to be effective against Phytophthora root rot in chili pepper, including plant growth promoting rhizobacteria (PGPR) (Hyder et al., 2020), Trichoderma (Tomah et al., 2020, Nawaz et al., 2018) and arbuscular mycorrhizal (AM) fungi (Kumar et al., 2018; Reyes-Tena et al., 2017).
Most terrestrial plants, including agricultural crops, form mycorrhizas with AM fungi from Glomeromycota, and plant growth promotion and stress alleviation are common traits of these mycorrhizal associations (Smith & Read, 2008). AM fungi are well known for their biocontrol traits especially against root diseases caused by nematodes, fungi, and oomycetes (Banuelos et al., 2014; Li et al., 2007; Ravnskov et al., 2020). Biocontrol traits of AM fungi have been reported in chili pepper (Reyes-Tena et al., 2017, 2022). Different general modes of action for the biocontrol traits of AM fungi have been proposed, including plant defense induction (Banuelos et al., 2014), improved stress tolerance (Begum et al., 2019; Thygesen et al., 2004), increased nutrient uptake (Whipps, 2004; Whiteside et al., 2019), altered root exudation (Dowarah et al., 2021) and antagonism from mycorrhiza-associated bacteria (Kamal et al., 2014; Li et al., 2007).
PGPR such as Bacillus, Burkholderia, Rhizobium, Pseudomonas, and Azospirillum, among others, are also well known as biocontrol agents (Ali et al., 2020; Verma et al., 2019). The mechanisms by which they stimulate plant growth can be through soil structure improvement, increased resistance to abiotic stress, biological nitrogen fixation, production of vegetative growth regulators, phosphorus solubilization, and siderophore production (Etesami & Adl, 2020; Attia et al., 2020; Karthika et al., 2020; Damam et al., 2016; Son et al., 2014). Various strains of these PGPR are commercialized such as A. brasilense. The plant growth promoting traits of A. brasilense seem to be mainly related to alterations in root structure through the production of plant hormones (Méndez-Gómez et al., 2021; Spaepen et al., 2008).
Microbial cooperation in the rhizosphere has been proposed as an important factor in relation to plant nutrition and health (Antoniou et al., 2017; Petipas et al., 2021; Van Der Heijden et al., 2016). Profound information regarding the interactions between microbial biocontrol agents and other plant beneficial microorganisms such as AM fungi are required for a successful and safe application of the biocontrol agent in question. Limited information, however, is available on the interactions between AM fungi and the PGPR A. brasilense in relation to plant growth and health.
The objective of this research was to examine individual and combined effects of R. irregularis and A. brasilense on plant growth and health of chili pepper in relation to root rot caused by P. capsici. The main hypothesis was that individual inoculation with R. irregularis and A. brasilense would increase plant growth and reduce root rot caused by P. capsici and to higher extend when combined.
Materials and methods
Experimental design
The first experiment had a fully factorial design with three factors: 1) R. irregularis (with and without), 2) A. brasilense (with and without) and 3) P. capsici (with and without). Two harvest times were included in the study before and after inoculation with P. capsici. For the first harvest (67 days after sowing), only two factors were examined: 1) R. irregularis (with and without) and 2) A. brasilense (with and without). Each of the 4 treatments had 3 replicates. For the second harvest (102 days after sowing), all three aforementioned factors were included, and each of the 8 treatments included 3 replicates. The second experiment had 3 treatments: 1) Control without microbial inoculation, 2) P. capsici, and 3) P. capsici + R. irregularis each with 5 replicates.
Experimental set-up
In both experiments, plants were grown under greenhouse conditions. In the first experiment, 3 chili pepper seeds (Var. Camino Real) were sown in each pot and thinned to one assuring a uniform starting point, leaving one plant per pot. In the second experiment, plants were established as seedlings in seed germination trays for 6 weeks and hereafter seedlings were transplanted to their respective pots. Watering was done daily by weighing each pot individually to maintain 80% field capacity. Greenhouse temperature was approximately 10–15 °C at night and 25–35 °C during the daytime throughout the period of the experiments.
Soil and substrate
The soil used in the first experiment was a Luvisol soil with the following chemical characteristics: organic matter content (1.91%), inorganic nitrogen (13.7 mg kg−1 soil), available phosphorus (7.01 mg kg−1 soil) and a pH (H2O) of 6.66. The soil was mixed with quartz sand (1:1, w/w) and autoclaved twice at 120 °C, 15 psi for 40 min on two consecutive days.
