Abstract
Citrus black spot (CBS) caused by the fungus Phyllosticta citricarpa occurs in tropical and sub-tropical citrus production regions and affects all varieties of citrus. In Florida, the disease cycle is unique, having only the asexual spore. This work examines incidence and severity of CBS (hard spot symptoms) on fruit in two citrus groves during 2013–2014, 2014–2015 (Grove III) and 2015–2016 (Grove II) citrus seasons. Disease incidence and severity on fruit were analyzed based on citrus season, side of tree evaluated, height within the canopy, tree health, and tree age. Results indicate an increase in CBS incidence in Grove III between 2013–2014 and 2014–2015 seasons, with more infected or symptomatic fruit on the road side of the canopy and a higher incidence above 2 meters. Tree health status affected incidence but not severity and tree age had a significant effect on severity of CBS in Grove II. Analysis of weather data conducive for infection, between 2010 and 2017, indicated an average of 172 days per year (range: 104–261 days) when the temperature (15–35 °C) and relative humidity (RH ≥ 90% for 8 consecutive hours) were conducive for infection of fruit and an average of 98 days per year (range: 72–123 days) when the fruit were susceptible to infection.
Similar content being viewed by others
Introduction
Citrus black spot caused by the fungus Phyllosticta citricarpa was first discovered on sweet orange in southwest Florida in April 20101. The disease has a worldwide distribution affecting all varieties of citrus within tropical and sub-tropical citrus production regions, particularly in warm, humid climates. The disease has been reported in Australia, South Africa2, and Argentina3,4 since the early 1900’s. It has more recently been introduced into Brazil, Cuba5, Uganda6, Ghana7, Italy, Malta, and Portugal8. The fungus invades and colonizes the fruit rind producing five distinct lesion types9,10. Lesions do not affect the internal quality of the fruit10, however in markets where fruit is quarantined it is precluded from sale in the fresh market, as opposed to endemic areas where less blemished fruit are sold. Fruit symptoms include hard spots (also known as shot hole spots), freckle spots, virulent spots, and false melanoses (see Supplementary Fig. S1). Interaction with spider mites produces cracked spots which have been observed in Brazil11 and Florida1. Yield losses due to premature fruit drop occurs under severe infections7. Florida’s citrus affected by this pathogen are placed under quarantine by the United States Department of Agriculture, restricting interstate movement of citrus materials. This places an additional economic cost due to the implementation of further phytosanitary practices in order to treat and move material within and outside of the state and country. Since the initial find in 2010, the quarantine zone has been extended to encompass 1160.32 km2 (286,720 acres) within Lee, Charlotte, Collier, Hendry and Polk counties as of July 201912.
The life cycle of P. citricarpa consists of a sexual (ascospores) and asexual (conidia) phase. The fungus forms latent infection in citrus tissues. Leaves produce ascospores within 40 to 180 days of leaf fall2,10 depending on environmental conditions. Leaves, twigs, fruit, and thorns produce conidia. Rainfall, irrigation, or heavy dew is sufficient to trigger the release of spores - ascospores from mature asci are ejected into the air and dispersed by wind currents, constituting long distance travel, and gelatinous masses of conidia are splashed dispersed over shorter distances. Fruit are susceptible to infection for up to 6 months (24 weeks) after petal fall13 and leaves up to 10 months from flush14,15. Twig and thorn susceptibility windows have yet to be determined. Conidia have been considered less important than the ascospore in disease spread due to shorter dispersal distance by splash and reduced viability compared to the ascospore, however, recent work suggest that water and wind may disperse conidia over longer distances than previously reported16.
Both the sexual and asexual spores of CBS are present on citrus17 with the exception of Florida. In Florida, only one mating type has been found17,18 and genomic analysis using 15 simple sequence repeat markers for P. citricarpa revealed a clonal population17. In Brazil, the conidia have been shown to be a major inoculum source in infected groves19. In Florida, the conidia appear to be the sole spore type spreading the disease. Florida isolates produce conidia in vitro between 15 and 27 °C, with time to emergence between 24 to 14 days20. Brazilian isolates of P. citricarpa germinate and form appresoria at temperatures between 10 and 40 °C when exposed to moisture for 48 hours21. Following storage of citrus fruit at 4.5 and 10 °C, conidia can remain viable22 and approximately 40% percent will remain viable at 25 °C for up to 4 days, but are 100% non-viable at 30 days9.
The majority of the literature attributes the incidence and severity of CBS on fruit to a combination of ascospore and conidia inocula. The situation in Florida gives a unique opportunity to study the incidence and severity of CBS hard spot lesions in a system lacking ascospores. This work examines the incidence and severity of CBS on fruit spatially distributed within the tree canopy over a 3-year period (2013/14 to 2015/16) in one grove with 100% tree incidence of CBS at the beginning of the study and for a single citrus season (2015/16) in a second grove with 26.6% tree incidence.
