Abstract
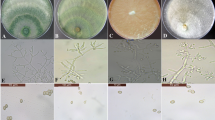
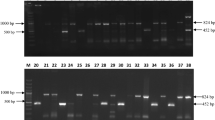
Egyptian clover suffers from high yield losses due to crown rot (Sclerotinia trifoliorum) disease. Varieties with complete resistance to crown rot are not available and application of fungicides is not an ecologically viable option in Egyptian clover. In such a scenario, knowledge based and integrated use of different biocontrol agents can be explored, as this strategy has not been utilized for disease management in Egyptian clover. In this study, Trichoderma isolates were isolated from the rhizosphere of Egyptian clover. Low and medium molecular weight chitosan was evaluated for their antagonistic ability against clover rot pathogens and compatibility with Trichoderma isolates. Among different Trichoderma isolates, Trichoderma harzianum TBR-7 was found to be highly antagonistic against clover rot pathogens and also found compatible with low molecular weight chitosan. Mycelial biomass accumulation and protein leakage studies clearly showed the differential effect of low molecular weight chitosan on clover rot pathogens and Trichoderma isolates. Seed treatment with Trichoderma harzianum TBR-7 followed by foliar spray of chitosan (0.05%) led to consistent upregulation of various defense enzymes (PAL, Phenols, SOD, H2O2 and Proline) in Egyptian clover. Field trials showed that seed treatment with Trichoderma harzianum TBR-7 followed by a foliar spray of chitosan (0.05%) decreased crown rot incidence, and increased green fodder and seed yield significantly compared to other treatments. Through this study, an effective and knowledge-based utilization of different biocontrol agents (Trichoderma and chitosan) for disease management in Egyptian clover has been proposed.







Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Abawi, G. S., & Grogan, R. G. (1979). Epidemiology of diseases caused by Sclerotinia species. Phytopathology, 69, 899–904.
Abdulkarim, A., Isa, M. T., Abdulsalam, S., Muhammad, A. J., & Ameh, A. O. (2013). Extraction and characterisation of chitin and chitosan from mussel shell. Civil and Environmental Research, 3, 108–114.
Abreu, M. J., & Souza, E. A. (2015). Investigation of Sclerotinia sclerotiorum strains variability in Brazil. Genetics and Molecular Research, 14, 6879–6896.
Adams, P., De-Leij, F. A., & Lynch, J. M. (2007). Trichoderma harzianum Rifai 1295–22 mediates growth promotion of Crack willow (Salix fragilis) saplings in both clean and metal-contaminated soil. Microbial Ecology, 54, 306–313.
Agrawal, G. K., Rakwal, R., Tamogami, S., Yonekura, M., Kubo, A., & Saji, H. (2002). Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiology and Biochemistry, 40, 1061–1069.
Ahn, I. P., Lee, S. W., & Suh, S. C. (2007). Rhizobacteria-induced priming in Arabidopsis is dependent on ethylene, jasmonic acid, and NPR1. Molecular Plant Microbe Interactions, 20, 759–768.
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001). The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environment, 24, 1337–1344.
Ali, A., Noh, N. M., & Mustafa, M. A. (2015). Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packaging and Shelf Life, 3, 56–61.
Badawy, M. E., Rabea, E. I., Rogge, T. M., Stevens, C. V., Smagghe, G., Steurbaut, W., & Hofte, M. (2004). Synthesis and fungicidal activity of new N, O-acyl chitosan derivatives. Biomacromolecules, 5, 589–595.
Bakeer, A. R. T., El-Mohamedy, R. S., Saied, N. M., & Abd-El-Kareem, F. (2016). Field suppression of Fusarium soil borne diseases of Tomato plants by the combined application of bio agents and Chitosan. British Biotechnology Journal, 13, 1–10.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Bautista-Banos, S., Hernandez-Lopez, M., Bosquez-Molina, E., & Wilson, C. L. (2003). Effect of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Protection, 22, 1087–1092.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acryl amide gels. Analytical Biochemistry, 44, 276–287.
Beausejour, C. M., Krtolica, A., Galimi, F., Narita, M., Lowe, S. W., Yaswen, P., & Campisi, J. (2003). Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO Journal., 22, 4212–4222.