In the second experiment, plants were transplanted to pots with 300 g of substrate with peat, sand, and vermiculite in a 3:1:1 ratio. In the first experiment plants were not fertilized. In the second experiment plants were fully fertilized, except for P, with the following nutrients (mg kg−1 dry soil): K2SO4, 75.0; CaCl2 × 2H2O, 30; CuSO4 × 5H2O, 2.1; ZnSO4 × 7H2O, 5.4; MnSO4 × H2O, 10.5; CoSO4 × 7H2O, 0.39; MgSO4 × 7H2O, 45.0; Na2MoO4 × 2H2O, 0.18; and NH4NO3, 86.2. The nutrients were mixed carefully into the growth substrate prior plant sowing or seedling transplanting.
Microbial inoculants
In both experiments, the same microbial strains and inoculants were used. The inoculum of R. irregularis (isolate BEG87) was obtained from a pot culture produced the Agroecology lab at IIES-UNAM, which included soil and root segments. In treatments with R. irregularis, 80 g of inoculum was mixed with the soil. For A. brasilense, we used a commercial product, and the treatments with A. brasilense received 106 CFU g−1 of soil. Both MPGP were mixed into the soil before sowing. The inoculum of P. capsici isolate CH-11 was obtained from the Laboratorio de Patología Vegetal-Universidad Michoacana de San Nicolás de Hidalgo and is a MX3 virulence phenotype of the pathogen. This strain has been described to be very virulent against chili pepper (Reyes-Tena et al., 2019).
To inoculate plants with the pathogen, slices of cucumber were first infected with P. capsici, kept in Petri dishes, and incubated at room temperature (15 °C—28 °C) for 48 h. Several sets of cucumber slices were maintained under the same conditions but were not inoculated with P. capsici and used for the control treatments without the pathogen. To infect the plants, two holes were made in the substrate 2 cm from the stem, and one slice of cucumber was placed in each hole. In the first experiment, 10-week-old plants were infected. For the second experiment, the infection with P. capsici occurred 1 month after the transplant. In both experiments, after inoculation with P. capsici, plants were watered to 100% field capacity for 3 days to promote disease development.
Harvest and response variables
In the first experiment, plants were harvested 67 and 102 days after sowing. In the second experiment, plants were harvested 90 days after transplant. In both experiments, the same variables were determined including shoot dry weight, root fresh weight, total fresh weight, plant response to microorganisms (PRM), AMF root colonization and disease severity index.
The shoot and the root of each plant were separated. Roots were washed free of soil, and 2 g of subsample was taken to determine R. irregularis root colonization. Shoot dry weight was determined after drying at 70 °C for 48 h.
The variable PRM was calculated for A. brasilense, R. irregularis and P. capsici. This was done by dividing the dry plant weight variables in question with the mean on the corresponding control of non-inoculated control plants. The formula was presented by Johnson (2010) as a measure of response of the host plant to mycorrhizal fungus inoculation. Here we did the same for all microorganisms: PRM = loge (PM/PNM) where PM is the weight of plants with microorganisms and PNM is the mean of the weight of the control treatment without microorganisms for the same treatments.
Root colonization by AMF was done using the line-intercept method by Giovannetti and Mosse (1980) after clearing and staining the roots following the methods by Kormanik and McGraw (1982) except that acid fuchsin was replaced by trypan blue. For this, 2 g of root was placed in 10% KOH, and the solution was renewed daily for 7 days. After this, the root segments were placed in hydrogen peroxide for 20 min at room temperature and stained using 0.05% trypan blue for 5 min at 90 °C in a water bath.
The shoot and root disease severity index (DSI) were determined using a five-level-scale: 1) Without damage; 2) Leaf chlorosis and roots with superficial lesions; 3) Loss of turgor in leaves and roots with a some damage; 4) Loss of leaves and necrotic roots with high damage and 5) Dead plant.
Statistics
Levels of significance of the main treatments and their interactions were calculated through analysis of variance (ANOVA) after testing for variance homogeneity with Bartlett test. Comparisons between treatment means were performed with LSD test. Multifactor analyses of variance were done with either two- or three-way analyses depending on the variable in question. Kruskal–Wallis analysis and a Duncan test were performed to compare differences between medians for the DSI variable. All statistical analyses were performed using Statgraphics Plus, version 5.1 and graphs were designed with GraphPad Prism 7.