Results
Weather and susceptibility
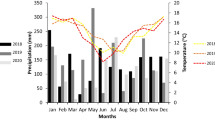
The number of days for fruit susceptibility with appropriate weather conditions for infection by conidia of P. citricarpa is given in Table 1. Defining fruit susceptibility as days with temperatures between 15 and 30 °C and a total daily rainfall ≥0.25 mm, with fruit on the trees within the first 24 weeks post fruit set (wpfs), IN3 (IN = potential infection period) gave consistently higher number of susceptible days than IN1, IN2, IN4 or IN5, with the exception of 2013, when there were more days with RH ≥ 90% (Table 1). Since the buildup of inoculum in the grove is an important factor to consider, we looked at the weather conditions conducive for conidial infection across the entire year, defining IN6 to IN10 as weather conditions conducive to infection of citrus tissue other than the fruit. Overall there were more days throughout the years when conditions were conducive for the infection of citrus tissue defined as days with temperatures between 15 and 30 °C and RH ≥ 90% in all years examined, especially in 2013 where 261 days out of the year were conducive to tissue infection based on this definition (Table 1).
Effect of citrus season and fruit location (height and side) within the canopy on CBS incidence and severity in Grove III
Data on incidence and severity of hard spots on fruits are given in Table 2 and in Supplementary Table S1. Disease incidence was significantly different between citrus seasons (LSM ± SEM – 49.13 ± 2.92% in 2013–2014 vs. 67.96 ± 2.86% in 2014–2015 citrus season; P < 0.0001). Additionally, the overall incidence of CBS on fruit was significantly greater on the road side than the swale side of the tree, 61.49 ± 2.13% vs. 56.20 ± 2.44%, respectively (LSM ± SEM; Table 3), and there was a significant interaction between citrus season and the height within the canopy (P = 0.0041; Table 3). This interaction was due on average to a higher incidence of disease in the 2014–2015 citrus season than in the previous season, 2013–2014 season, while the average incidence of disease at the >3 m height was about the same between each season (Table 2, Fig. 1). Simple effect comparisons of the least square means of the interaction between citrus season and height within the canopy by citrus season revealed a statistical difference in disease incidence between <1 m vs. >3 m (43.81 ± 3.69% vs. 59.09 ± 4.90%, Adjusted P = 0.0129), 1–2 m vs. >3 m (46.49 ± 2.98% vs. 59.09 ± 4.90%, Adjusted P = 0.0408) but not 2–3 m vs. >3 m (47.09 ± 3.05% vs. 59.09 ± 4.90%, Adjusted P = 0.0590) during the 2013–2014 season. Similarly, no statistical difference (P > 0.5) was found in the different heights within the canopy during the 2014–2015 season.
Incidence of Citrus Black Spot on fruit in Grove III for the 2013–2014 and 2014–2015 citrus season. (A) Citrus season and height of citrus black spot infected fruit within the canopy of ‘Valencia’ orange trees. (B) Least square means for the interaction between citrus season and height of citrus black spot infected fruit within the canopy with the 95% confidence interval.
Disease severity for Grove III was 3.32 ± 0.22% and 4.06 ± 0.20% for the 2013–2014 and 2014–2015 citrus seasons, respectively (Table 2). A statistically significant interaction between citrus season and location of fruit (road versus swale side of the tree) was found (Table 3). Multiple comparisons of the interaction revealed significant differences between the severity of fruit symptoms on the road vs. the swale side of the canopy during the 2013–2014 season (Adjusted P = 0.0151); and between the swale side of the canopy in the 2013–2014 season vs. (i) the road side of the canopy (Adjusted P = 0.0159) and (ii) the swale side of the canopy (Adjusted P = 0.0247) during the 2014–2015 season.
Effect of tree health on CBS incidence and severity in Grove III during the 2014–2015 citrus season
A total of 54 trees were scored as healthy or declining based on tree canopy characteristics during the 2014–2015 citrus season. Thirty-nine were classified as declining and 15 as healthy (Table 4). Health status significantly impacted disease incidence (LSM ± SEM – 72.99 ± 3.25% vs. 59.86 ± 4.52%; P = 0.0202) but not disease severity, the percentage of fruit area covered by symptoms, (P = 0.1884; Table 5). CBS severity was not affected by health status or the side of the tree evaluated (Table 5).
Disease incidence, severity and age
In Grove III a total of 598 fruit were evaluated with an average incidence of 59.03% (range: 0–100%) exhibiting hard spot symptoms within individual tree canopies. The average CBS Severity Index was 31.43% (Range 0–68.06%). In Grove II a total of 4036 fruit were evaluated with an average incidence of 13.68% (Range 0–100%) in the trees surveyed and an average CBS severity index of 4.47% (Range 0–75.0%). Incidence, severity, and CBS Severity Index of hard spot on fruits and the number of fruit in each Grade category for Grove II and III is given in Table 6.
