Abstract
Root-knot nematodes (Meloidogyne spp.) have been reported to be responsible for large economic losses of agricultural crops due to their wide host range and variety of suitable climates. The control measures of these parasitic nematodes depend upon synthetic nematicides and a small number bio-based products. Chemical nematicides are eliciting adverse effects on the environment and human health. In the present study, an alternative tool, nano-chitosan was tested for the control the root-knot nematodes, Meloidogyne incognita, and Tobacco mosaic tobamovirus (TMV) in greenhouse-cultivated tomato. The effect of nano-chitosan on morphological (weight and length of shoot and root systems) and biochemical responses (Polyphenol oxidase, Peroxides, Total soluble phenol and Total protein) was assessed. The obtained results indicated that densities of Meloidogyne incognita alone or in the presence of TMV were decreased by nano-chitosan at a range of 45.89 to 66.61%, while root gall desntiy was reduced between 10.63 and 67.87%. Moreover, the density of TMV on tomato leaves singly or in the presence of M. incognita was suppressed at range of 10.26 to 65.00% after 20 days of infection, and reached up to 58.00% after 40 days of infection. However, soil application of nano-chitosan pre infection reduced TMV density only by 5.48%. Morphogenesis of tomato plants such as shoot and root systems were significantly improved. The impacts of nano-Chitosan applications on total soluble phenol, total protein, polyphenol oxidase and peroxides after 20 and 40 days of infections varied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Egypt, tomatoes (Solanum lycopersicum, Mill) are very popular vegetables which are utilized for both local consumption and exportation (Rakha, 2014). Tomato is one of the richest sources of minerals, vitamins and carbohydrates (Ibrahim et al., 2010). There are about 159 countries globally that produce tomatoes commercially. China is one of the biggest producers of tomato with over 59 million tons in 2017 followed by Turkey, USA, Egypt, Italy, Spain, Mexico, Nigeria, Brazil and Russia (Ong et al., 2020).
Root-knot nematodes (Meloidogyne spp.) are a serious disease/pest which parasitize the roots of tomato plants causing damages leading to significant yield losses (Abd El-Monem et al., 2016; Niu et al., 2020).
Farmers depend on synthetic nematicides to manage nematodes. Such nematicides can impact on human healthy and the environment. Nanotechnology is increasingly being investigated for use in agriculture, food industry, pharmaceuticals and medicine (Giongo et al., 2016; Alfy et al., 2020). A Nanoparticle has a size range from 1 to 100 nm and many are investigated for their ability to manage pests and diseases (Sun et al., 2014; Alfy et al., 2020).
Chitosan is a natural biopolymer which is formed by chitin deacetylation that exists in the outer shell of shrimp, shellfish, lobster or crab, as well as infungal cell walls. It was discovered in 1811 by Henri Braconnot (Chakraborty et al., 2020). Chitosan is an eco-friendly and biodegradable material (Park et al., 2008; Khalil, 2016), is cheap and easy to work with (Anitha et al., 2014; Malerba & Cerana, 2016). Moreover, chitosan is able to control viruses, bacteria, fungi, insects, plant nematodes and other pests locally and systemically (Abd El-Aziz & Khalil, 2020; Alfy et al., 2020).
Tobacco mosaic tobamovirus (TMV) is a positive-sense single stranded RNA virus in the genus Tobamovirus and known as one of the most stable viruses (Silva et al., 2011; Hashemi et al., 2014; Ong et al., 2020). TMV causes severe economic losses in several crops included tomato (Abo-Zaid et al., 2020). The management of TMV is difficult because it is easily dispersed, it is transmitted mechanically, and symptoms are appearing 7 to 14 days after infction (Ara et al., 2012).
Therefore, in this study we aimed to investigate the impact of nano-chitosan on the presence of root-knot nematode (Meloidogyne incognita) and Tobacco mosaic tobamovirus (TMV) or their combination. We also studied their effect on morphological and physiological characteristics of tomato plants under greenhouse conditions.
Materials and methods
Nematode inoculum
The egg inoculum of root-knot nematode (Meloidogyne incognita) was isolated from egg-plant roots (cv. Balady). The roots were cut into small segments (1–2 cm long), and shaken for 3 min in sodium hypochlorite (5%). The suspension was passed through 200 and 400 mesh sieves to obtain free eggs (Hussey & Barker, 1973). The eggs were washed several times with water and then counted under a stereo-microscope. The species of root-knot nematode (M. incognita) was identified by using the perineal patterns method according to Taylor and Nelscher (1974).
Source of tobacco mosaic virus inoculum
Leaf samples of tomato plants showing mosaic symptoms (Fegla et al., 2000; Aghamohammadi et al., 2011) were collected separately in plastic bags from locations in Alexandria governorate during the 2019–2020 growing seasons. Virus inoculum was prepared by grinding infected leaf tissues 1:10 (w/v) with a mortar and pestle in 0.1 M phosphate buffer (pH 7.0), containing 0.5% of 2-mercaptoethanol. To identify TMV, the leaves of healthy Nicotiana glutinosa plants in the seedling stage were first dusted with carborundum (600 mesh) and then inoculated with a freshly prepared viral inoculum using the forefinger method, and kept in an insect proof greenhouse (Hamza et al., 2018).