Bhardwaj, N.R., Atri, A., Rani, U., & Roy, A.K. (2021). A logistic regression model for predicting Sclerotinia stem rot in Egyptian clover (Trifolium alexandrinum L.). Legume Research, https://doi.org/10.18805/LR-4492
Bhaskar, R. B., Hasan, N., Pandey, K. C., & Melkania, N. P. (2003). Management of root-rot disease complex of berseem (Trifolium alexandrinum L.). Forage Research, 29, 84–87.
Bisen, K., Keswani, C., Mishra, S., Saxena, A., Rakshit, A., & Singh, H. B. (2015). Unrealized potential of seed biopriming for versatile agriculture. In A. Rakshit, H. B. Singh, & A. Sen (Eds.), Nutrient Use Efficiency: From Basics to Advances (pp. 193–206). Springer.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Chandrika, K. S. V. P., Prasad, R. D., & Varsha, G. (2019). Development of chitosan-PEG blended films using Trichoderma: Enhancement of antimicrobial activity and seed quality. International Journal of Biological Macromolecules, 126, 282–290.
Chen, J.L., Sun, S.Z., Miao, C.P., Wu, K., Chen, Y.W., Xu, L.H., Guan, H.L., & Zhao, L. X. (2015). Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng Journal of Ginseng Research, https://doi.org/10.1016/j.jgr.2015.09.006
Chittenden, C., & Singh, T. (2009). In vitro evaluation of combination of Trichoderma harzianum and chitosan for the control of sapstain fungi. Biological Control, 50, 262–266.
Christopher, D. J., Raj, T. S., Rani, S. U., & Udhayakumar, R. (2010). Role of defense enzymes activity in tomato as induced by Trichoderma virens against Fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici. Journal of Biopesticides, 3, 158–162.
Conrath, U., Beckers, G. J. M., Flors, V., Garcia-Agustin, P., Jakab, G., Mauch, F., Mari-Anne Newman, M. A., Pieterse, C. M. J., Poinssot, B., Pozo, M. J., Pugin, A., Schaffrath, U., Ton, J., Wendehenne, D., Zimmerli, L., & Mauch-Mani, B. (2006). Priming: Getting ready for battle. Molecular Plant Microbe Interactions, 19, 1062–1071.
Contreras-Cornejo, H. A., Macias-Rodriguez, L., Cortes-Penagos, C., & Lopez-Bucio, J. (2009). Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiology, 149, 1579–1592.
Corradini, E., de Moura, M. R., & Mattoso, L. H. C. (2010). A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polymer Letters, 4, 509–515.
Du, Y., Zhao, Y., Dai, S., & Yang, B. (2009). Preparation of water-soluble chitosan from shrimp shell and its antibacterial activity. Innovative Food Science & Emerging Technologies, 10, 103–107.
El Ghaouth, A., Arul, J., Grenier, J., & Asselin, A. (1992). Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology, 82, 398–402.
Eweis, M., Elkholy, S. S., & Elsabee, M. Z. (2006). Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. International Journal of Biological Macromolecules, 38, 1–8.
Frame, J. (2005). Forage Legumes for temperate grasslands. Italy and Science Publishers Inc, Enfield, USA.
Hadwiger, L. A., Beckman, J. M., & Adams, M. J. (1981). Localization of fungal components in the Pea-Fusarium interaction detected immunochemically with anti-chitosan and anti-fungal cell wall antisera. Plant Physiology, 67, 170–175.
Hafez, Y. M., El-Nagar, A. S., Elzaawely, A. A., Kamel, S., & Maswada, H. F. (2018). Biological control of Podosphaera xanthii the causal agent of squash powdery mildew disease by upregulation of defense-related enzymes. Egyptian Journal of Biological Pest Control, 28, 1–8.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Hermosa, R., Viterbo, A., Chet, I., & Monte, E. (2012). Plant beneficial effects of Trichoderma and of its genes. Microbiology, 158, 17–25.
Joshi, B. B., Bhatt, R. P., & Bahukhandi, D. (2010). Antagonistic and plant growth activity of Trichoderma isolates of Western Himalayas. Journal of Environmental Biology, 31, 921–928.
Kappel, L., Kosa, N., & Gruber, S. (2022). The multilateral efficacy of Chitosan and Trichoderma on Sugar Beet. Journal of Fungi, 8, 137.
Kaushal, P., Malaviya, D. R., Roy, A. K., Kumar, B., & Tiwari, A. (2005). Trifolium alexandrinum x T. resupinatum- interspecific hybrids developed through embryo rescue. Plant Cell Tissue Organ Culture, 83, 137–144.
