Abstract
Avocado sunblotch viroid (ASBVd) is found in many avocado-growing regions of the world, where it affects fruit yield and quality. The trees develop two types of infections: symptomatic and symptomless infections. Symptoms are most obvious as yellow streaks on leaves, fruit and green stems of symptomatic trees and symptomless carrier trees do not display any such symptoms. Symptomless carrier trees are considered the primary source of disease transmission in orchards. Hence, this study investigated the impact of ASBVd-infected symptomless carrier trees on tree morphology, fruit maturity, yield and quality of ‘Hass’ avocado from 2019 until 2021. Differences were observed in the orchard between infected and healthy trees; trees with medium and high viroid concentrations excessively produced flowers, lost leaves during flowering and ultimately bore few to no fruit at the end of the season. The dry matter content results showed that ASBVd did not affect the rate of maturity of the fruit, as fruit from infected and healthy trees matured at the same time. Yield counts indicated that medium and highly infected trees produced between 83 and 96% lower yields compared to healthy trees. Postharvest studies showed that medium and highly infected fruit significantly lost firmness and coloured up more rapidly than healthy fruit. Infected, non-stored fruit also developed external rots and shrivels, however, these disorders were reduced in fruit stored at 5 °C for 28 days. Therefore, flower overbearing with the shedding of leaves and lower yields can be used as indicators of ASBVd infection in ‘Hass’ orchards but confirmation with molecular testing is required. These observations can be incorporated into an ASBD management strategy in ‘Hass’ orchards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avocado sunblotch viroid (ASBVd) is found in many avocado-growing regions of the world, where it affects fruit yield and quality. (Palukaitis et al., 1979). Avocado sunblotch viroid is a small, infective, single-stranded circular RNA molecule of 246–251 nucleotides (Kuhn et al., 2017). Avocado is the only known natural host for ASBVd, and all avocado cultivars are susceptible (Saucedo Carabez et al., 2019). The use of infected propagative material is the most important mode of spreading ASBVd, however, there is no sufficient evidence of the natural infection from an infected- to a healthy tree (Wallace, 1958). ASBVd can be transmitted via seed from infected trees, from infected propagative rootstocks, infected scions used for grafting and via root grafts and pollen. To date, no vector has been reported for the transmission of ASBVd, however, honeybees were experimentally proven to carry ASBVd from an infected to a healthy tree and thus have been suspected as a possible vector (Desjardins et al., 1979). ASBVd has been present in South Africa since 1954 and to date has become one of the common diseases in South African avocado orchards (Da Graça & Van Vuuren, 2003).
There are three ASBVd symptom-associated variants namely symptomless carrier (ASBVd-Sc), a bleached symptom associated (ASBVd-B) and variegated symptom associated (ASBVd-V) variants (Semancik & Szychowski, 1994). The different ASBVd variants arise from slight sequence variations on the ASBVd sequence (Running et al., 1996). Most of the changes occur between UA bases leading to the sequence variations (Schnell et al., 1997). Semancik and Szychowski (1994) demonstrated that the terminal loops are the predominant sites of sequence variation of ASBVd, ASBVd-B was demonstrated to have an enlarged poly (A) right loop which introduces a unique structural modification and an UA exchange on the left terminal loop. The infected trees can either express symptoms or show no symptoms or signs of infection but still be carriers of ASBVd, and these latter trees are known as symptomless carrier trees (Acheampong et al., 2008). Characteristic symptoms of include the appearance of irregular, sunken areas of white, yellow, or reddish colour in infected fruit; white/yellow sunken streaks on green stems and variegated or bleached symptoms on the leaves. Symptomless carrier trees do not exhibit any symptoms, however, these trees can exhibit symptoms when exposed to stress, e.g. fire, when the trees are cut back, or when a healthy scion is grafted onto a symptomless carrier rootstock (Dodds, 2001). The composition of sequence variants in the tree is fluid over time.
Symptomless carrier trees play an essential role in the epidemiology of ASBVd, as they have been described as the primary sources of infection for spreading the disease through budding and grafting practices (Saucedo Carabez et al., 2019). Studies have shown that ASBD can significantly reduce the yield and quality of avocado fruit (Da Graça, 1985; Saucedo-Carabez et al., 2014). Avocado sunblotch viroid–infected symptomless carrier trees could lead to between 50 to 80% yield reductions compared with the symptomatic trees, which cause a yield reduction between 15 to 30% (Saucedo-Carabez et al., 2014).