Results
Experiment 1
In Tables 1 and 2, the p values of all the measured variables for experiment 1 are shown. Hereafter, only individual effects, or significant interactions are presented.
Plant growth variables
In the first harvest of experiment 1, A. brasilense significantly reduced the shoot dry weight by 43.2% compared with the non-inoculated control treatment (Fig. 1A). In the second harvest, inoculation with R. irregularis and A. brasilense decreased the shoot dry weight by 22.4% and 28.1%, respectively, when compared with the non-inoculated control treatment (Fig. 2A and B). In the second harvest, inoculation with P. capsici reduced the shoot dry weight with 35.4% compared with the non-inoculated control (Fig. 2C).
Results of plant variables in the first harvest of the first experiment: A Shoot dry weight with and without A. brasilense, B Root fresh weight with and without R. irregularis, C Root-Shoot ratio with and without A. brasilense, D Root-shoot relationship with and without R. irregularis. Different letters indicate significant differences. Error bars represent standard error of the mean
Results of plant variables from the second harvest of the first experiment. A Shoot dry weight with or without R. irregularis, B Shoot dry weight with or without A. brasilense, C Shoot dry weight with and without P. capsici, D Root fresh weigh with and without P. capsici in the different treatments control, R. irregularis, A. brasilense and the combination with R. irregularis and A. brasilense, E Total fresh weight with or without P. capsici in the different treatments control, R. irregularis, A. brasilense and the combination of R. irregularis and A. brasilense, F Root-shoot ratio with and without R. irregularis. Different letters indicate significant differences. Error bars represent standard error of the mean
The root fresh weight in the first harvest was 41.4% lower with R. irregularis than without (Fig. 1B). In the second harvest, a three-way interaction was found between R. irregularis, A. brasilense and P. capsici for root fresh weight (Table 1). Without P. capsici the co-inoculation with R. irregularis and A. brasilense reduced the root fresh weight by 48.8% compared with the non-inoculated control treatment (Fig. 2D). Furthermore, A. brasilense increased the root fresh weight compared with R. irregularis and R. irregularis + A. brasilense by 51.7% and 64.1%, respectively (Fig. 2D). Inoculation with P. capsici in addition to the individual and combined inoculation with R. irregularis and A. brasilense reduced the root fresh weight compared with the control treatment, R. irregularis with 79.3%, A. brasilense with 46.9% and the combination with 85.5% (Fig. 2D). Without P. capsici, the combination of R. irregularis + A. brasilense decreased the root fresh weight by 72.8% compared with the individual inoculation with A. brasilense (Fig. 2D). The inoculation with A. brasilense and the infection with the pathogen reduced the root fresh weight by 53% compared with the treatment without the pathogen (Fig. 2D).
In the second harvest, the total plant fresh weight of plants inoculated with P. capsici was lower after inoculation with R. irregularis and A. brasilense individually and in combination. The reduction of total root fresh weight in comparison with the control was 73.3% with R. irregularis, 61.6% with A. brasilense and 78.5% with R. irregularis + A. brasilense (Fig. 2E). All the treatments with MPGP showed a significant decrease in total fresh weight when infected with the P. capsici as compared with the corresponding treatments without the pathogen. In the R. irregularis + P. capsici treatment, the decrease was 63.3% compared with R. irregularis alone. In the case of A. brasilense + P. capsici, the decrease was 55.1% compared with A. brasilense alone. Moreover, there was a decrease of 65.3% with R. irregularis + A. brasilense + P. capsici as compared with only R. irregularis + A. brasilense (Fig. 2E).
Root/shoot ratio
In the first harvest, significant individual factor effects were found for root-shoot ratio, where the inoculation with R. irregularis decreased the root-shoot ratio by 26.9%, while the inoculation with A. brasilense increased the root-shoot ratio by 29.4% compared with the control treatments without microbial inoculation (Fig. 1C and D). In the second harvest, the effect of the inoculation with R. irregularis was maintained, which reduced the root-shoot ratio with 54.2% compared to the treatment without inoculation with R. irregularis (Fig. 2F).