Keswani, C., Mishra, S., Sarma, B. K., Singh, S. P., & Singh, H. B. (2014). Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Applied Microbiology and Biotechnology, 98, 533–544.
Laflamme, P., Benhamou, N., Bussieres, N., & Dessureault, M. (2000). Differential effect of chitosan on root rot fungal pathogens in forest nurseries. Canadian Journal of Botany, 77, 1460–1468.
Liu, H., Du, Y., Wang, X., & Sun, L. (2004). Chitosan kills bacteria through cell membrane damage. International Journal of Food Microbiology, 95, 147–155.
Liu, C. X., Zhang, D. R., He, Y., Zhao, X. S., & Bai, R. B. (2010). Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. Journal of Membrane Science, 346, 121–130.
Lopes, F. A. C., Stendorff, A. S., Geraldine, A. M., Brandao, R. S., Monteiro, V. N., Junior, M. L., Coelho, A. S. G., Ulhon, C. J., & Silva, R. N. (2012). Biochemical and metabolic profiles of Trichoderma strains isolated from common bean crops in the Brazilian Cerrado, and potential antagonism against Sclerotinia sclerotiorum. Fungal Biology, 116, 815–824.
Lopez-Diez, E. C., & Bone, S. (2000). An investigation of the waterbinding properties of protein+sugar systems. Physics in Medicine & Biology, 45, 3577–3588.
Lynch, J., Wilson, K., Ousley, M., & Whipps, J. (1991). Response of lettuce to Trichoderma treatment. Letters in Applied Microbiology, 12, 59–61.
Malaviya, D. R., Roy, A. K., Kaushal, P., Kumar, B., & Tiwari, A. (2004). Development and characterization of interspecific hybrids of Trifolium alexandrinum x T. apertum using embryo rescue. Plant Breeding, 123, 536–542.
Malaviya, D. R., Roy, A. K., Kaushal, P., Chakraborti, M., Yadav, A., Khare, A., Dhir, R., Khairnar, D., & George, G. P. (2018). Interspecific compatibility barriers, development of interspecific hybrids through embryo rescue and lineage of Trifolium alexandrinum (Egyptian clover)-important tropical forage legume. Plant Breeding, 137, 655–672.
Morton, D. T., & Stroube, N. H. (1955). Antagonistic and stimulatory effect of microorganism upon Sclerotium rolfsii. Phytopathology, 45, 419–420.
Muhammad, D., Misri, B., Nahrawy, E. L., Khan, M. S., & Serkan, A. (2014). Egyptian clover (Trifolium alexandrinum L.): King of forage crops (p. 127). Cairo: FAO Regional.
Ngo, B. H., Vu, D. N., & Tran, D. Q. (2006). Analyze antagonist effects of Trichoderma spp. for controlling southern stem rot caused by Sclerotium rolfsii on peanut. Plant Protection, 1, 12–14.
Nitu, N. J., Masum, M.M.I., Jannat, R., Bhuiyan, M.K.A., & Sultana, S. (2016). Application of chitosan and Trichoderma against soil-borne pathogens and their effect on yield of tomato (Solanum lycopersicum L.). International Journal of Biosciences, 9, 1024
Pande, P. P., Rathi, A. S., & Avtar, R. (2008). Management of stem rot of Egyptian clover using bio-agents and chemicals. Forage Research, 34, 83–86.
Park, S. Y., Marsh, K. S., & Rhim, J. W. (2002). Characteristics of different molecular weight chitosan films affected by the type of organic solvents. Journal of Food Science, 67, 194–197.
Patel, S., & Saraf, M. (2017). Biocontrol efficacy of Trichoderma asperellum MSST against tomato wilting by Fusarium oxysporum f. sp. lycopersici. Archives of Phytopathology and Plant Protection, 50, 228–238.
Piras, A. M., Maisetta, G., Sandreschi, S., Gazzarri, M., Bartoli, C., Grassi, L., Esin, S., Chiellini, F., & Batoni, G. (2015). Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Frontiers in Microbiology, 28, 372.
Prasad, R. D., Chandrika, K. S. V. P., & Godbole, V. (2020). A novel chitosan biopolymer based Trichoderma delivery system: Storage stability, persistence and bio efficacy against seed and soil borne diseases of oilseed crops. Microbiology Research, 237, 126487.