Effect of grove and canopy side on CBS incidence and severity index during the 2015–2016 citrus season
The incidence and severity (square-root CBS Severity Index) of fruits with hard spot symptoms within a 1 m2 area of the canopy for Grove II and Grove III during the 2015–2016 citrus seasons were significantly different (P < 0.0001). There was no effect of side of the tree evaluated on incidence (P = 0.2223) or severity (P = 0.3874) and no interaction between the main effects on incidence (P = 0.6004) or severity (P = 0.6127).
Effect of tree age and canopy side on CBS incidence and severity index during the 2015–2016 citrus season in Grove II
Of a total of 89 trees evaluated in Grove II, 13 were classified as reset trees that were fruiting for the first time and 76 as mature trees (>4 years) and fruiting during the 2014–2015 citrus season. A significant effect of age (reset vs. mature tree) on disease and severity (P = 0.0066) and a tendency for age to have an effect on incidence (P = 0.0834) was found. However, the interaction between age and side was not significant for either disease incidence or severity (Table 7, Fig. 2). Fruits on resets had a lower incidence and severity of hard spots than fruits in mature trees.
Incidence of Citrus Black Spot on fruit in Grove II and Grove III for the 2015–2016 citrus season. (A–D) Disease incidence as a percentage of fruits exhibiting hard spots symptoms on either the road or swale side of the tree and graded on a scale 0 to 3 based on severity of the disease on the fruit (Rating 0 = no hard spots; Rating 1 = 1–5 hard spots; Rating 2 = 5–50 hard spots and Grade 3 ≥ 50 hard spots). Disease incidence on fruits in newly fruiting resets (C) and in mature citrus trees (D).
Discussion
This is the first study in Florida that examines the disease incidence and severity of CBS on fruit in affected groves since the disease became established in 2010. The incidence and severity of the hard spot lesions on the exposed surface of the fruit within the tree canopy was evaluated in a single grove over a 3-year period and in a second grove during a single citrus season. Grove III had a 100% tree incidence of CBS at the onset of the study and showed a significant increase in incidence of fruits with hard spot lesions from the 2013–2014 to the 2014–2015 citrus seasons. This increase may have been due to the build-up of inoculum in the grove with the high tree incidence of CBS and the conducive weather conditions in 2013 for conidia production and dispersal. In 2013, there were 261 days with temperatures between 15 and 35 °C and RH > 90% for 8 consecutive hours which impacted the 2014–2015 incidence and severity of hard spot lesions on fruit.
It has been reported that hard spot lesions of CBS usually develop on the surface of the fruit exposed to sunlight23 and that symptom development is strongly influenced by light24. More recent work has shown that hard spot lesions are typically expressed close to color break25, a potential effect of ripening that triggers latent infections to develop26. In this study, the groves were oriented in a north-south direction, allowing for equal exposure of both the east and west sides of the tree to direct sunlight. If exposure to sunlight is fairly equal on both sides of the tree, one would expect equal expression of the disease on fruit on the road and swale side, however this was not the case. More fruits with lesions were seen on the road side than on the swale side of the tree. Additionally, the severity of the disease was also more pronounced on the fruit found on the road side of the tree during the 2013–2014 citrus season. The ascospore has not been found in Florida to date18 and genetic studies also supported this17,18, which removes the aspect of wind driven sexual spores from the disease cycle in Florida. This is in contrast to other citrus producing areas where Phyllosticta citricarpa is an endemic pathogen on citrus such as in Taiwan27, South Africa2,10, Australia9, and Brazil28. More recently, clonal populations of P. citricarpa were found in Italy, Malta, and Portugal8. The increase in incidence and severity of CBS seen on the road side of trees suggests increased exposure to the conidial inoculum. This could be due to movement of inoculum on vehicle and/or personnel either by direct contact with the spores or as a consequence of the movement of infected plant material.
In the absence of wind-driven ascospores, we investigated the effect of height at which fruits with hard spot lesions were found as a means of assessing conidial dispersal within the tree. In Grove III, there was an interaction on disease incidence between height and season during the 2013–2014 season which was not found in the 2014–2015 season. This could indicate progression of the disease between seasons, with irrigation and rainfall events (with and without high wind) propelling the conidial inoculum upwards and outwards throughout the canopy and downwards due to drip. Weather data conducive to spread of the disease within the canopy, defined as temperature between 15 and 35 °C with relative humidity (RH) ≥ 90% for 8 consecutive hours, were 261 days in 2013. If one considered the presence of susceptible citrus fruit, this was reduced to 160 days. In either scenario, it is conceivable that spread of inoculum within the canopy was possible leading to the progression of the disease from 55% to 68% incidence and the reduction in severity differences between swale and road side of the tree from the 2013–2014 to 2014–2015 citrus seasons (road: 3.7% vs. swale: 2.8% in 2013–2014 to 4.1% vs. 4.0% in 2014–2015 citrus season). The increase in severity on fruit on the swale side of the tree to equal that of the road side of the tree may indicate disease progression from road to swale and indicate the direction from which the inoculum spread. Indeed, it has been shown that wind driven splash droplets can propel conidia upwards and outwards from the point of origin and this distance increases under the influence of wind16. Clearly the ascospore is not required for the spread of the disease into the topmost sections of the canopy (>3 m).