Plant response to microorganisms
In the second harvest, a negative PRM was obtained for shoot dry weight. The treatments with the highest decrease in PRM were the combinations of P. capsici with both MPGP individually and in combination (Fig. 3A). R. irregularis + P. capsici and the combination of the three microorganisms caused the highest negative MPGR for the root fresh weight (Fig. 3B). For total plant fresh weight, the treatments that caused highest negative PRM were the combination of P. capsici + R. irregularis and the combination of the three R. irregularis + A. brasilense + P. capsici (Fig. 3C).
Disease severity
Combined inoculation with A. brasilense and R. irrregularis in combination with P. capsici was the treatment with the highest disease severity index for shoot (Fig. 4A), root (Fig. 4B) and total plant (Fig. 4C) in comparison with the corresponding control treatments without P. capsici.
Experiment 2
In Table 3, the p values of all the measured variables in the second experiment are shown. Hereafter, only the results of the significant effects are presented.
Plant growth variables
The shoot dry weight suffered a decrease of 27.9% when infected with P. capsici, and the decrease was even greater with the combination of P. capsici and R. irregularis (39.8%) as compared with the control without any of the microorganisms (Fig. 5A).
For the root fresh weight, the combination of P. capsici with R. irregularis resulted in a 71% reduction, whereas P. capsici individually reduced the root fresh weight by 38.4% compared with the control without any of the microorganisms (Fig. 5B).
Single inoculation with P. capsici caused a 36% reduction in total plant fresh weight, whereas dual inoculation with P. capsici and R. irregularis resulted in 61.2% reduction in the total plant fresh weight compared with the control treatment without microbial inoculation (Fig. 6C).
Plant response to microorganisms
Negative PRM was observed for root fresh weight, which was highest when R. irregularis was present (Fig. 6A).
Disease severity
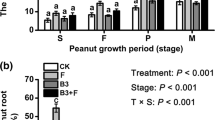
The shoot disease symptoms after P. capsici infection were highest in the plants inoculated with R. irregularis (Fig. 7). The DSI corroborates this for the shoot, which showed a greater DSI when the pathogen was combined with R. irregularis (Fig. 6B). Regarding the roots, P. capsici alone and in combination with R. irregularis obtained a higher DSI (Fig. 6C). The total DSI showed differences between treatments, with the combination of P. capsici with R. irregularis resulting in the highest DSI followed by P. capsici alone (Fig. 6D).
Discussion
Here we show that the well-known plant growth promoters R. irregularis (isolate BEG87) and a commercial product with A. brasilense not only decreased plant growth but also increased root rot caused by P. capsici (isolate CH11) in chili pepper plants (variety Camino Real), contrary to our main hypothesis. At first, we ascribed these surprising results to the low level of nutrients applied to favor functional plant microbial associations. However, this does not seem to be the case since similar results were obtained when plants were fully fertilized.
Our results showing plant growth suppression in chili pepper after inoculation with R. irregularis differ from other studies showing plant growth promotion in chili pepper after inoculation with AMF, which included the chili pepper variety Camino Real and the AMF genotype R. irregularis BEG 87 also used in the present study (Reyes-Tena et al., 2022). However, both soil and fertilization differed in these studies, which should be further examined to better understand these contrasting results of chili pepper growth response to AMF.
Plant growth suppression with AMF, however, is known to occur when the cost of hosting the AMF is higher than the benefits (Johnson & Graham, 2013), as reported in different vegetable crops like cucumber (Li et al, 2011) and tomato (Larsen et al., 2012), as well as cereals like maize (Raya-Hernández et al., 2020) and wheat (Ryan et al., 2005).
A possible explanation for the observed plant growth suppression in chili pepper associated with R. irregularis observed in the present study could be linked to imbalanced nutrient availability, which has been suggested as one of the main reasons for AMF plant growth suppression (Johnson et al., 2015; López-Carmona et al., 2019). Interestingly, the observed plant growth suppression with AMF obtained in the present study occurred in unfertilized plants, which grew only from the nutrient available in the soil.
Similarly, our results showing increased root rot development with P. capsici in mycorrhizal chili pepper contrasts with the consensus that AMF improve root health, reducing root diseases caused by nematodes, fungi, and oomycetes (Reyes-Tena et al., 2017; Reyes-Tena et al., 2016; Jamiołkowska & Michałek, 2019; Bernardo et al., 2021; Ozgonen & Erkilic, 2007). In fact, to the best of our knowledge, this is the first report that reveals increased root rot in chili pepper associated with mycorrhizal fungi. Here it is important to mention that no obvious root disease symptoms were observed in chili pepper plants inoculated with R. irregularis without pathogen inoculation, which indicates that the observed plant growth suppression was not related to pathogen contamination of the AMF inoculum employed.