The purpose of this study was to assess the impact of avocado sunblotch viroid (ASBVd) on tree morphology, fruit maturity, yield and quality of ‘Hass’ avocado trees in South Africa.
Material and methods
Orchard description
The experimental field was located at a commercial farm in Mbombela, province of Mpumalanga, South Africa. The experimental ‘Hass’ trees were obtained from an orchard, established in 2009 (longitude: 30.9298773 latitude: -25.429164). Thirty trees were utilised for all the investigations of the study including 15 ASBVd infected symptomless carrier trees (selected by molecular screening) and 15 ASBVd negative trees (confirmed negative with molecular screening and hereafter referred to as ‘healthy’ trees).
Field selection of symptomless carrier trees
Since ASBVd infected symptomless carrier trees are difficult to identify in the field, infected trees showing fruit symptoms were first identified. In their immediate vicinity, trees were screened using molecular testing. Screening was also conducted in the immediate areas where confirmed positive trees had been removed.
Leaf samples were collected by selecting a combination of 20 leaves (both young and old) from the main branches of individual avocado trees. The stack of leaves was then punched four times using an 8 mm punch creating a representative sample of 400 mg leaf material per tree. The double-stranded RNA (ds-RNA) was extracted from 400 mg leaf samples using a cellulose column-chromatography technique (Luttig & Manicom, 1999).
Trees used in the study were tested for the presence or absence of ASBVd using a quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay. A fluorescent-based real-time one-step qRT-PCR assay was optimised to amplify ASBVd using the qPCRBIO SyGreen 1-Step Go Lo-ROX kit (PCRBIOSYSTEMS, UK), according to the manufacturer's instruction in a Rotor-Gene Q instrument (Qiagen, Germany). The Rotor-gene Q software 2.3.1 (Qiagen) was utilized to calculate PCR efficiency, quantification cycle (Cq)-, and quantification values for establishing the standard curve and for determining the concentration of samples. The efficiency and sensitivity of the real-time qRT-PCR assay was validated using a 10 ng/µl g-block® Gene Fragment (GenBank® S73860-Sc) in a ten-fold, seven-point dilution standard curve.
An ASBVd-specific primer set, (5’- AGAGAAGGAGGAGTCGTGGTGAAC -3’; 5’- TTCCCATCTTTCCCTGAAGAGAC -3’), amplifying a 99 bp product, was used at a final concentration of 400 nM in a 12.5 µl reaction volume. A standardised volume, 1.2 µl of the dsRNA template, was added to the reaction. The cycling conditions included reverse transcription at 50 °C for 10 min, followed by polymerase activation at 95 °C for 2 min. The PCR step included 35 cycles with a denaturation step at 95 °C for 5 s and an annealing step at 56 °C for 30 s.
Seasonal monitoring of trees
Infected and healthy trees were monitored for three consecutive seasons from 2019 until 2021, from the flowering stage until harvest. These included observations of differences in tree morphology (branches, leaves and reproductive structures), flowering patterns and fruit sets that could be physical indicators of ASBVd symptomless carrier trees in avocado orchards.
Determination of the dry matter content (% DM) of fruit
The degree of ripeness of avocado fruit was determined by measuring the % DM content of fruit at four different intervals before harvest in the years 2019 and 2020 (Woolf et al., 2003). The % DM measurements were conducted by randomly selecting a single fruit from each of the 30 experimental trees (15 infected and 15 healthy trees) at each interval. For each fruit, the skin was removed by peeling; the flesh was grated into a Petri dish and measured to a standard weight of 10 g. The Petri dishes containing the fresh flesh were then placed in an oven at 30 °C for 48 h, after which, the dry mass of each sample was recorded. The percentage of dry mass was calculated as follows: Dry mass (%) = (dry flesh weight/fresh flesh weight) × 100. The fruit was considered to reach full maturity once a moisture content of 77% was reached, as recommended by the South African Avocado Growers' Association minimum maturity requirements expressed as maximum allowable moisture content (Kruger & Claassens, 2001).
Yield
The yield per tree was determined by counting the number of fruit on each tree in the years 2020 and 2021. Two individuals conducted the counting of fruit, each repeating the count on every tree three times. The average count was used to determine the number of fruits on each tree.
Determination of fruit ripening and quality
Thirty fruit were harvested from each of the 30 trees in this study (infected and healthy), except in the case of highly infected trees because they produced less than 30 fruit and all available fruit were harvested. The fruit were transported to the ARC-TSC postharvest laboratory on the day of harvest and were divided equally into two parts, non-stored and stored fruit. Non-stored fruit was immediately taken to the darkroom at 21 ± 2 °C for ripening. Stored fruit was taken to the cold storage at 5 °C for 28 days, there after removed from the storage and moved to the darkroom for ripening under the same conditions as mentioned above. Ripening was determined by measuring firmness and colour every second day during storage. Fruit quality was determined when the fruit had ripened.