An important distinction in the disease cycle in Florida is the absence of the ascospore. The severity on symptomatic fruit was more or less uniformly distributed throughout the canopy during the 2014–2015 citrus season. If the dripping and splashing of conidia in dew, rain, or irrigation water is the sole source of spread of the disease, we would expect that the severity of symptoms on low hanging fruit would be significantly greater than that of fruit higher in the canopy, but this was not the case as equal (2014–2015) or higher (2013–2014) incidence was found in the topmost height of the trees examined. This information, in addition to the lack of the ascospore, paints Kiely9 and McOnie (1965) findings in a different light. McOnie concluded that conidia originating from leaf litter was not relevant to the severity of the disease, based on the relative uniform vertical distribution of symptomatic fruits and the observation that fruit located within the splash zone of these leaves did not show an increase in severity. Perhaps in their case the relative uniform vertical distribution of symptomatic fruits was either due to the stage of the disease establishment within the examined groves or a function of both the conidia and ascospore. The current research on the severity and incidence of the disease indicates the significant role of the conidia in disease establishment and spread, confirming work conducted in Brazil19,28.
It is intuitive to expect that a healthy plant would perform better than a diseased plant against a pathogen. We examined the impact of other citrus diseases and the general health of citrus trees on the outcome of CBS. Florida citrus has been dealing with the impact of citrus greening (huanglongbing, HLB) since its introduction in 200529,30,31 and other citrus pathogens such as citrus canker, Xanthomonas citri32, which together have caused devastating losses to the citrus industry32,33. The study of the interaction between these diseases and CBS was not feasible during this study. All trees within this study demonstrated foliar symptoms of HLB, which did not allow for a comparison of trees with and without HLB. Additionally, the incidence and severity of citrus canker was very low in the tree studies (data not shown). However, the overall health of the tree was assessed and classified as either healthy or declining. Purely from the point of symptom expression, declining trees produce less fruit30 and thus have less chance for the expression of fruit symptoms on CBS affected trees. Despite this, the data bears out this hypothesis with respect to the development of hard spots on citrus fruit. There was a significant effect on the incidence of CBS: declining citrus trees had a higher incidence of CBS than trees defined as healthy. No effect of tree health on the severity of the disease was found.
In a review of the epidemiology of CBS in South Africa from 1946 to the late 1970s, it was observed that tree condition and age influenced the development of CBS, with older trees being more severely affected than younger trees10. This was true in Grove II where reset trees fruiting for the first time had a lower incidence of fruits with hard spot symptoms and lower severity of the disease. A plausible explanation for this is that older trees have been (i) exposed to CBS inoculum for a longer period of time and/or (ii) have more dead or dying canopy material capable of producing inoculum (conidia). Research in Brazil has shown that green twigs colonized by P. citricarpa in the grove, once damaged and senescence occurs, produce viable conidial inoculum within 45 days34. This implies than any activity that leads to senescence of infected tissue, whether physiological or pathological, can lead to the release of viable inoculum within 45 days, provided that the climatic conditions are conducive. If temperature (15–35 °C) and moisture (RH ≥ 90% or rainfall >0.25 mm) conditions in Florida are conducive for tree tissue infection at least one-third of the year, the disease will continue to spread throughout infected groves into clean citrus areas and continually pose a problem on citrus fruit. A more comprehensive look at the reduction of the inoculum year-round is required to control the disease. A steady buildup of inoculum within the canopy of the citrus tree will continue to occur in Florida if steps are not taken to manage this disease year-round.
Fungicide active ingredients currently recommended and labeled for the control of CBS in Florida are copper, strobilurins, fenbuconazole, and premix combinations, such as azoxystrobin/difenoconazole and pyraclostrobin/boscalid. Recommendations entail the application of these fungicides on a 21–28 day cycle from early May to mid-September35 depending on the citrus variety, spanning approximately 130 days. Recent research suggests that extending the application time to protect the fruit to 180 or 220 days36 would improve control of the disease, reducing incidence and severity of CBS symptoms by up to 96% and crop loss due to premature fruit drop by up to 77%. Extending the fungicide application period would better control the continuous conidia inoculum within the canopy and secondary inoculum on fruits in Florida and potentially slow the spread of the disease while other modes of control are developed.
Methods
Data collection
For the purpose of this study, disease incidence is defined as the number of CBS positive fruit (hard spot symptoms)/total number of fruits examined, and disease severity is defined as the percentage of fruit surface covered by hard spot lesions. The disease incidence and severity of CBS was examined over a 3-year period (2013 to 2016) in three commercial citrus groves in Florida. The groves are organized with irrigation ditches (swales) and roads (drivable surface) alternating between rows of citrus (row-swale-row-road-row-swale), and citrus rows were oriented in roughly a north-south direction in each grove. The cultivar-rootstock combination was ‘Valencia’ grafted onto Swingle, planted on average 3.36 m (Grove II) and 3.33 m (Grove III) apart within rows and 6.70 m between rows. Tree age varied from resets (1.5–2 years, with or without fruit) to mature trees (>4 years and fruiting). Initial plot maps were prepared and findings on the spatial and temporal distribution of CBS on fruit in Grove II and another grove not discussed in this paper (Grove I) has been previously published37. The data on the incidence and severity of CBS on fruit in the canopy was taken from Grove II and III.