Our results showing increased root rot in mycorrhizal chili pepper differ from those reported in Reyes-Tena et al. (2022), which, while using the same plant, AMF, and P. capsici genotypes, as in the present study, showed neutral effects of AMF on root rot caused by P. capsici. A possible explanation for these differences could be the use of different soil types. However, general information about how soil types affect the influence of AMF on root diseases is limited. The pathogen inoculation method also differed between the two studies. In our study, we used pre-inoculated cucumber slices, whereas zoospores were employed in the study of Reyes-Tena et al. (2022). As for the soil type, further studies examining different inoculum sources, such as zoospores, oospores, and pre-infected fresh plant tissues, are needed.
A. brasilense is well known for promoting growth development in chili pepper plants, which however contrasts with our results showing plant growth suppression after inoculation with A. brasilense. This growth suppression in chili pepper plants after inoculation with A. brasilense could be attributed to the lack of nutrients resulting in competition between plant and microorganism, which has been reported in maize (López-Carmona et al., 2019; Sarabia et al., 2017).
Our results showing that individual and combined inoculation with R. irregularis and A. brasilense increased root rot caused by P. capsici contrast the consensus of these microorganisms as plant growth and health promoters. In this study, we did not evaluate alterations in root architecture, hormone production or plant defense, which should be performed in future studies to better understand the mechanisms behind the results shown.
The observed differential changes in shoot/root resource allocation after the inoculation with R. irregularis and A. brasilence, resulting in increased and decreased shoot/root ratio, respectively, agree with the consensus about root microbe interactions and host plant resource allocations. It is well known that mycorrhizal plants allocate less resources for root development, which is compensated by root and soil mycorrhizal colonization (Johnson et al., 2008; Yao et al., 2009). Similarly, inoculation with A. brasilense is known to cause alterations in root architecture promoting lateral root formation and total root biomass (Méndez-Gómez et al., 2021; Spaepen et al., 2008).
In conclusion, our experiments showed that the well-known microbial plant growth promoters R. irregularis and A. brasilense not only decreased plant growth but also increased root rot caused by P. capsici in chili pepper plants. These results call for further studies on possible negative effects of commercial rhizosphere microorganisms on plant growth and health.
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
References
Ali, S., Hameed, S., Shahid, M., Iqbal, M., Lazarovits, G., & Imran, A. (2020). Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiological Research, 232, 126389. https://doi.org/10.1016/j.micres.2019.126389
Antoniou, A., Tsolakidou, M. D., Stringlis, I. A., & Pantelides, I. S. (2017). Rhizosphere microbiome recruited from a suppressive compost improves plant fitness and increases protection against vascular wilt pathogens of tomato. Frontiers in Plant Science, 8, 2022. https://doi.org/10.3389/fpls.2017.02022
Attia, M. S., El-Sayyad, G. S., AbdElkodous, M., & El-Batal, A. I. (2020). The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Scientia Horticulturae, 266, 109289. https://doi.org/10.1016/j.scienta.2020.109289
Banuelos, J., Alarcón, A., Larsen, J., Cruz-Sánchez, S., & Trejo, D. (2014). Interactions between arbuscular mycorrhizal fungi and Meloidogyne incognita in the ornamental plant Impatiens balsamina. Journal of Soil Science and Plant Nutrition, 14, 63–74. https://doi.org/10.4067/S0718-95162014005000005
Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., Ahmed, N., & Zhang, L. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Frontiers in Plant Science, 10, 1068. https://doi.org/10.3389/fpls.2019.01068
Bernardo, V. F., Garita, S. A., Arango, M. C., Ripodas, J. I., Saparrat, M. C. N., & Ruscitti, M. F. (2021). Arbuscular mycorrhizal fungi against the false root-knot nematode activity in Capsicum annuum: Physiological responses in plants. Biocontrol Science and Technology, 31, 119–131. https://doi.org/10.1080/09583157.2020.1833304
Damam, M., Kaloori, K., Gaddam, B., & Kausar, R. (2016). Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. InTernational Journal of Pharmaceutical Sciences Review and Research, 37, 130–136.