Fruit firmness
Fruit firmness was measured using an automated Sinclaire IQ instrument (51DFTB, International Ltd, Jorrold, Bowthorpe Norwich, NR5, 9.D, England). The fruit was rated using the IQ score, the fruit receiving an IQ score of 70 was considered hard and an IQ of 10 was soft (Howarth et al., 2003). The fruit was considered ripe after reaching an IQ score of ≤ 25.
Colour rating
The colour change was monitored visually using a six-point rating where: 1, emerald; 2, forest green; 3, approximately 25% coloured; 4, approximately 75% coloured; 5, purple; 6, black (White et al., 2009).
Determination of fruit quality
External rot, flesh bruising, diffuse flesh discolouration, stem-end and body rot were rated according to the International Avocado Quality Manual (White et al., 2009) using a scale of zero (0%) to three (≥ 50% of the fruit surface affected). Shrivel was determined using a rating scale between zero (no shrivel) to three (fruits appear shrivelled) (White et al., 2009).
Data analysis
Statistical analyses of variance were obtained using STATISTICA 8 and the means were separated using the Post-hoc Tukey HSD test at a significance level of p ≤ 0.05.
Results
Field screening of symptomless carrier trees
Detection was confirmed in the qRT-PCR assay using the seven-point, ten-fold dilution, standard curve that showed an amplification efficiency of 1.03 with a linearity correlation coefficient (R2) of 0.999. The qRT-PCR result confirmed 15 trees as ASBVd positive symptomless carriers. The ASBVd titre varied considerably between trees. Based on the cycling threshold (Ct) values, the infected trees were divided into three infection levels; low, medium and high (Table 1). All trees with Ct values equal to or less than 10 were rated as highly infected, Ct values from 10.1 to 18 were rated as medium and Ct values from 18.1 to 25.4 were rated as low/slightly infected. Seven of the15 trees were highly infected with ASBVd, five trees had a medium infection and three trees had a low infection level.
Seasonal monitoring of trees
Observations of the infected symptomless carrier trees in the orchard were conducted over three years (2019–2021). Morphological differences were found between infected symptomless carrier trees with medium and high infection levels, and healthy trees in the orchard. The first observation was that a significantly large number of flowers were produced by the infected trees when trees showed alternate bearing (‘on’ and ‘off’ seasons); the flowers appeared clustered and more liable to sunburn (Fig. 1B, C). Secondly, a large number of leaves were lost, leaving trees looking unhealthy and completely dominated by flowers (Fig. 1B, C). The trees lost the majority of flowers (Fig. 1C), and the fruit set was delayed compared to the healthy trees (Fig. 2). The ASBVd infected trees started recovering the lost leaves instead of producing fruit (Fig. 2), and ultimately recovered most of the leaves, looking healthier but with very little to no fruit. Additionally, the bud-wood of symptomless carrier trees was bigger with larger buds compared to that of healthy trees; this was consistently observed with more infected trees in the field over the three years (Fig. 3). However, trees with low infection levels produced a mixture of bigger bud-wood with large buds and normal bud-wood.
Determining fruit dry matter content (DM)
The dry matter content of the fruit was determined to measure the fruit's maturity. The % DM measurements were conducted for the 2019 and 2020 harvests. In 2019, the % DM measurements were taken at four intervals between 15 April and 06 May, there were no significant differences between the dry matter content of the healthy and infected trees in all four intervals (Table 2). The measurements were repeated in 2020 from 23 April until 13 May. The % DM ratings of highly infected fruit were significantly higher than that of healthy fruit for the first two intervals (p ≤ 0.05, Table 2). In the first interval, the % DM of the highly infected fruit was rated 30.9 ± 3.3%, 4.2% higher than that of healthy fruit which was rated 26.7 ± 2.0%. Again, in the second interval, the DM of highly infected fruit rated 30.1 ± 3.6%, 3.7% higher than that of healthy fruit, which rated 26.4 ± 1.7%. There were, however, no significant differences recorded for the % DM of all fruit between the third and the fourth intervals.