The study area in Grove II consisted of 37 rows of citrus containing 124 to 127 citrus trees per row. During the 2015–2016 citrus season, there were 4339 trees with 167 open spaces (no tree) and 26.57% (1153/4339) were classified as positive for CBS. The study area in Grove III was comprised of 27 rows of citrus containing 98 to 111 citrus trees per row. In 2013–2014 citrus season, there were 2849 tree spaces, with 1659 trees and 1190 open spaces and 100% were positive for CBS.
Weather data
The Florida Automated Weather Network’s (FAWN) Immokalee station, located at 26°27′43.5″N 81°26′25.9″W is approximately 11.08 km SSE from Grove II and 25.92 km WNW from Grove III and was used to gather 15 minute data on soil temperature, air temperature at 0.6, 2 and 10 m, relative humidity (RH) at 2 m (%), dew point (DP) at 2 m, rainfall, wind speed at 10 m and solar radiation between 1 Jan 2010 and 15 Jun 2017. This station was chosen due to its close proximity to the groves and availability of archived data. A 24-hr period, rainy day, and wet canopy were defined as previously described37. Briefly, a 24-hr period started at 12:00 am and ended at 11:59 pm; a rainy day had a total daily rainfall (TDR) greater than or equal to 0.25 mm; and a wet canopy was a day in which RH was greater than or equal to 90% for at least 8 consecutive hours38. A secondary definition of a wet canopy was defined as a day when the dew point, DP, the temperature at which water vapor in the air condenses, was greater than the temperature (T) at 0.6 m. Data used to estimate susceptibility period of fruit within the canopy to CBS were generated as described in Hendricks et al.37, however fruit susceptibility was calculated as occurring in the first 168 days (24 weeks) following fruit set (wpfs) according to work done by Baldassari et al.13 on ‘Valencia’ and ‘Natal’ orange varieties. To date there is no evidence to suggest that the sexual spore (ascospore) is present in Florida17,18,39, hence only published literature values relevant to the conidia were used to determine susceptibility of tree tissues, including fruit. Conservatively, the temperature range selected was 15 to 35 °C, allowing for the production and germination of conidia. Susceptibility of tree tissues was considered throughout the entire year and defined as periods in which temperature and moisture were sufficient for infection based on literature values for the conidia. For T, RH and DP, a minimum period of 8 consecutive hours was required to be included in the data used to develop the ten categories for potential infection period (IN). These categories were designated as IN1 to IN5 for fruit and IN6 to IN10 for other citrus tissue. These were defined as:
FRUIT SUSCEPTABILITY (IN1 to IN5)
TREE TISSUE SUSCEPTABILITY (IN6 to IN10)
If there was a 24-hr period in which the conditions for infection were met, that day counted towards the susceptibility data.
2013–2015 disease incidence and severity of citrus black spot on fruit
During the 2013–2014 and 2014–2015 citrus seasons, two trees from each row of Grove III, totaling 54 trees, were randomly chosen for the assessment of CBS incidence and severity on fruit. The road and swale side of a tree was assessed at four heights (<1 m, 1–2 m, 2–3 m and >3 m), and fruits were evaluated within a 1 m2 section at each height. Severity assessments were based on a rating system devised by Sposito et al.40; ratings ranged from 0.5% to 49% hard spot coverage on the visible surface of each fruit28,40. The mean severity was based on an average of all the positive fruits evaluated within a 1 m2 section of the canopy. For example, if there were 20 fruits in a 1 m2 sample area and 5 were positive, each with a severity rating of 1.5%, the mean severity rating at that height was 1.5%. Additionally, disease severity and incidence for the entire tree (combining road and swale side) was calculated from this data. During 2014–2015, trees examined were classified as either healthy or declining. Citrus were defined as declining if they exhibited a combination of the following canopy features: sectored within the canopy or as a whole, thinning; reduced growth flushes, die back, reduced fruiting and/or vegetative sprouts on the trunk or on larger interior branches.
CBS severity index
During the 2015–2016 citrus season, a severity rating for Grove III was done on fruit from 20 randomly selected trees, two from each row. Additionally, 89 randomly selected CBS positive trees in Grove II were evaluated for CBS severity. Fruit within a single 1 m2 sampling area taken on the swale and road sides of the tree were graded based on the number of lesions on the fruit using a 0 to 3 grading system. Fruits with no lesions were given a grade of 0; fruits with 1–5 hard spots were given a grade of 1; fruits with 6–50 spots were given a grade of 2 and grade 3 was reserved for fruits having greater than 50 spots.