Diagnostics of tobacco mosaic virus
Hosts and symptomology test
Chenopodium amaranticolor, Datura metal, Nicotiana rustica, N. glutinosa and Solanum lycopersicum (Abd El-Aziz, 2000) and Solanum melongena (Aghamohammadi et al., 2011) were used to confirm the identification of isolated virus because they give distinct symptoms. Plantlets (five replicates for each host) of each plant species were mechanically inoculated with TMV and kept under greenhouse conditions. Plants were assessed daily for about 4 weeks for symptoms expression. Inoculated plants which didn’t show any symptoms were checked for latent infection by back-inoculation to the indicator host N. glutinosa (Fegla et al., 2001).
Serological diagnostic test
Serological diagnostics was used to confirm the identification of TMV by indirect ELISA according to Abd El-Aziz and Younes (2019). Antiserum of Tobacco mosaic tobamovirus (TMV) used in the serological diagnosis was supplied by the Lab. of Prof. Fath-Allah Mervat (Plant Pathology Institute, Agricultural Research Center). This Antiserum was used to discriminate Cucumber mosaic cucumovirus (CMV) and TMV (Abd El-Aziz & Younes, 2019).
Enzyme linked immunosorbent assay (indirect ELISA) was carried out as described by Abd El-Aziz, 2019 and Abd El-Aziz and Younes (2019). The infected and healthy leaves of tomato plants, 20 and 40 days of infection, were extracted to detect the infection by TMV. The obtained values of ELISA (Sunrise ELISA plat reader™ instrument) were expressed as absorbance at 405 nm. The absorbance values considered positive when the value was at least twice that in uninfected plants. In each set of tests, wells lacking antigen (coating buffer only) were included as controls (Abd El-Aziz, 2019).
Nano-chitosan source, preparation and SEM
Chitosan was obtained from ChitoLytic, Canada with deacetylation of 88.3% and a viscosity 70 (at 20 °C). The nanoparticles of chitosan were prepared according to the method described by Qi et al. (2004). The SEM of nano-chitosan was carried out in the electron microscopy unit of the Faculty of Science, Alexandria University. The solution of nano-chitosan was prepared by dissolving 0.225 g nano-chitosan in 150 ml distilled water blended with 0.1% glacial acetic acid (Khan et al., 2021).
The efficacy of nano-chitosan on some parameters related to induced resistance against Meloidogyne incognita, Tobacco mosaic tobamovirus (TMV) and their mixture in the tissue of tomato plants
Total soluble phenol contents determination
The total soluble phenol content of tomato leaves, was extracted as described by Slinkard and Singleton (1997). The absorbance was carried out on 765 nm by using a spectrophotometer (Tuner, model 390). Total soluble phenol content was standardized against tannic acid and absorbance values were converted to μg of phenols per gram fresh weight of tomato leaves. Each value reported was the average of three replicates. The results were expressed as tannic equivalents according to the following equation:
μg tannic acid/g fresh weight = (((OD/K) ∗ (10/0.2)/0.5))
Where: OD = absorbance at 765 nm, K = the extension coefficient = 0.016898 μg-1.
Protein content
Total soluble protein was extracted from tomato leaves according to Dixon (1985) and determined according to Lowry et al. (1951). The blue color was measured at 700 nm by using a spectrophotometer (Tuner, model 390). The reading was related to standard curve prepared from known concentration of bovine serum albumin (BSA) protein.
mg protein/g fresh weight = ((OD/K) *100)/0. 5)/1000), Where: OD = the absorption at 700 nm, K = 0.0383.
Polyphenol oxidase (PPO)
Polyphenol oxidase activity (expressed as OD/mg protein), was determined according to Gauillard et al. (1993). The samples were repeated three times and absorbed on 420 nm by using a spectrophotometer (Tuner, model 390).
Peroxidase (POD)
Peroxides activity (expressed as OD / mg protein), was determine according to Murage and Masuda (1997) with some modification. The reaction mixture contained 200 μl of enzyme extract, 0.1 M sodium phosphate buffer (pH 6.1, 1.5 ml of 20% H2O2 and 1.5 ml of catechol 0.04 M). The initial rate of increase in absorbance was measured within 1 min at 470 nm determined by using a spectrophotometer (Tuner, model 390).
The experimental design
A pot experiment was carried out under greenhouse conditions to evaluate the performance of nano-chitosan against M. incognita, Tobacco mosaic tobamovirus (TMV) and their combination on tomato plants. All plastic pots (15 cm diameter) were filled with 1 kg of autoclaved loamy sand soil. A single plantlet of S. lycopersicum L. (cv. 086) of 40 days old was transplanted in each pot. The nano-chitosan was applied as foliar and soil application (drench) 5 days before infection as well as at the same time of infection. Each pot received 5000 nematode eggs by pouring the nematode suspension into holes made 2–4 cm below the soil surface around the base of the plants. All pots including controls (inoculated and uninoculated plants) were replicated four times and arranged in a completely randomized design on a bench under greenhouse conditions. During the course of the experiment, irrigation and fertilization were applied as needed. After 47 days, plants were uprooted and the roots were washed free of soil. The shoot and root lengths, in addition to their fresh weights were recorded. Furthermore, the number of galls / root system and number of J2 / 250 g soil were estimated at the end of the experiment. The second stage juveniles (J2) were extracted from the soil by using sieving and the Baermann plate technique (Ayoub, 1980) and then counted. Meanwhile, to inoculate TMV, the leaves of the tomato plants were dusted with carborundum (600 mesh) and then inoculated with a freshly prepared viral inoculum using forefinger method (Hamza et al., 2018).