Dowarah, B., Gill, S. S., & Agarwala, N. (2021). Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. Journal of Plant Growth Regulation, 41, 1429–1444. https://doi.org/10.1007/s00344-021-10392-5
Etesami, H., Adl, S.M. (2020). Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In: Kumar, M., Kumar, V., Prasad, R. (Eds), Phyto-Microbiome in Stress Regulation. Environmental and Microbial Biotechnology. Singapore: Springer. https://doi.org/10.1007/978-981-15-2576-6_9
Giovannetti, M., & Mosse, B. (1980). An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist, 84, 489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Hyder, S., Gondal, A. S., Rizvi, Z. F., Ahmad, R., Alam, M. M., Hannan, A., Ahmed, W., Fatima, N., & Inam-ul-Haq, M. (2020). Characterization of native plant growth promoting rhizobacteria and their anti-oomycete potential against Phytophthora capsici affecting chilli pepper (Capsicum annum L.). Scientific Reports, 10, 1–15. https://doi.org/10.1038/s41598-020-69410-3
Jamiołkowska, A., & Michałek, W. (2019). Effect of mycorrhiza inoculati of pepper seedlings (Capsicum annuum L.) on the growth and protection against Fusarium oxysporum infection. Acta Scientiarum Polonorum Hortorum Cultus, 18, 161–169. https://doi.org/10.24326/asphc.2019.1.16
Johnson, N. C. (2010). Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist, 185, 631–647. https://doi.org/10.1111/j.1469-8137.2009.03110.x
Johnson, N. C., & Graham, J. H. (2013). The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant and Soil, 363, 411–419. https://doi.org/10.1007/s11104-012-1406-1
Johnson, N. C., Rowland, D. L., Corkidi, L., & Allen, E. B. (2008). Plant winners and losers during grassland N-eutrophication differ in biomass allocation and mycorrhizas. Ecology, 89, 2868–2878. https://doi.org/10.1890/07-1394.1
Johnson, N. C., Wilson, G. W., Wilson, J. A., Miller, R. M., & Bowker, M. A. (2015). Mycorrhizal phenotypes and the l aw of the minimum. New Phytologist, 205, 1473–1484. https://doi.org/10.1111/nph.13172
Kamal, R., Gusain, Y. S., & Kumar, V. (2014). Interaction and symbiosis of AM fungi, actinomycetes and plant growth promoting rhizobacteria with plants: Strategies for the improvement of plants health and defense system. International Journal of Current Microbiology and Applied Sciences, 3, 564–585.
Karthika, S., Varghese, S., & Jisha, M. S. (2020). Exploring the efficacy of antagonistic rhizobacteria as native biocontrol agents against tomato plant diseases. 3 Biotech, 10, 1–17. https://doi.org/10.1007/s13205-020-02306-1
Kormanik, P. P., & McGraw, A. C. (1982). Quantification of vesicular arbuscular mycorrhiza in plant roots. In N. C. Schenck (Ed.), Methods and principles of mycorrhizal research (pp. 37–45). American Phytopathological Society.
Kumar, M. R., Ashwin, R., & Bagyaraj, D. J. (2018). Screening arbuscular mycorrhizal fungi in order to select the best for alleviating wilt disease complex of capsicum. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 88, 679–684. https://doi.org/10.1007/s40011-016-0804-1
Larsen, J., Graham, J. H., Cubero, J., & Ravnskov, S. (2012). Biocontrol traits of plant growth suppressive arbuscular mycorrhizal fungi against root rot in tomato caused by Pythium aphanidermatum. European Journal of Plant Pathology, 133, 361–369. https://doi.org/10.1007/s10658-011-9909-9
Li, B., Ravnskov, S., Xie, G., & Larsen, J. (2007). Biocontrol of Pythium damping-off in cucumber by arbuscular mycorrhiza-associated bacteria from the genus Paenibacillus. BioControl, 52, 863–875. https://doi.org/10.1007/s10526-007-9076-2
Li, B., Ravnskov, S., Xie, G., & Larsen, J. (2011). Differential effects of organic compounds on cucumber damping-off and biocontrol activity of antagonistic bacteria. Journal of Plant Pathology, 93, 43–50. https://www.jstor.org/stable/41998936