The CBS severity index was calculated based on the following equation:
where n0, n1, n2 and n3 represent the number of fruits graded 0, 1, 2 and 3 respectively and ntotal the total number of fruits evaluated.
Statistical analysis
Effect of citrus season and fruit location (height and side) within the canopy on CBS incidence and severity in Grove III
The data on disease incidence were fit to a generalized linear mixed model assuming a binomial distribution with a repeated measures effect of tree based on compound symmetry. Disease incidence was modelled as a function of the citrus season (2013–2014 and 2014–2015), side of the tree evaluated (swale or road), height within the canopy at which diseased fruit was found (<1, 1–2, 2–3, <3 m) and the interactions between these factors. Non-significant effects were removed hierarchically to obtain the final model of citrus season, side of the tree evaluated, height within the canopy, and the interaction between citrus season and height.
Mean severity was calculated for each side of the tree by averaging the observed percentage for the four heights within the canopy. These data were transformed by square-root to obtain approximate normality of the residuals. Square-root mean severity was modelled as a function of the fixed effects of citrus season (2013–2014 and 2014–2015), side of the tree evaluated (swale or road), and the interactions between these factors and a random effect for the repeated measures on trees.
Effect of health on CBS incidence and severity in Grove III during the 2014–2015 citrus season
The data on disease incidence were fit to a generalized linear mixed model assuming a binomial distribution with a repeated measures effect of tree using compound symmetry. Disease incidence was modelled as a function of the health, side of the tree evaluated (swale or road), height within the canopy at which diseased fruit was found (<1, 1–2, 2–3, >3 m) and the interactions between these factors. Non-significant effects were removed hierarchically to obtain the final model of health, side of the tree evaluated, and height within the canopy.
Mean severity was calculated as described above and transformed by square-root to obtain approximate normality of the residuals. Square-root mean severity was modelled as a function of the fixed effects of health, side of the tree evaluated (swale or road), and the interactions between these factors and a random effect for the repeated measures on trees.
Effect of grove and tree side on CBS incidence and severity index during the 2015–2016 citrus season
Incidence of CBS affected fruit within 1 m2 area of the canopy was calculated for each side of the tree based on grading data (Incidence = (Grade 1 + Grade 2 + Grade 3)/(Grade 0 + Grade 1 + Grade 2 + Grade 3)). Incidence was fit to a generalized linear mixed model assuming a binomial distribution and modelled as a function of the fixed effect of grove, side of the tree evaluated (swale or road) and their interaction and the random effect of tree.
Disease severity was assessed based on a CBS severity index adapted by Truter (2010)41 from previous work by De Wet (1987). These data were transformed by square-root to obtain approximate normality of the residuals. Square-root CBS Severity Index was fit to a generalized linear mixed model with a repeated measures effect of tree assuming a normal distribution and modelled as a function of grove, side of the tree evaluated (swale or road) and the interaction.
Effect of tree age on CBS incidence and severity index during the 2015–2016 citrus season in Grove II
A subset of data from the 2015–2016 season taken solely from Grove II containing tree age was used to model the effect of age on the incidence of CBS affected fruit and on CBS Severity Index. Disease incidence (Incidence = (Grade 1 + Grade 2 + Grade 3)/(Grade 0 + Grade 1 + Grade 2 + Grade 3)) were fit to a generalized linear mixed model assuming a binomial distribution and modelled as a function of age, side of the tree evaluated (swale or road) and their interactions.
As above, CBS severity index data were transformed by square-root to obtain approximate normality of the residuals and the data were fit to a generalized linear mixed model. Square-root CBS Severity Index was modelled as a function of age, side of the tree evaluated (swale or road) and their interactions. Square-root CBS Severity Index was modelled as a normal distribution random variable.
Values of P ≤ 0.05 was considered statistically significant for all models. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schubert, T. S. et al. First report of Guignardia citricarpa associated with citrus black spot on sweet orange (Citrus sinensis) in North America. Plant Dis. 96, 1225–1225, https://doi.org/10.1094/Pdis-01-12-0101-Pdn (2012).
Kotze, J. M. Studies on the black spot disease of citrus caused by Guignardia citricarpa Kiely, with particular reference to its epiphytology and control at Letaba. Thesis, 148 (1963).
Duran, E. L. et al. The foliar fungal endophytes of Citrus limon in Argentina. Canadian Journal of Botany-Revue Canadienne De Botanique 83, 350–355, https://doi.org/10.1139/b05-009 (2005).
EPPO/CABI. EPPO Reporting Service no. 07-1998 Guignardia citricarpa is present in Argentina, https://gd.eppo.int/reporting/article-3632 (1998).
Hidalgo Góngora, E. I. & Pérez Vicente, L. Diferenciación morfológica, cultural y biológica de Guignardia citricarpa y Guignardia mangiferae en frutos cítricos de Cuba. Fitosanidad 14, 141–152 (2010).
Reeder, R., Kelly, P. L. & Harling, R. First confirmed report of citrus black spot caused by Guignardia citricarpa on sweet oranges (Citrus sinensis) in Uganda. Plant Pathol 58, 399–399, https://doi.org/10.1111/j.1365-3059.2008.01966.x (2009).