Statistical analysis
The statistical analysis of the data was carried out using Costat (2005) version 6.303. Statistically significant differences between means were compared using analysis of variance (ANOVA) with the least significant differences (LSD) and P-values at 0.05 probability.
Results
Nematicidal impact of nano-chitosan on Meloidogyne incognita, alone or in the combination with TMV on tomatoes
Application of nano-chitosan (ranged from 19.11 upto 22.79 nm, Fig. 1) was effective against the root-knot nematode (M. incognita) singly or in the coexist of Tobacco mosaic tobamovirus (TMV) as soil and foliar application on tomato plants under greenhouse conditions (Table 1).
The results indicate that nano-chitosan as soil drench suppressed the second stage juveniles (J2) in the soil by 64.50 to 58.28%. While, foliar applicatios showed a reduction of 55.23 and 46.62%, respectively, without significant differences. For the infection with M. incognita and TMV the soil application of nano-chitosan decreased J2 density by 66.61 and 45.89%, and while foliar application 61.61 and 57.50% when applied after or before infection, respectively.
Root gall density was reduced with soil application of nano-chitosan by 67.87 and 56.40% pre and post infection, respectively, without significance difference. The foliar application showed a significant reduction of galls by 40.45 and 26.07% post and pre infections, respectively (Table 1). In dual infections (M. incognita + TMV), nano-chitosan decreased root gall density by 50.79 and 30.71% as soil application pre and post infection, respectively. Foliar application showed a reduction by 29.53 and 10.63% post and pre infections, respectively.
Serological detection of tobacco mosaic tobamovirus (TMV)
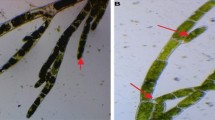
The TMV used in this study was isolated from naturally infested tomato plants collected from various locations in the Governorate of Alexandria. TMV was isolated from tomato plants showing mosaic, severe mosaic and yellowing with vein clearing (Fig. 2b) and yellowing mosaic on Nicotiana gluca (Fig. 2a). Necrotic local lesions induced after mechanical inoculations with TMV on S. melongena, C. amaranticolor, Datura metal and N. glutinosa were shown (Fig. 3). To identify the infection of TMV serologically specific antisera and indirect ELISA tests were utilized (Table 2). The obtained results showed that with antiserum of TMV the optical density of infected plants was 0.554 while healthy plants had an optical density of 0.196. Meanwhile, with antiserum of CMV, the optical density was 0.319 and healthy plant gave 0.280, this means that the recognized virus was TMV only and there is no CMV contamination.
a Necrotic local lesion (NLL) induced on Solanum melongena by TMV, b Reddish necrotic local lesion (RLL) on Chinopodium amaranticolor inoculated leaf induced by TMV, c Necrotic local lesion (NLL) caused by TMV on inoculated leaf of Datura metal d Necrotic local lesion (NLL) on inoculated leaf of Nicotiana glutinosa by TMV
Efficacy of nano-chitosan on TMV alone or in the presence of M. incognita
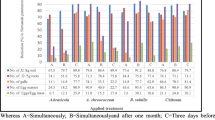
Table 3 shows the impact of nano-chitosan as soil and foliar applications before and 5 days after infection in the presence of TMV alone or combined with the root-knot nematode (M. incognita). Healthy plants (uninfected untreated control) recorded natural density of plant fractions estimated by 0.16 (at 20 days of infection) and 0.18 (at 40 days of infection). In plants infected with TMV alone or in the presence of M. incognita (untreated controls) the densities of virus reached up to 0.78 and 0.80 (at 20 days of infection), and up to 0.73 and 0.73 (at 40 days of infection), respectively.
After 20 days of infections, in the case of dual infection results showed that nano-chitosan as soil application recorded reduction in virus densities by 50 and 10%, while foliar application recorded 16.67 and 21.79% with and before infections, respectively. However, soil application of nano-chitosan against TMV alone decreased the virus densities in leaves tissues by 45.00 and 65.00% with and before infections, respectively. Suppression in virus densities were noticed in foliar application by 12.50 and 40.00% with and before infections, respectively.
In respect to the dual infection after 40 days, results indicated that application of nano-chitosan with infection as soil drench minimized the density of virus by 42.47%, while application before infection recorded increasing in virus density by 5.48%. Meanwhile, foliar application with infection recorded a reduction by 13.70%, whereas the application prior infection showed no suppression. In the presence of TMV only, soil application showed reductions by 28.77 and 58.90%, with and prior infections, consecutively. The implementation of nano-chitosan as foliar achieved reductions in virus densities estimated by 15.07 and 21.92% with and prior infections, respectively.
Impact of nano-chitosan on the morphogenesis of tomato plants
Data shown in Table 4 revealed the impact of soil (drench) and foliar applications of nano-chitosan with and without infection of root-knot nematode (M. incognita) and Tobacco mosaic tobamovirus (TMV) singly or in combination on the vigor of tomato plants. The healthy plants showed increases in all plant indices in comparison to untreated controls (inoculated plants). The use of nano-chitosan as soil application showed increases in shoot height by 4.34, 9.45 and 9.95%, while foliar application recorded 8.50, 23.01 and 1.91% in the presence of M. incognita alone, M. incognita + TMV and TMV alone, respectively. With respect to shoot weight, soil application of nano-chitosan achieved a mean increase by 25.84, 12.50 and 14.80%, whilst foliar application gave 23.17, 24.97 and 24.49% in the presence of M. incognita alone, M. incognita + TMV and TMV alone, respectively.