Yield
The yield of the infected trees was determined by counting the number of fruits per tree. The values represent the average number of fruits counted in trees from different infection levels (Fig. 4). Yield counts for 2020 averaged 221.0 ± 55.1 fruit per tree for the healthy trees, significantly different from the medium and highly infected trees, which averaged between 38.0 ± 8.6 and 12.0 ± 6.9 fruit per tree, respectively (p ≤ 0.05, Fig. 4). This indicates that infected trees with medium and high infection levels produced between 83 and 95% lower yields compared to the healthy trees, while trees with low infection levels produced an average yield of 177.0 ± 28.5 fruit per tree; not significantly different from the yield of healthy trees (Fig. 4). Yield counts were repeated in 2021 where healthy trees produced an average of 437.0 ± 117.3 fruit per tree, which was significantly higher compared to 41.0 ± 19.1 and 19.0 ± 11.8 fruit per tree produced by medium and highly infected trees, respectively (p ≤ 0.05, Fig. 6). At this time, the high and medium infected trees produced yields between 91 and 96% lower than healthy trees. Yet again, the low infected trees produced an average of 395.0 ± 22.5 fruit per tree, not significantly different from the yield of healthy trees (Fig. 4).
Effect of ASBVd on avocado fruit ripening and postharvest quality
Fruit firmness
Non-stored fruit
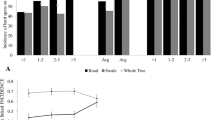
Fruit firmness was measured using the Sinclair IQ score to determine fruit ripening from day of harvest. The average IQ scores of all treatments were over 70 on the day of harvest, implying the fruit was still very hard. Healthy fruit had an IQ score of 75.7 ± 7.0, low infected fruit had an IQ score of 76.0 ± 7.2, medium infected fruit had an IQ score of 74.4 ± 6.4 and highly infected fruit an IQ score of 72.9 ± 10.0 The IQ scores of all treatments had decreased on day five suggesting that the fruit started losing firmness, the IQ score for healthy fruit decreased to 49.8 ± 6.0, significantly different from medium infected fruit which had decreased to 45.0 ± 9.6 and highly infected fruit which decreased to 36.1 ± 14.5 (p ≤ 0.05, Fig. 5A). Further firmness was lost on day seven; the highly infected fruit had an IQ score of 25.4 ± 12.7 suggesting that the fruit had ripened. The IQ score of medium infected fruit was reduced to 33.9 ± 10.1 and was significantly different from the firmness of the healthy fruit with the IQ score of 42.2 ± 6.5 (p ≤ 0.05). The IQ score of medium infected fruit had decreased to 20.3 ± 8.8 on day nine suggesting that the fruit were ripe, significantly different from the IQ score of medium infected fruit (26.3 ± 7.1) and healthy fruit (28.0 ± 7.7) which had not yet reached the minimum IQ score of ≤ 25 to be considered ripe (Fig. 5A).
Loss of firmness for non-stored (A) and cold stored fruit at 5 °C for 28 days (B). ‘Hass’ avocado fruit was ripened in the dark at 21 °C and the IQ scores were recorded from the Sinclair instrument. Values represent means ± standard errors (SE). Bars with the same letters did not differ significantly at P ≤ 0.05 (Tukey HSD)
Stored fruit
The fruit was removed from the 5 °C storage after 28 days, and firmness was determined on the first day of removal from storage. Fruit had already lost firmness during storage. Highly infected fruit had an IQ score of 26.1 ± 6.8, medium infected fruit a firmness of 33.6 ± 10.1 IQ and had significantly lower IQ scores compared to the firmness of low infection fruit at 45.0 ± 6.5 IQ and healthy fruit firmness at 46.9 ± 8.3 IQ (p ≤ 0.05, Fig. 5B). The high and medium infected fruit had IQ scores of 14.7 ± 3.2 and 16.8 ± 4.0, respectively, on day three of ripening suggesting that they had ripened. The healthy and low infected fruit remained with IQ scores of 29.2 ± 9.2 and 26.3 ± 6.6 respectively, not yet reaching the IQ score of ≤ 25 to be considered ripe. Similar to the non-stored fruit, the stored fruit from the highly and moderately infected group ripened before the healthy and low infected fruit.
Colour rating
Non-stored fruit
The colour change was monitored visually and rated using a six-point rating scale. The first colour ratings on the day of harvest showed that all fruit had the same emerald colour with a rating of one (Fig. 6A). The fruit started to develop colour on day five post-harvest changing from emerald to forest green, however, highly infected fruit significantly differed from other fruit as they already started developing a purple colour (p ≤ 0.05, Fig. 6A). By day seven, all highly infected fruit had already developed a 25% colour rating of 3.0 ± 0.9, and some of the moderately infected fruit had also started to develop a purple colour with an average rating of 2.5 ± 0.7, all significantly different from the healthy fruit which remained forest green with a rating of 2.1 ± 0.3 (p ≤ 0.05, Fig. 6A). The healthy and fruit from trees with a low viroid concentration only developed a purple colour on day nine with a rating of 2.6 ± 0.6 and 3.0 ± 0.7, respectively (Fig. 6A).