Pratt, R. G., McLaughlin, M. R., Pederson, G. A., & Rowe, D. E. (1998). Pathogenicity of Macrophomina phaseolina to mature plant tissues of alfalfa and white clover. Plant Disease, 82, 1033–1038.
Qi, L., Xu, Z., Jiang, X., Hu, C., & Zou, X. (2004). Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research, 339, 2693–2700.
Qing, W., Jin-hua, Z., Qian, W., Yang, N. A., & Li-pu, G. (2015). Inhibitory effect of chitosan on growth of the fungal phytopathogen, Sclerotinia sclerotiorum and sclerotinia rot of carrot. Journal of Integrative Agriculture, 14, 691–697.
Rathi, A. S., Avtar, R., & Jhorar, B. S. (2007). Prevalence and severity of stem rot disease of berseem in Haryana a survey report. Forage Research, 32, 260–261.
Reddy, M. V. B., Belkacemi, K., Corcuff, R., Castaigne, F., & Arul, J. (2000). Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biology and Technology, 20, 39–51.
Ross, W. W., & Sederoff, R. R. (1992). Phenylalanine ammonia lyase from loblolly pine: Purification of the enzyme and isolation of complementary DNA clones. Plant Physiology, 98, 380–386.
Saharan, V., Mehrotra, A., Khatik, R., Rawal, P., Sharma, S. S., & Pal, A. (2013). Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. International Journal of Biological Macromolecules, 62, 677–683.
Saharan, V., Sharma, G., Yadav, M., Choudhary, M. K., Sharma, S. S., Pal, A., Raliya, R., & Biswas, P. (2015). Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. International Journal of Biological Macromolecules, 75, 346–353.
Saharan, V., Kumaraswamy, R. V., Choudhary, R. C., Kumari, S., Pal, A., Raliya, R., & Biswas, P. (2016). Cu-Chitosan nanoparticle mediated sustainable approach to enhance seedling growth in Maize by mobilizing reserved food. Journal of Agricultural and Food Chemistry, 64, 6148–6155.
Shibuya, N., & Minami, E. (2001). Oligosaccharide signalling for defence responses in plant. Physiological and Molecular Plant Pathology, 59, 223–233.
Stockwell, V. O., Johnson, K. B., Sugar, D., & Loper, J. E. (2011). Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology, 101, 113–123.
Swain, J., & Hillis, W. E. (1959). The Phenolic constitutents of Prunus domestica. I. The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10, 63–68.
Tran, T. T. (1998). Antagonistic effectiveness of Trichoderma against plant fungal pathogens. Plant Protection, 4, 35–38.
Van, C.N., Van, B.N., & Ming-Fa, H. (2013). Curcumin-loaded chitosan/gelatin composite sponge for wound healing application. International Journal of Polymer Science, 106570, https://doi.org/10.1155/2013/106570
Van-Loon, L. C., & Van-Strien, E. A. (1999). The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiological and Molecular Plant Pathology, 55, 85–97.
Vinale, F., Ghisalberti, E., Sivasithamparam, K., Marra, R., Ritieni, A., Ferracane, R., Woo, S., & Lorito, M. (2009). Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Letters in Applied Microbiology, 48, 705–711.
Wang, Q., Jin-hua, Z., Qian, W., Yang, N. A., & Li-pu, G. A. O. (2015). Inhibitory effect of chitosan on growth of the fungal phytopathogen, Sclerotinia sclerotiorum, and sclerotinia rot of carrot. Journal of Integrative Agriculture, 14, 691–697.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols: A Guide to Methods and Applications (pp. 315–322). Academic Press.
Woo, S. L., Scala, F., Ruocco, M., & Lorito, M. (2006). The Molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathology, 96, 181–185.
Wu, Z. H., Wang, T. H., Huang, W., & Qu, Y. B. (2001). A simplified method for chromosome DNA preparation from filamentous fungi. Mycosystema, 20, 575–577.
Funding
The study was funded by ICAR-IGFRI, Jhansi under the project “Development of Trichoderma-Chitosan combination for management of root and stem rot disease in Berseem” (Project code- CRSCIGFRISIL20170101xxxxx).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhardwaj, N.R., Rana, M., Koli, P. et al. Rhizospheric Trichoderma harzianum TBR-7 in combination with chitosan for eco-friendly management of crown rot disease in Egyptian clover (Trifolium alexandrinum L.). Eur J Plant Pathol 167, 677–698 (2023). https://doi.org/10.1007/s10658-023-02709-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02709-9