On the other hand, the lengths of the root system was affected by soil application of nano-chitosan showing a mean increase of 18.77, 37.61 and 34.33%, while foliar application recorded 18.19, 43.46 and 21.17%, in the presence of M. incognita alone, M. incognita + TMV and TMV alone, respectively. In the same context, soil application of nano-chitosan recorded chanes in root weight by 26.13, 68.21and 10.76%, in addition to foliar application recorded general mean enhancement by 29.08, 75.66 and 14.71%, in the presence of M. incognita alone, M. incognita + TMV and TMV alone, respectively.
The effect of nano-chitosan on some parameters related to induced resistance
The efficacy of Nano-chitosan on total soluble protein and phenol content of tomato leaves
The efficacy of nano-chitosan as soil and foliar application on total soluble protein was estimated in fresh tissues of tomato leaves after 20 and 40 days of infection with M. incognita, TMV or their mixture (Table 5). Soil application of nano-chitosan showed a mean increase in the total soluble protein (TSP) by 3.65 and 9.97% after 20 days of infection with M. incognita and TMV alone, respectively, while the mixture of M. incognita + TMV reduced the level of TSP by 0.71%. Also, the foliar application of nano-chitosan increased the levels of TSP by 0.56 and 9.88%, in the presence of TMV alone and M. incognita + TMV, respectively, whilst TSP decreased by 4.05% in the presence of M. incognita alone. Similar trends hold for the infection after 40 days (Table 4).
Total soluble phenol (TSN) content of the tissue of tomato leaves was affected by the application of nano-chitosan (Table 5). The soil application of nano-chitosan after 20 days of infection gave general mean increasing in the levels of TSN estimated by 6.51 and 6.27% in the presence of M. incognita + TMV and TMV alone, respectively, while a reduction by 8.41% was recorded in the presence of M. incognita alone. Also, the foliar application of nano-chitosan increased the levels of TSN by 9.19 and 5.67%, in the presence of M. incognita + TMV and M. incognita alone, respectively. Application of nano-chitosan in the presence of TMV alone diminished TSN level by 11.67% in tomato leaves. Similar trends hold for the infaction after 40 days (Table 5).
The effect of nano-chitosan on polyphenol oxidase and peroxidase activity
The impact of nano-chitosan on the levels of Polyphenol oxidase (PPO) after 20 and 40 days of infection with M. incognita, TMV and their mixture was shown in Table 6. The soil application of nano-chitosan after 20 days of infection by M. incognita alone and M. incognita + TMV increased the levels of PPO by 11.61 and 4.67%, respectively, while a reduction (6.16%) was noticed in the presence of TMV alone. Foliar application of nano-chitosan showed an increase by 15.31 and 20.79% in the presence of M. incognita and TMV alone, respectively, while a decrease was recorded in PPO levels of 1.56% in the presence of M. incognita + TMV. Similar trends hold for the infection after 40 days (Table 6).
The levels of Peroxides (POD) were affected by application of nano-chitosan after 20 and 40 days of infection in the presence of M. incognita, TMV or their mixture (Table 6). The soil application of nano-chitosan after 20 days of infection augmented the levels of POD by 6.76 and 23.17% in the presence of M. incognita alone and M. incognita + TMV, respectively, while in the presence of TMV alone the POD level was decreased by 16.59%. Meanwhile, applied nano-chitosan as foliar increased the levels of POD by 10.17 and 8.71% in the presence of both M. incognita and TMV alone, respectively. Nano-chitosan in the presence of binary mixture of M. incognita + TMV minimized the level of POD by 8.42%. Similar trends were found for the infection after 40 days (Table 6).
DISSCUSION
Our results indicate that nano-chitosan was very effective against the plant parasitic nematode (Meloidogyne incognita) singly or in the presence of TMV. The results of the present study are in agreement with a recent study by Alfy et al. (2020) who found that nano-chitosan at 500 to 2000 mg/l induced a mortality in juveniles of the root-knot nematode (M. incognita) ranging from 85.2 to 97.5% after 72 hours of exposure, and inhibited egg hatching by 73 to 95.3%. Meanwhile, the pot experiment under greenhouse conditions revealed that nano-chitosan at 2000 mg/l recorded suppression in galls, egg masses and juveniles/250 g soil by 90, 78 and 98%, respectively. The low, medium and high molecular weights of chitosan decreased the soil population at range of 37.67 to 68.60%, and from 70.30 to 83.33% in root galls (Khalil & Badawy, 2012). Similalry, high and low molecular weights of chitosan were minimized root galls by 90 and 93%, respectively (El-Sayed & Mahdy, 2015). da Silva et al. (2014) found that using chitosan in different molecular weights suppressed the severity of the pine wilt disease caused by Bursaphelenchus xylophilus.