Stored fruit
The fruit was removed from the cold storage after 28 days and moved to the dark ripening room. Upon removal, colour was rated and fruit had already changed colour from emerald (harvest day) to forest green with healthy and low infected fruit rating 2.1 ± 0.1 and 2.1 ± 0.3, respectively. This is significantly different from medium and high infected fruit with ratings of 2.5 ± 0.6 and 2.6 ± 0.5, respectively, which had already started to develop a purple colour (p ≤ 0.05). By day three, almost all the fruit had developed 25% of the colour, all having a rating of approximately 3 (Fig. 6B).
External quality
Non-stored fruit
After ripening, the fruit were analysed for external damage. The fruit was rated for external rots using a rating scale between 0 and 50%. High, medium and low infected fruit developed significantly higher incidences of rots between 27 and 30%, significantly higher than healthy fruit with an average incidence of 4.2 ± 2.9% (p ≤ 0.05, Table 3). Shrivel was rated using a rating scale between zero and three, and there were no significant differences between fruit that developed shrivel.
Stored fruit
There were lower incidences for both external rots and shrivel for the stored fruit. Only the fruit with a high infection rate developed 5.0 ± 4.5% of external rots, which was significantly different from all other fruit that developed no rots. The fruit with high infection rated 1.0 ± 0.6 for shrivel and was the only fruit that had shrivel which was significantly different (p ≤ 0.05) from other fruit that did not develop any shrivel (Table 3).
Internal fruit quality
Non-stored fruit
Flesh bruising, diffuse flesh discolouration, stem-end rot and body rots were rated using a percentage between 0 to 50%. Fruit with a high level of infection were more prone to flesh bruising, the fruit developed an average of 30.8 ± 15.9% bruising, which was significantly different (p ≤ 0.05) from the healthy fruit which only developed an average of 8.7 ± 8.1% bruising (p ≤ 0.05; Table 4). The diffuse discolouration of highly infected fruit was significantly higher (20.5%) than that of healthy fruit (p ≤ 0.05). Stem-end rot significantly differed for highly infected fruit with a 17.1% difference from healthy fruit (p ≤ 0.05, Table 4). Highly infected fruit had the highest incidences of rot, averaging 41.7 ± 12.9%, which differed significantly from the healthy fruit with an average of 10.7 ± 9.9% (p ≤ 0.05; Table 4).
Stored fruit
The fruit’s internal injury was reduced at 5 °C for 28 days. The fruit only developed flesh bruising and body rots and there were no significant differences between the injuries for all fruit (Table 4).
Discussion
The ASBVd infection had a dramatic effect on the phenology of the trees, quantity and quality of fruit. The impact of infection was related to the titre of viroid, as measured by RT-qPCR, the higher the viroid titre, the higher was the impact on the trees. Semancik and Szychowski (1994) described ASBVd as a chronic infection with the continuous and measured production of high titres of ASBVd-Sc throughout the host. Symptomless carrier trees are not visually distinguishable from the healthy avocado trees because they do not display any visible symptoms, however, infected trees are characterised by lower yields and increased seed transmission in other cultivars as described by Desjardins, (1987). ASBVd detection methods are limited by the uneven distribution of ASBVd in the trees that phenotypically display symptoms (Bruening et al., 1982; Running et al., 1996). However, previous studies on ASBVd-Sc infected trees have demonstrated an even distribution of ASBVd (Jooste & Zwane, 2021; Semancik & Szychowski, 1994).
We observed a clear difference between the numbers of flowers of infected and healthy trees. The trend of avocado trees producing excessive flowers to attract pollinators has been discussed (Ish-Am, 2005). Even though massive bearing was observed on the symptomless ‘Hass’ carrier trees in this experiment, the flowers were excessive for trees with medium and high infection levels compared with healthy and trees with low infection levels. The loss of canopy and excessive flowering, because of disease stress, has been associated with Phytophthora disease in avocado (Wolstenholme, 2001). However, the difference with ASBVd infection is that the infected trees recover their canopy completely; unlike with Phytophthora disease infection, where canopy recovery is rare and the tree would end up dying from severe infections (Marais, 2007). Given the above-mentioned observations on ASBVd infected symptomless carrier trees, it is possible that ASBVd-infected trees have developed a survival strategy where they first excessively bear flowers to ensure successful transmission. Subsequently, they save energy for survival to the following season by producing less fruit while, at the same time, recovering the lost leaf canopy as a disguise to appear healthy.