López-Carmona, D. A., Alarcón, A., Martínez-Romero, E., Peña-Cabriales, J. J., & Larsen, J. (2019). Maize plant growth response to whole rhizosphere microbial communities in different mineral N and P fertilization scenarios. Rhizosphere, 9, 38–46. https://doi.org/10.1016/j.rhisph.2018.11.004
Méndez-Gómez, M., Barrera-Ortiz, S., Castro-Mercado, E., López-Bucio, J., & García-Pineda, E. (2021). The nature of the interaction Azospirillum-Arabidopsis determines the molecular and morphological changes in root and plant growth promotion. Protoplasma, 258, 179–189. https://doi.org/10.1007/s00709-020-01552-7
Nawaz, K., Shahid, A. A., Bengyella, L., Subhani, M. N., Ali, M., Anwar, W., Iftikhar, S., & Ali, S. W. (2018). Diversity of Trichoderma species in chili rhizosphere that promote vigor and antagonism against virulent Phytophthora capsici. Scientia Horticulturae, 239, 242–252. https://doi.org/10.1016/j.scienta.2018.05.048
Ozgonen, H., & Erkilic, A. (2007). Growth enhancement and Phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper. Crop Protection, 26, 1682–1688. https://doi.org/10.1016/j.cropro.2007.02.010
Petipas, R. H., Geber, M. A., & Lau, J. A. (2021). Microbe-mediated adaptation in plants. Ecology Letters, 24, 1302–1317. https://doi.org/10.1111/ele.13755
Ravnskov, S., Cabral, C., & Larsen, J. (2020). Mycorrhiza induced tolerance in Cucumis sativus against root rot caused by Pythium ultimum depends on fungal species in the arbuscular mycorrhizal symbiosis. Biological Control, 141, 104133. https://doi.org/10.1016/j.biocontrol.2019.104133
Raya-Hernández, A. I., Jaramillo-Lopez, P. F., Lopez-Carmona, D. A., Diaz, T., Carrera-Valtierra, J. A., & Larsen, J. (2020). Field evidence for maize-mycorrhiza interactions in agroecosystems with low and high P soils under mineral and organic fertilization. Applied Soil Ecology, 149, 103511. https://doi.org/10.1016/j.apsoil.2020.103511
Reyes Tena, A., Quiñones Aguilar, E. E., Rincón Enríquez, G., & López Pérez, L. (2016). Mycorrhizae in Capsicum annuum L. to promote growth and biosecurity against Phytophthora capsici L. Revista Mexicana de Ciencias Agrícolas, 7, 857–870.
Reyes-Tena, A., Rincón-Enríquez, G., López-Pérez, L., & Quiñones-Aguilar, E. E. (2017). Effect of mycorrhizae and actinomycetes on growth and bioprotection of Capsicum annuum L. against Phytophthora capsici. Pakistan Journal of Agricultural Sciences, 54, 513–522. https://doi.org/10.21162/PAKJAS/17.4245
Reyes-Tena, A., Castro-Rocha, A., Rodríguez-Alvarado, G., Vázquez-Marrufo, G., Pedraza-Santos, M. E., Lamour, K., Larsen, J., & Fernández-Pavía, S. P. (2019). Virulence phenotypes on chili pepper for Phytophthora capsici isolates from Michoacán, Mexico. HortScience, 54, 1526–1531. https://doi.org/10.21273/HORTSCI13964-19
Reyes-Tena, A., Ortega, J. M. G., Sarabia, M., Lopez, P. J., Pavia, S. P. F., Dorantes, N. G., Rodríguez-Alvarado, G., & Larsen, J. (2022). Differential response of chili pepper genotypes to single and combined association with the mycorrhizal fungus Rhizophagus irregularis and the root pathogen Phytophthora capsici. Rhizosphere, 23, 100579. https://doi.org/10.1016/j.rhisph.2022.100579
Reyes-Tena, A., Rodríguez-Alvarado, G., de Jesús Luna-Ruíz, J., Arreola-Romero, V., Arriaga-Solorio, K. L., Gómez-Dorantes, N., & Fernández-Pavía, S. P. (2021). Tolerance to virulence phenotypes of Phytophthora capsici in pasilla pepper cultivars. HortScience, 56, 1239–1243. https://doi.org/10.21273/HORTSCI15998-21
Ryan, M. H., Herwaarden, A. F. V., Angus, J. F., & Kirkegaard, J. A. (2005). Reduced growth of autumn-sown wheat in a low-P soil is associated with high colonisation by arbuscular mycorrhizal fungi. Plant and Soil, 270, 275–286. https://doi.org/10.1007/s11104-004-1611-7