The action of chitosan as soil application against plant parasitic nematodes (PPNs) are assumed to be linked with its stimulation of the reduplication of chitinolytic microorganisms which degrade chitin in organs of the PPNs, in addition to suppressing egg hatching and the viability of larvae and adults for both root-knot and cyst nematodes (Kalaiarasan et al., 2006; El-Sayed & Mahdy, 2015; Westerdahl et al., 1992). The parasitism by Pochonia chlamydosporia of the eggs of Meloidogyne javanica was increased by chitosan (El-Sayed & Mahdy, 2015). Meanwhile, chitosan has a high nitrogen content which releases ammonia causing toxicity to PPNs (Fan et al., 2020). Chitosan and its derivatives induce systemic acquired resistance in plants against a range of pathogens, in addition to root-knot nematode M. incognita (Chakraborty et al., 2020). Also, several reports mentioned that glucanase, chitinase, peroxidase, phenylalanine ammonia-lyase, polyphenol oxidase, superoxide dismutase and catalase were triggered with the application of chitosan or its derivatives in certain crops (Burkhanova et al., 2007; Eilenberg et al., 2009; Yin et al., 2010; Chang & Kim, 2012; Orzali et al., 2014; Xing et al., 2015; Li et al., 2016). The levels of protein and some secondary metabolites linked to the protection from pathogens, such as phenolics and phytoalexins, were increased in treated plants (Lin et al., 2005; Hadwiger, 2013; Zhang et al., 2015). The positive charge of chitosan molecules may be linked with negative charges on the surfaces of wide array pathogens and causing damage in cell structure (Chakraborty et al., 2020).
Viral diseases such as; alfalfa mosaic alfamovirus, peanut stunt cucumovirus, potato virus X potexvirus, tobacco mosaic tobamovirus, Tobacco necrosis alphanecrovirus and cucumber mosaic cucumovirus are affected by chitosan applications (Nagorskaya et al., 2014; Jia et al., 2016). TMV has a range of effects on fruit crops including the fall of flowers or failure of flowering leading to decreased production (El Shafie et al., 2005). The efficacy of chitosan as anti-viral agent may be due to chitosan being able to inducing hypersensitive response in infected plants (Chirkov, 2002). In addition, the antiviral activity of chitosan depends on the average polymerization frequency, degree of N-deacetylation, quality of positive charge, and character of the molecule’s chemical modifications (Chakraborty et al., 2020).
Our study revealed that application of nano-chitosan in the presence of root-knot nematode or TMV alone and in combination improved plant growth (shoot and root heights and weights) significantly which is in agreement with previous studies of Alfy et al. (2020) and Khalil and Badawy (2012). The enhancement in plant vigor with nano-chitosan or its derivatives may be linking to certain necessary nutrients, trace elements and improved index of photosynthesis (Chakraborty et al., 2020). However, chitosan can invigorate cell elongation, the division of root cells via stimulation plant hormones including auxin and cytokinin (John et al., 1997; Amin et al., 2007; Dzung et al., 2011). The positive charge of nano-chitosan can easily penetrate plant cell walls’ or adhere to plant surfaces and enhance seed germination and biophysical properties. Also, crop yield was increased substantially by enhancing stomatal function and chlorophyll content (Chakraborty et al., 2020).
Different physiological processes in plants such as nutrient uptake, enzymatic activation and synthesis of protein that can eventually lead to increase yield are affected by chitosan applications (Chakraborty et al., 2020). Chitosan influenced the soil biodiversity positively (Hassan & Chang, 2017). Chitosan can also be utilized as natural plant elicitor of defense responses and as a tool to combat pathogenic diseases post-harvest (Wang et al., 2017). Functionally, chitosan is working as antifungal (Sathiyabama & Charles, 2015), antibacterial (Sathiyabama et al., 2014), antiviral (Kulikov et al., 2006), and antinematodal agent (Khalil & Badawy, 2012; da Silva et al., 2014; Abd El-Aziz & Khalil, 2020).
Foliar applications of chitosan enhanced plant growth parameters, yield and improved the production of some important metabolites e.g. polyphenolics, flavonoids, lignin and phytoalexins (Emami et al., 2017; Xoca-Orozco et al., 2017). Chitosan and its derivatives induced the activity of polyphenol oxidase (PPO), chitinase, phenylalanine ammonia-lyase (PAL), tyrosine ammonialyase (TAL) and catalase (CAT), superoxide dismutase in orange, tomato, cucumber, table grapes, strawberries, sweet cherries and pears (Romanazzi, 2010; Chang & Kim, 2012; Xing et al., 2015), in addition to sugar and proline concentration (Guan et al., 2009). Furthermore, the level of peroxidase (POD) which is responsible for scavenging of reactive oxygen species in plants was enhanced significantly with chitosan application (Ahmad et al., 2013). The structural barriers in root tissues of tomato plants as defense mechanism were induced with chitosan application (Benhamou et al., 1998).
References
Abd El-Aziz, M. H. (2019). Three modern serological methods to detect plant viruses. Journal of Plant Science and Phytopathology, 3, 101–106.
Abd El-Aziz, M. H., & Younes, H. A. (2019). Detection of cucumber mosaic cucumovirus in infected cowpea plants (Vigna unguiculata L.) from northern Egypt. Novel Research Microbiology Journal, 3, 326–340.
Abd El-Aziz, M. H., & Khalil, M. S. (2020). Antiviral and Antinematodal potentials of chitosan: Review. Journal of Plant Science and Phytopathology, 4, 055–059.