A study on the dry matter and oil content of ASBVd-infected asymptomatic and symptomatic fruit showed no significant differences in dry matter and oil content between both fruit types (Saucedo-Carabez et al., 2014). These findings agreed with the findings obtained in 2019 in this study between asymptomatic and healthy fruit. However, there was a slight difference in the maturity between fruit of infected and healthy trees in the 2020 results and it is suspected that fruit from infected trees would have a higher DM content than those of healthy trees, because the infected trees produce fewer fruit and therefore do not have to divide carbohydrate sources across as many carbohydrate sinks (the fruit). Da Graça (1980) conducted a similar study and found that the dry matter for fruit obtained from recovery growth was different to the other infected fruit (infected or healthy ‘Edranol’ fruit).
The reduced yield detected in asymptomatic trees was observed in several studies that evaluated yield losses associated with ASBVd infections worldwide (Da Graça, 1985; Running et al., 1996; Randles, 2003; Tondo et al., 2010; Saucedo-Carabez et al., 2014). In terms of yield reduction, Da Graça (1985) conducted a three-year trial testing the effect of ASBVd in ‘Fuerte’, both in symptomless carrier trees and symptomatic trees. Symptomatic trees showed a yield reduction of 14%, while the yield of symptomless carrier trees was reduced by 82% (Da Graça, 1985). Cultivar type appears to have no influence on ASBVd severity given that our study gave a similar result of approximately 90% fruit yield reduction from the infected symptomless carrier trees. Symptomless trees had extremely low yields compared to healthy trees and this yield reduction should be regarded as a symptom. Therefore, infected trees can be identified based on the number of fruit they produce. If the fruit yield is extremely low in a tree, the tree should be flagged and submitted for ASBVd indexing.
The higher the ASBVd concentration, the lower the yield produced for a specific tree. However, this was not detected for the trees with low infection levels; as these trees produced yields almost similar to healthy trees and, given the fact that these trees had no sign of ASBVd infection, it would be very difficult to convince a grower to remove good yielding trees that show no sign of infection from the orchard. It is uncertain whether trees with low infection levels will progress to having medium and high viroid titres within time. This research question is yet to be explored. These findings also emphasise the importance of indexing all propagation material.
The post-harvest effects of ASBVd infection were determined in this study and a clear difference were seen between fruit harvested from infected trees. Saucedo-Carabez et al. (2014) conducted a similar study in Mexico where the effect of ASBVd on the postharvest quality of avocado in both symptomatic and asymptomatic fruit were studied. They found that ASBVd affected firmness, weight loss and colour change in symptomatic fruit. Asymptomatic fruit was not affected by the variables evaluated in the Mexican study and developed similar conditions to that of fruit from healthy trees and fulfilled quality standards required by national and international markets. We confirmed that ASBVd affected the firmness and colour change of fruit from high and medium infected asymptomatic trees. The only difference detected was with fruit trees with a low infection level where firmness and colour change were similar to fruit from healthy trees. In this study, the ASBVd-infected symptomless ‘Hass’ trees were divided into different infection levels which was not done in previous studies.
The study confirmed that the presence of ASBVd contributes to yield reduction in symptomless carrier trees regardless of whether trees alternate between off and on-crop years.
Conclusions
Results clearly showed that trees with high and medium ASBVd infection levels had a lower yield than healthy trees. A few signs of infected trees that can enable their identification were observed. Firstly, ASBVd- infected symptomless carrier ‘Hass’ trees produced excessive flowers and shed leaves in the process. Therefore, the flowering stage of ‘Hass’ can be useful in identifying the symptomless carrier trees in the field, before trees recover their canopy. Secondly, infected ‘Hass’ trees also tend to remain in the flowering stage longer than healthy trees that are already at the fruiting stage at the same time interval. Lastly, infected ‘Hass’ trees produce very little to no fruit at all and by the end of the season, the trees will have recovered from the loss of canopy appearing greener and healthier just like healthy trees. These observations can be incorporated as part of ASBD management strategies in ‘Hass’ orchards and those of other cultivars. Postharvest, fruit from infected trees ripened faster than normal fruit and were more prone to fungal infections during the ripening process. Because infected trees do not display symptoms, regular systematic indexing of orchards, especially trees for bud-wood and seed sources, is required. All the field observations, yield and postharvest outcomes from this experiment are specific to the ‘Hass’ cultivar, and similar studies on other important cultivars will benefit the understanding of the relationship between ASBVd infection and yield losses.