Brentu, F. C. et al. Crop loss, aetiology, and epidemiology of citrus black spot in Ghana. Eur. J. Plant Pathol. 133, 657–670, https://doi.org/10.1007/s10658-012-9944-1 (2012).
Guarnaccia, V. et al. First report of Phyllosticta citricarpa and description of two new species, P. paracapitalensis and P. paracitricarpa, from citrus in Europe. Stud. Mycol., 161–185, https://doi.org/10.1016/j.simyco.2017.05.003 (2017).
Kiely, T. B. Preliminary studies on Guignardia citricarpa, n sp: the ascigerous stage of Phoma citricarpa McAlp and its relation to black spot of Citrus. Proc. Linn. Soc. N. S. W. 73, 249–292 (1948).
Kotze, J. M. Epidemiology and control of citrus black spot in South Africa. Plant Dis. 65, 945–950, https://doi.org/10.1094/PD-65-945 (1981).
De Goes, A., Baldassari, R. B., Feichtenberger, E., Spósito, M. B. & Aguilar-Vildoso, C. I. In 9th International Citrus Congress of the International Society of Citriculture, University of Florida: Orlando, FL. 145.
United States Department of Agriculture - Animal and Plant Health Inspection Service, Citrus Black Spot, https://www.aphis.usda.gov/plant_health/plant_pest_info/citrus/downloads/black_spot/DA-2019-16.pdf (2017).
Baldassari, R. B., Reis, R. F., Goes, Ad & de Goes, A. Susceptibility of fruits of the ‘Valencia’ and ‘Natal’ sweet orange varieties to Guignardia citricarpa and the influence of the coexistence of healthy and symptomatic fruits. Fitopatol. Bras. 31, 337–341, https://doi.org/10.1590/S0100-41582006000400002 (2006).
Truter, M., Kotze, J. M., van Rensberg, T. N. J. & Korsten, L. A sampler to determine available Guignardia citricarpa inoculum on citrus leaf litter. Biosyst. Eng. 89, 515–519, https://doi.org/10.1016/j.biosystemseng.2004.08.018 (2004).
Truter, M., Labuschagne, P. M., Kotzé, J. M., Meyer, L. & Korsten, L. Failure of Phyllosticta citricarpa pycnidiospores to infect Eureka lemon leaf litter. Australas. Plant Pathol. 36, 87–93, https://doi.org/10.1071/ap06087 (2007).
Perryman, S. A. M., Clark, S. J. & West, J. S. Splash dispersal of Phyllosticta citricarpa conidia from infected citrus fruit. Sci Rep-Uk 4, https://doi.org/10.1038/srep06568 (2014).
Carstens, E. et al. A global perspective on the population structure and reproductive system of Phyllosticta citricarpa. Phytopathology 107, 758–768, https://doi.org/10.1094/PHYTO-08-16-0292-R (2017).
Wang, N. Y., Zhang, K., Huguet-Tapia, J. C., Rollins, J. A. & Dewdney, M. M. Mating type and simple sequence repeat markers indicate a clonal population of Phyllosticta citricarpa in Florida. Phytopathology 106, 1300–1310, https://doi.org/10.1094/PHYTO-12-15-0316-R (2016).
Sposito, M. B., Amorim, L., Bassanezi, R. B., Filho, A. B. & Hau, B. Spatial pattern of black spot incidence within citrus trees related to disease severity and pathogen dispersal. Plant Pathol 57, 103–108, https://doi.org/10.1111/j.1365-3059.2007.01705.x (2008).
Er, H. L., Roberts, P. D., Marois, J. J. & van Bruggen, A. H. C. Potential distribution of citrus black spot in the United States based on climatic conditions. Eur. J. Plant Pathol. 137, 635–647, https://doi.org/10.1007/s10658-013-0276-6 (2013).
Noronha, M. A. Escala diagramática para avaliaçao da mancha preta em folhas de citros e efeito da temperatura e da duraçao do molhamento na pré-penetraçao de conidios de Guignardia citricarpa Kiely [Phyllosticta citricarpa (McAlp.) van der Aa]., Universidade de Sao Paulo (2003).
Korf, H. J. G., Schutte, G. C. & Kotze, J. M. Effect of packhouse procedures on the viability of Phyllosticta citricarpa, anamorph of the citrus black spot pathogen. Afr. Plant Prot. 7, 103–109, doi: https://hdl.handle.net/10520/EJC87836 (2001).
EFSA. Scientific opinion of the panel on plant health on a request from the European Commission on Guignardia citricarpa Kiely. The EFSA Journal 925, 1–108 (2008).
Brodrick, H. T. & Rabie, C. J. Light and temperature effects on symptom development and sporulation of Guignardia citricarpa Kiely, on Citrus sinensis (Linn.) Osbeck. Phytophylactica 2, 157–164, doi:https://hdl.handle.net/10520/AJA03701263_444 (1970).