Abd El-Aziz, M. H. (2000). Detection of certain plant viruses. Ms. C. Thesis. Fac. of agriculture (Saba–Basha) Alexandria University, Egypt. Pp. 118.
Abd El-Monem, M. A., Sharaf, A. M., Kailla, M. S., & Nofal, M. M. (2016). Induced resistance in tomato plants against root knot nematode using biotic and abiotic inducers. International Journal of Advance Research Biology Science, 3(11), 31–46.
Abo-Zaid, G. A., Matar, S. M., & Abdelkhalek, A. (2020). Induction of plant resistance against tobacco mosaic virus using the biocontrol agent Streptomyces cellulosae isolate Actino 48. Agronomy, 10(11), 1620. https://doi.org/10.3390/agronomy10111620
Aghamohammadi, V., Rakhshandehroo, F., & Shams-bakhsh, M. (2011). First report of tomato mosaic virus in eggplant in Iran. Journal of Plant Pathology, 93, 63–89.
Ahmad, I., Basra, S. M. A., Afzal, I., Farooq, M., & Wahid, A. (2013). Growth improvement in spring maize through exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide. International Journal of Agriculture and Biology, 15, 95–100.
Alfy, H., Ghareeb, R. Y., Soltan, E., & Farag, D. A. (2020). Impact of chitosan nanoparticles as insecticide and nematicide against Spodoptera littoralis, Locusta migratoria, and Meloidogyne incognita. Plant Cell Biotechnology Molecular Biology, 21, 126–140.
Amin, A. A., Rashad, E. M., & EL-Abagy, H. M. H. (2007). Physiological effect of indole-3-butyric acid and salicylic acid on growth, yield and chemical constituents of onion plants. Journal of Applied Sciences Research, 3, 1554–1563.
Anitha, A., Sowmya, S., Kumar, P. T. S., Deepthi, S., Chennazhi, K. P., Ehrlich, H., Tsurkan, M., & Jayakumar, R. (2014). Chitin and chitosan in selected biomedical applications. Progress in Polymer Science, 39, 1644–1667.
Ara, I., Bukhari, N. A., Aref, N. M., Shinwari, M. M. A., & Bakir, M. A. (2012). Antiviral activities of treptomycetes against tobacco mosaic virus (TMV) in Datura plant: Evaluation of different organic compounds in their metabolites. African Journal of Biotechnology, 11, 2130–2138.
Ayoub, S. M. (1980). Plant nematology, an agricultural training aid. Secramanto, California, USA, Nema aid Publications, P. 195.
Benhamou, N., Kloepper, J. W., & Tuzun, S. (1998). Induction of resistance against fusarium wilt of tomato by combination of chitosan with endophytic bacteria strain: Ultrastructure and cytochemistry of the host response. Planta, 204, 153–168.
Burkhanova, G. F., Yarullina, L. G., & Maksimov, I. V. (2007). The control of wheat defense responses during infection with Bipolaris sorokiniana by chitooligosaccharides. Russian Journal of Plant Physiology, 54, 104–110.
Chakraborty, M., Hasanuzzaman, M., Rahman, M., Khan, M. A. R., Bhowmik, P., Mahmud, N. U., Tanveer, M., & Islam, T. (2020). Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture. https://doi.org/10.3390/agriculture10120624
Chang, T., & Kim, B. S. (2012). Application of chitosan preparations for eco-friendly control of potato late blight. Research in Plant Disease, 18, 338–348.
Chirkov, S. N. (2002). The antiviral activity of chitosan (review). Applied Biochemistry and Microbiology, 38, 1–8.
CoStat Software (2005). Microcomputer program analysis, CoHort software, Version 6.303, Monterey, CA, USA.
da Silva, N. M., Cardoso, A. R., Ferreira, D., Pintado, M. M. E., & Vasconcelos, M. W. (2014). Chitosan as a biocontrol agent against the pinewood nematode (Bursaphelenchus xylophilus). Forest Pathology, 44, 420–423.
Dixon, R. A. (1985). Plant cell culture, a practical approach IRLPRESS. Oxford. Washington DC.1-235.
Dzung, N. A., Khanh, V. T. P., & Dzung, T. T. (2011). Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydrate Polymers, 84, 751–755.
Eilenberg, H., Pnini-Cohen, S., Rahamim, Y., Sionov, E., Segal, E., Carmeli, S., & Zilberstein, A. (2009). Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany, 61, 911–922.
El Shafie, E., Daffalla, G., Gebre, K., & Marchoux, G. (2005). Mosaic-inducing viruses and virus like- agents infecting tomato and pepper in Sudan. International Journal of Virology, 1, 28–28.
El-Sayed, S. M., & Mahdy, M. E. (2015). Effect of chitosan on root-knot nematode, Meloidogyne javanica on tomato plants. International Journal of Chemistry and Technology Research, 7, 1985–1992.
Emami, B. Z., Siadat, S. A., Bakhshandeh, A., Ghasemi, P. A., & Hashemi, M. (2017). Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop Journal, 5, 407–415.