Saltos, L. A., Monteros-Altamirano, Á., Reis, A., & Garcés-Fiallos, F. R. (2022). Phytophthora capsici: The diseases it causes and management strategies to produce healthier vegetable crops. Horticultura Brasileira, 40, 5–17. https://doi.org/10.1590/s0102-0536-20220101
Sarabia, M., Cornejo, P., Azcón, R., Carreón-Abud, Y., & Larsen, J. (2017). Mineral phosphorus fertilization modulates interactions between maize, rhizosphere yeasts and arbuscular mycorrhizal fungi. Rhizosphere, 4, 89–93. https://doi.org/10.1016/j.rhisph.2017.09.001
Smith, S. E., & Read, D. J. (2008). Mycorrhizal symbiosis (3rd ed.). Academic Press. https://doi.org/10.1016/B978-0-12-370526-6.X5001-6
Son, J. S., Sumayo, M., Hwang, Y. J., Kim, B. S., & Ghim, S. Y. (2014). Screening of plant growth-promoting rhizobacteria as elicitor of systemic resistance against gray leaf spot disease in pepper. Applied Soil Ecology, 73, 1–8. https://doi.org/10.1016/j.apsoil.2013.07.016
Spaepen, S., Dobbelaere, S., Croonenborghs, A., & Vanderleyden, J. (2008). Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant and Soil, 312, 15–23. https://doi.org/10.1007/s11104-008-9560-1
Thygesen, K., Larsen, J., & Bødker, L. (2004). Arbuscular mycorrhizal fungi reduce development of pea root-rot caused by Aphanomyces euteiches using oospores as pathogen inoculum. European Journal of Plant Pathology, 110, 411–419. https://doi.org/10.1023/B:EJPP.0000021070.61574.8b
Tomah, A. A., AbdAlamer, I. S., Li, B., & Zhang, J. Z. (2020). A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biological Control, 145, 104261. https://doi.org/10.1016/j.biocontrol.2020.104261
Van Der Heijden, M. G., Bruin, S. D., Luckerhoff, L., Van Logtestijn, R. S., & Schlaeppi, K. (2016). A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. The ISME Journal, 10, 389–399. https://doi.org/10.1038/ismej.2015.120
Verma, P. P., Shelake, R. M., Das, S., Sharma, P., & Kim, J. Y. (2019). Plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF): Potential biological control agents of diseases and pests. In D. Singh, V. Gupta, & R. Prabha (Eds.), Microbial interventions in agriculture and environment (pp. 281–311). Springer.
Whipps, J. M. (2004). Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Canadian Journal of Botany, 82, 1198–1227. https://doi.org/10.1139/b04-082
Whiteside, M. D., Werner, G. D. A., Caldas, V. E. A., Van’tPadje, A., Dupin, S. E., Elbers, B., et al. (2019). Mycorrhizal fungi respond to resource inequality by moving phosphorus from rich to poor patches across networks. Current Biology, 29, 2043–2050. https://doi.org/10.1016/j.cub.2019.04.061
Yao, Q., Wang, L. R., Zhu, H. H., & Chen, J. Z. (2009). Effect of arbuscular mycorrhizal fungal inoculation on root system architecture of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Scientia Horticulturae, 121, 458–461. https://doi.org/10.1016/j.scienta.2009.03.013
Acknowledgements
We thank the Mexican Research Council (CONAHCYT) for the graduate scholarship to the first author AIRH (708064) and the project PRONACE-CONACYT 316049 for financial support. We also thank UNAM-DGPA for financial support for the project PAPIIT-UNAM IG200921. Also, the first author AIRH (516012162) thanks the post graduate program in biological sciences (PCB) at UNAM for PhD training. Finally, this paper is presented as a part of the requirements for obtaining the doctoral degree at PCB-UNAM for the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving humans and/or animals
This research did not involve human participants or animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raya-Hernández, A.I., López-Carmona, D., Jaramillo-López, P. et al. Well known microbial plant growth promoters provoke plant growth suppression and increase chili pepper wilt caused by the root pathogen Phytophthora capsici. Eur J Plant Pathol 167, 787–799 (2023). https://doi.org/10.1007/s10658-023-02711-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02711-1