Data availability
Available.
References
Acheampong, A. K., Akromah, R., Ofori, F. A., Takrama, J. F., & Zeidan, M. (2008). Is there avocado sunblotch viroid in Ghana? African Journal of Biotechnology, 7, 3540–3545. https://doi.org/10.5897/AJB08.122
Bruening, G., Gould, A. R., Murphy, P. J., & Symons, R. H. (1982). Oligomers of avocado sunblotch viroid are found in infected avocado leaves. Febs Letters, 148, 71–78. https://doi.org/10.1016/0014-5793(82)81245-3
Da Graça, J. V. and van Vuuren (2003). Viroids in Africa. In Hadidi, A., Flores, R., Randles, J. W. & Semancik, J. S (Eds) Viroids (1st ed., pp-291). Australia.
Da Graça, J. V. (1980). General studies on avocado sunblotch disease. In a study on avocado sunblotch disease. Thesis (Ph.D.)-University of Natal, Pietermaritzburg. http://hdl.handle.net/10413/11185
Da Graça, J. V. (1985). Sunblotch- associated reduction in fruit yield in both symptomatic and symptomless carrier trees. South African Growers’ Association Yearbook 8, 59-60. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.576.6211&rep=rep1&type=pdf
Desjardins, P.R., Drake, R.J., Atkins, E.L., & Bergh, B.O. (1979). Pollen transmission of avocado sunblotch virus experimentally demonstrated. California Agriculture 33, 14–15. http://www.avocadosource.com/Journals/CA/CA_1979_V33_N11_PG_14_15.pdf
Desjardins, P. R., Saski, P. J., & Drake, R. J. (1987). Chemical inactivation of avocado sunblotch viroid on pruning and propagation tools. California Avocado Society Yearbook, 71, 259–262. http://www.avocadosource.com/cas_yearbooks/cas_71_1987/cas_1987_pg_259-262.pdf
Dodds, J. A., Mathews, D., Arpaia, M. L. & Witney, G. W. (2001). Recognizing avocado sunblotch disease. Avoresearch. https://www.avocadosource.com/Journals/AvoResearch/avoresearch_01_03_2001_Dodds_Sunblotch.pdf
Howarth, M. S., Shmulevich, I., Raithatha, C., & Ioannides, Y. (2003). Online non-destructive avocado firmness assessment based on low-mass impact technique. Proceedings V World Avocado Congress (Actas V Congreso Mundial del Aguacate), 679–685. https://www.researchgate.net/publication/229048318_Online_non-destructive_avocado_firmness_assessment_based_on_low-mass_impact_technique
Ish-Am, G. (2005). Avocado pollination - A review. New Zealand and Australia Growers’ Conference ’05, 1–9. https://www.researchgate.net/publication/239534954_AVOCADO_POLLINATION_-_A_REVIEW
Jooste, A.E.C., & Zwane, Z.R. (2021). Avocado sunblotch viroid (ASBVd) symptom identification and detection in avocado orchards, South African Avocado Growers’ Association Yearbook 44, 66–71. http://avocadosource.com/Journals/SAAGA/SAAGA_2021/SAAGA_2021_44_PG_66_6.pdf
Kruger, F. J., & Claassens, N. J. F. (2001). Packhouse procedures. In De Villiers, E. A (Eds.), The cultivation of avocado (1st ed., pp 319–330). South Africa.
Kuhn, D. N., Geering, A. D.W., Dixon, J. (2017). ‘Avocado Sunblotch Viroid’. In Hadidi, A., Flores, R., Randles, J. W and Palukaitis P (Eds.) Viroids and Satellites (1st ed., pp. 297–305). London.
Luttig, M. and Manicom, B.Q., (1999). Application of a highly sensitive avocado sunblotch viroid indexing method. South African Avocado Growers' Association Yearbook 22, 55–60. https://www.avocadosource.com/Journals/SAAGA/SAAGA_1999/SAAGA_1999_PG_055-060.pdf
Marais, L. (2007). Avocado Diseases of Major Importance Worldwide and their Management. In. Naqvi S. A. M. H (Eds), Diseases of fruits and vegetables (1st ed., pp. 1–36). Netherlands.