Fan, Z., Qin, Y., Liu, S., Xing, R., Yu, H., & Li, P. (2020). Chitosan oligosaccharide fluorinated derivative control root-knot nematode (Meloidogyne incognita) disease based on the multi-efficacy strategy. Marine Drugs, 18(5), 273. https://doi.org/10.3390/md18050273
Fegla, G. I., El-Samra, I. A., Younes, H. A., & Abd El-Aziz, M. H. (2000). Optimization of dot Immunobinding assay (DIA) for detection of tomato mosaic virus (ToMV). Journal of Advance Agriculture Research, 5, 1495–1506.
Fegla, G. I., El-Samra, I. A., Younes, H. A., & Abd El-Aziz, M. H. (2001). Comparative studies for detection of tomato mosaic tobamovirus (TOMV), cucumber mosaic cucumovirus (CMV) and potato Y potyviruses (PVY). Advance Agriculture Research, 6, 239–254.
Gauillard, F., Richard-Forget, F., & Nicolas, J. (1993). New spectrophotometric assay for polyphenol oxidase activity. Analytical Biochemistry, 215, 59–65.
Giongo, A. M. M., Vendramim, J. D., & Forim, M. R. (2016). Evaluation of neem-based nanoformulations as alternative to control fall armyworm. Ciência e Agrotecnologia, 40, 26–36.
Guan, Y., Hu, J., Wang, X., & Shao, C. (2009). Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. Journal of Zhejiang University Science B, 10, 427–433.
Hadwiger, L. A. (2013). Multiple effects of chitosan on plant systems: Solid science or hype. Plant Science, 208, 42–49.
Hamza, K. A., Abd El-Aziz, M. H., Behiry, S. I., & Younes, H. A. (2018). Isolation and purification of potato virus y isolate infecting potato (Solanum tuberosum l.) in al-nubaria region. Middle East Journal of Agricultural Research, 7(4), 1201–1207.
Hashemi, S., Rakhshandehroo, S. F., & Shahraeen, N. (2014). First report of tomato mosaic virus on common sow thistle in Iran. Plant Disease, 98(8), 1151–1164. http://apsjournals.apsnet.org/loi/pdis. https://doi.org/10.1094/PDIS-03-14-0220-PDN
Hassan, O., & Chang, T. (2017). Chitosan for eco-friendly control of plant disease. Asian Journal of Plant Pathology, 11, 53–70.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula on Meloidogyne spp., including a new technique. Plant Disease Report, 57, 1025–1028.
Ibrahim, H. S., Saad, A. S. A., Massoud, M. A., & Khalil, M. S. H. (2010). Evaluation of certain agrochemicals and biological agents against Meloidogyne incognita on tomatoes. Alexandria Science Exchange Journal, 31, 10–17.
Jia, X., Meng, Q., Zeng, H., Wang, W., & Yin, H. (2016). Chitosan oligosaccharide induces resistance to tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signaling pathway. Scientific Reports, 6, 26144. https://doi.org/10.1038/srep26144
John, M., Röhrig, H., Schmidt, J., Walden, R., & Schell, J. (1997). Cell signaling by oligosaccharides. Trends in Plant Science, 2, 111–115.
Kalaiarasan, P., Lakshmanan, P., Rajendran, G., & Samiyappan, R. (2006). Chitin and chitinolytic biocontrol agents for the management of root knot nematode, Meloidogyne arenaria in groundnut (Arachis hypogaea L.) cv. Co3. Indian Journal of Nematology, 36, 181–186.
Khalil, M. S. (2016). Utilization of biomaterials as soil amendments and crop protection agents in integrated nematodes management. In K. R. Hakeem, M. S. Akhtar, & S. N. A. Abdullah (Eds.), Plant, soil and microbes. 1: Interactions and implications in crop science (pp. 203–224). Springer International Publishing.
Khalil, M. S., & Badawy, M. E. I. (2012). Nematicidal activity of a biopolymer chitosan at different molecular weights against root-knot nematode, Meloidogyne incognita. Plant Protection Science, 48(4), 170–178.
Khan, A., Tariq, M., Ahmad, F., Mennan, S., Khan, F., Asif, M., Nadeem, H., Ansari, T., Shariq, M., & Siddiqui, M. A. (2021). Assessment of nematicidal efficacy of chitosan in combination with botanicals against Meloidogyne incognita on carrot. Acta Agriculturae Scandinavica, Section B — Soil and Plant Science, 71, 225–236. https://doi.org/10.1080/09064710.2021.1880620
Kulikov, S. N., Chirkov, S. N., Ilina, A. V., Lopatin, S. A., & Varlamov, V. P. (2006). Effect of the molecular weight of chitosan on its antiviral activity in plants. Applied Biochemistry and Microbiology, 42, 200–203 [CrossRef].
Li, P., Cao, Z., Wu, Z., Wang, X., & Li, X. (2016). The effect and action mechanisms of oligochitosan on control of stem dry rot of Zanthoxylum bungeanum. International Journal of Molecular Sciences, 17, 1044.
Lin, W., Hu, X., Zhang, W., Rogers, W. J., & Cai, W. (2005). Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. Journal of Plant Physiology, 162, 937–944.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Malerba, M., & Cerana, R. (2016). Chitosan effects on plant systems-a review. International Journal of Molecular Sciences, 17, 996.
Murage, N., & Masuda, M. (1997). Response of pepper and eggplant to continuous light in relation to leaf chlorosis and activities of antioxidative enzymes. Scientia Horticulturae, 70, 269–279.