Frare, G. F. et al. Sweet orange fruit age and inoculum concentration affect the expression of citrus black spot symptoms. Plant Dis., https://doi.org/10.1094/PDIS-03-18-0492-RE (2018).
Cruickshank, R. H. & Wade, G. C. The activation of latent infections of Monilinia fructicola on apricots by volatiles from the ripening fruit. Journal of Phytopathology 136, 107–112, https://doi.org/10.1111/j.1439-0434.1992.tb01288.x (1992).
Huang, C. S. & Chang, S. L. Leaf infection with citrus black spot and perithecial development in relation to ascospore discharge of Guignardia citricarpa Kiely. Journal of Taiwan Agricultural Research 21, 256–263 (1972).
Sposito, M. B. et al. Relative importance of inoculum sources of Guignardia citricarpa on the citrus black spot epidemic in Brazil. Crop Prot. 30, 1546–1552, https://doi.org/10.1016/j.cropro.2011.08.007 (2011).
Alvarez, S., Rohrig, E., Solis, D. & Thomas, M. H. Citrus greening disease (Huanglongbing) in Florida: Economic impact, management and the potential for biological control. Agric. Res. 5, 109–118, https://doi.org/10.1007/s40003-016-0204-z (2016).
Bove, J. M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37 (2006).
Halbert, S. E. In 2nd International Citrus Canker and Huanglongbing Research Workshop. (Florida Citrus Mutual).
Gottwald, T. R., Hughes, G., Graham, J. H., Sun, X. & Riley, T. The citrus canker epidemic in Florida: The scientific basis of regulatory eradication policy for an invasive species. Phytopathology 91, 30–34, https://doi.org/10.1094/PHYTO.2001.91.1.30 (2001).
Spreen, T. H., Baldwin, J. P. & Futch, S. H. An Economic Assessment of the Impact of Huanglongbing on Citrus Tree Plantings in Florida. Hortscience 49, 1052–1055 (2014).
de Oliveira Silva, A. et al. Epidemiological aspects of Phyllosticta citricarpa colonization and viability in Citrus sinensis. J Plant Dis Protect, 1–8, https://doi.org/10.1007/s41348-016-0046-8 (2016).
Dewdney, M. M., Schubert, T. S., Estes, M. R., Roberts, P. D. & Peres, N. A. In 2017–2018 Florida Citrus Production Guide Vol. 2017–2018 (University of Florida Institute of Food and Agricultural Sciences, Gainesville, FL, 2017).
Lanza, F. E., Metzker, T. G., Vinhas, T., Behlau, F. & Silva, G. J. Jr. Critical fungicide spray period for Citrus Black Spot control in São Paulo State, Brazil. Plant Dis. 102, 334–340, https://doi.org/10.1094/PDIS-04-17-0537-RE (2017).
Hendricks, K. E., Christman, M. & Roberts, P. D. Spatial and temporal patterns of commercial citrus trees affected by Phyllosticta citricarpa in Florida. Sci Rep-Uk 7, 1641, https://doi.org/10.1038/s41598-017-01901-2 (2017).
Rowlandson, T. et al. Reconsidering leaf wetness duration determination for plant disease management. Plant Dis. 99, 310–319, https://doi.org/10.1094/PDIS-05-14-0529-FE (2014).
Zavala, M. G. M. et al. Genetic variation among Phyllosticta strains isolated from citrus in Florida that are pathogenic or nonpathogenic to citrus. Trop Plant Pathol 39, 119–128, https://doi.org/10.1590/S1982-56762014000200002 (2014).
Sposito, M. B. et al. Elaboration and validation of diagrammatic scale to evaluate black spot severity in citrus fruits. Elaboracao e validacao de escala diagramatica para avaliacao da severidade da mancha preta em frutos citricos. Fitopatol. Bras. 29, 81–85, https://doi.org/10.1590/S0100-41582004000100012 (2004).
Truter, M. Epidemiology of citrus black spot disease in South Africa and its impact on phytosanitary trade restrictions PhD thesis, Pretoria, (2010).
Acknowledgements
This project was funded by the Specialty Crop Block Grant Program (project number 15SCBGPFL0038).
Author information
Authors and Affiliations
Contributions
K.E.H. and P.D.R. conceived the study, K.E.H. conducted the study, M.C. and K.E.H. analyzed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hendricks, K.E., Christman, M.C. & Roberts, P.D. The Effect of Weather and Location of Fruit within the Tree on the Incidence and Severity of Citrus Black Spot on Fruit. Sci Rep 10, 1389 (2020). https://doi.org/10.1038/s41598-020-58188-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58188-z
- Springer Nature Limited
This article is cited by
-
Climate suitability of the Mediterranean Basin for citrus black spot disease (Phyllosticta citricarpa) based on a generic infection model
Scientific Reports (2022)
-
Polyphasic identification and MAT1-2 isolates of Phyllosticta citricarpa in Cuba
European Journal of Plant Pathology (2022)