Palukaitis, P., Hatta, T., Alexander, D., & Symons, R. (1979). Characterization of a viroid associated with avocado sunblotch disease. Virology, 99, 145–151. https://doi.org/10.1016/0042-6822(79)90045-x
Randles, J. W. (2003). Economic impact of Viroid diseases. In Hadidi, A., Flores, R., Randles, J. W., & Semancik, J. S (Eds) Viroids (1st ed., pp-3). Australia.
Running, C. M., Schnell, R. J., & Kuhn, D. N. (1996). Detection of avocado sunblotch viroid and estimation of infection among accessions in the national germplasm collection for avocado. Proceedings of the Florida State Horticultural Society, 109, 235–237. https://doi.org/10.3390/v11060512
Saucedo Carabez, J. R., Téliz Ortiz, D., Vallejo Pérez, M. R., & Beltrán Peña, H. (2019). The avocado Sunblotch viroid, an invisible foe of avocado. Viruses, 11, 1–12. https://doi.org/10.3390/v11060491
Saucedo-Carabez, J. R., Teliz-Ortiz, D., Ochoa-Ascencio, S., Ochoa-Martinez, D., Vallejo-Perez, M. R., & Beltran-Pena, H. (2014). Effect of avocado sunblotch viroid (ASBVd) on avocado yield in Michoacan, Mexico. European Journal of Plant Pathology, 138, 799–805. https://doi.org/10.1007/s10658-013-0354-9
Schnell, R. J., Kuhn, D. N., Ronning, C. M., & Harkins, D. (1997). Application of RT-PCR for indexing avocado sunblotch viroid. Plant Disease, 81, 1023–1026. https://doi.org/10.1094/PDIS.1997.81.9.1023
Semancik, J. S., & Szychowski, J. A. (1994). Avocado sunblotch disease: A persistent viroid infection in which variants are associated with differential symptoms. Journal of General Virology, 75, 1543–1549. https://doi.org/10.1099/0022-1317-75-7-1543
Tondo, C. L., Schnell, R. J., & Kuhn, D. N. (2010). Results of the 2009 ASBVd Survey of Avocado Accessions of the National Germplasm Collection in Florida. Proceedings of the. Florida. State Horticultural. Society 123, 5–7. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Results+of+the+2009+ASBVd+Survey+of+Avocado+Accessions+of+the+National+Germplasm+Collection+in+Florida&btnG=
Wallace, J. M. (1958). The Sun-Blotch Disease of Avocado. Proceedings of the Rio Grande Valley Horticultural Society 12:69–74. http://www.avocadosource.com/Journals/RioGrande/rio%20grande%20proc_1958_12_pg_69-74.pdf
White, A., Woolf, A., Hofman, P., & Lu Arpaia, M. (2009). “The international avocado manual”. HortResearch, 2005. ISBN 0478068379,9780478068375
Wolstenholme, N. B. (2001). Understanding the avocado tree-Introductory ecophysiology. In De Villiers E. A (Ed). The cultivation of avocado (1st ed., pp 56 and 57). South Africa.
Woolf, A., Clark, C., Terander, E., Phetsomphou, V., Hofshi, R., Lu Arpaia, M., Boreham, D., Wong, M., & White, A. (2003). Measuring avocado maturity; ongoing developments. The orchardist, 40–45. http://www.avocadosource.com/Journals/Orchardist/woolfallan2003b.pdf
Acknowledgements
We further acknowledge the avocado grower in Mbombela for allowing access to avocado orchards and the provision of fruit used in this study.
Funding
Open access funding provided by Agricultural Research Council. Research funding was received from the South African Avocado Growers’ Association (SAAGA) and the Agricultural Research Council-Professional Development Programme (ARC-PDP) (PhD student, Z.R. Zwane). Drs. Akhona Mbatyoti, Grace Tefu, Fumani Kingsley and Elliosha Hajari are thanked for assistance with statistical analyses, postharvest work and critical evaluation of the manuscript.
Author information
Authors and Affiliations
Contributions
ZR Zwane: Conceptualization, Methodology, Formal Analysis, Writing Original Draft, Visualization.
A Gubba: Writing- Review and editing, Supervision.
AEC Jooste: Conceptualization, Resources, Writing- Review and Editing, Supervision, Project Administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zwane, Z.R., Gubba, A. & Jooste, A.E.C. The effect of avocado sunblotch disease (ASBD) on tree morphology, fruit maturity, yield and quality of ‘Hass’ avocado in South Africa. Eur J Plant Pathol 167, 287–299 (2023). https://doi.org/10.1007/s10658-023-02705-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02705-z