Nagorskaya, V., Reunov, A., Lapshina, L., Davydova, V., & Yermak, I. (2014). Effect of chitosan on Tobacco Mosaic Virus (TMV) accumulation, hydrolase activity and morphological abnormalities of the viral particles in leaves of N. tabacum L. cv. Samsun. Virologica Sinica, 29, 250–256.
Niu, B., Wang, W., Yuan, Z., Sederoff, R. R., Sederoff, H., Chiang, V. L., & Borriss, R. (2020). Microbial interactions within multiplestrain biological control agents impact soilborne plant disease. Frontiers in Microbiology, 11, 2452.
Ong, S. N., Taheri, S., Othman, R. Y., & Teo, C. H. (2020). Viral disease of tomato crops (Solanum lycopesicum L.): An overview. Journal of Plant Diseases and Protection, 127, 725–739.
Orzali, L., Forni, C., & Riccioni, L. (2014). Effect of chitosan seed treatment as elicitor of resistance to fusarium graminearum in wheat. Seed Science and Technology, 42, 132–149.
Park, Y., Kim, M. H., Park, S. C., Cheong, H., Jang, M. K., Nah, J. W., & Hahm, K. S. (2008). Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. Journal of Microbiology and Biotechnology, 18, 1729–1734.
Qi, L., Xu, Z., Jiang, X., Hu, C., & Zou, X. (2004). Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research, 339, 2693–2700.
Rakha, M. K. A. (2014). Growth, yield and fruit quality of eggplant (Solanum melongena l.) as affected by irrigation intervals and foliar application of some antitranspirants. Journal of Plant Production, 5(12), 2069–2083.
Romanazzi, G. (2010). Chitosan treatment for the control of postharvest decay of table grapes, strawberries and sweet cherries. Fresh Produce, 4, 111–115.
Sathiyabama, M., & Charles, R. E. (2015). Fungal cell wall polymerbased nanoparticles in protection of tomato plants from wilt disease caused by fusarium oxysporum f. sp. lycopersici. Carbohydrate Polymers, 133, 400–407.
Sathiyabama, M. G., Akila, R., & Einstein, C. (2014). Chitosan-induced defence responses in tomato plants against early blight disease caused by Alternaria solani (Ellis and Martin) Sorauer. Archives of Phytopathology and Plant Protection, 47, 1777–1787.
Silva, P. P., Freitas, R. A., Nascimento, W. M. (2011). Detection of tomato mosaic virus in tomato seed and treatment by thermotherapy article in acta horticulturae. December 2011. https://doi.org/10.17660/ActaHortic.2011.917.43.
Slinkard, K., & Singleton, V. L. (1997). Total phenol analysis: Automation and comparison with manual methods. American Journal of Enology and Viticulture, 28, 49–55.
Sun, C., Shu, K., Wang, W., Ye, Z., Liu, T., Gao, Y., Zheng, H., He, G., & Yin, Y. (2014). Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. International Journal of Pharmaceutics, 463, 108–114.
Taylor, D. P., & Nelscher, C. (1974). An improved technique for preparing perineal patterns of Meloidogyne spp. Nematology, 20, 268–269.
Wang, B., Zhang, S., Wang, X., Yang, S., Jiang, Q., Xu, Y., & Xia, W. (2017). Transcriptome analysis of the effects of chitosan on the hyperlipidemia and oxidative stress in high-fat diet fed mice. International Journal of Biological Macromolecules, 102, 104–110.
Westerdahl, B. B., Carlson, H. L., Grant, J., Radewald, J. D., Welch, N., Anderson, C. A., Darso, J., Kirby, D., & Shibuya, F. (1992). Management of plant-parasitic nematodes with a chitin-urea soil amendment and other materials. Journal of Nematology, 24, 669–680.
Xing, K., Zhu, X., Peng, X., & Qin, S. (2015). Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agronomy for Sustainable Development, 35, 569–588.
Xoca-Orozco, L. A., Cuellar-Torres, E. A., González-Morales, S., Gutiérrez-Martínez, P., López-García, U., Herrera-Estrella, L., Vega-Arreguín, J., & Chacón-López, A. (2017). Transcriptomic analysis of avocado hass (Persea americana mill) in the interaction system fruit-chitosan-Colletotrichum. Frontiers in Plant Science, 8, 956. https://doi.org/10.3389/fpls.2017.00956
Yin, H., Zhao, X., & Du, Y. (2010). Oligochitosan: A plant diseases vaccine-a review. Carbohydrate Polymers, 82, 1–8.
Zhang, D., Wang, H., Hu, Y., & Liu, Y. (2015). Chitosan controls postharvest decay on cherry tomato fruit possibly via the mitogen-activated protein kinase signaling pathway. Journal of Agricultural and Food Chemistry, 63(33), 7399–7404. https://doi.org/10.1021/acs.jafc.5b01566
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
We have no potential conflicts of interest.
Research involving human participants and / or animals
No human participants or animals were involved in this study.
Informed consent
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalil, M.S., Abd El-Aziz, M.H. & Selim, R.ES. Physiological and morphological response of tomato plants to nano-chitosan used against bio-stress induced by root-knot nematode (Meloidogyne incognita) and Tobacco mosaic tobamovirus (TMV). Eur J Plant Pathol 163, 799–812 (2022). https://doi.org/10.1007/s10658-022-02516-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02516-8