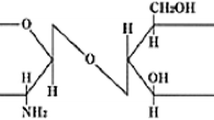
Abstract
In agriculture, current control of pathogens relies mainly on chemical fertilizers and pesticides. However, alternative solutions are needed due to concerns for public health, environmental protection, and development of resistant pests. Chitosan is a nontoxic, biodegradable biopolymer showing antimicrobial and plant-immunity eliciting properties. Here, we review chitosan antimicrobial activities, modes of action, and the elicitation of plant defense responses. The major points are the following: (1) Chitosan exhibits various inhibitory efficiency against bacteria, fungi, and viruses; (2) the five main modes of action of chitosan are electrostatic interactions, plasma membrane damage mechanism, chitosan-DNA/RNA interactions, metal chelation capacity of chitosan, and deposition onto the microbial surface; (3) the elicitation of plant defense responses by chitosan may be related to various pathogenesis-related proteins, defense-related enzymes, and secondary metabolites accumulation, as well as the complex signal transduction network. The facing problems and strategies for antimicrobial mechanism research and agricultural application of chitosan are also discussed.



Similar content being viewed by others
References
Abbasi NA, Iqbal Z, Maqbool M, Hafiz IA (2009) Postharvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak J Bot 41:343–357
Abro MA, Lecompte F, Bardin M, Nicot PC (2013) Nitrogen fertilization impacts biocontrol of tomato gray mold. Agron Sustain Dev. doi:10.1007/s13593-013-0168-3
Allan CR, Hadwiger LA (1979) The fungicidal effect of chitosan on fungi of varying cell composition. Exp Mycol 3:285–287. doi:10.1016/S0147-5975(79)80054-7
Alvarez MV, Ponce AG, Moreira MR (2013) Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT - Food Sci Technol 50:78–87. doi:10.1016/j.lwt.2012.06.021
Amborabé B-E, Bonmort J, Fleurat-Lessard P, Roblin G (2008) Early events induced by chitosan on plant cells. J Exp Bot 59:2317–2324. doi:10.1093/jxb/ern096
Atanasova-Penichon V, Pons S, Pinson-Gadais L, Picot A, Marchegay G, Bonnin-Verdal MN, Ducos C, Barreau C, Roucolle J, Sehabiague P, Carolo P, Richard-Forget F (2012) Chlorogenic acid and maize ear rot resistance: a dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol Plant Microbe Interact 25:1605–1616. doi:10.1094/MPMI-06-12-0153-R
Atkinson HA, Daniels A, Read ND (2002) Live-cell imaging of endocytosis during conidial germination in the rice blast fungus, Magnaporthe grisea. Fungal Genet Biol 37:233–244. doi:10.1016/S1087-1845(02)00535-2
Awadalla OA, Mahmoud YA-G (2005) New chitosan derivatives induced resistance to Fusarium wilt disease through phytoalexin (Gossypol) production. Sains Malays 34:141–146
Aziz A, Trotel-Aziz P, Dhuicq L, Jeandet P, Couderchet M, Vernet G (2006) Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 96:1188–1194. doi:10.1094/PHYTO-96-1188
Badawy ME, Rabea EI, Taktak NE (2014) Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydr Polym 111:670–682. doi:10.1016/j.carbpol.2014.04.098
Benhamou N (1992) Ultrastructural and cytochemical aspects of chitosan on Fusarium oxysporum f. sp. radices-lycopersici, agent of tomato crown and root rot. Phytopathology 82:1185–1193
Benhamou N (1996) Elicitor-induced plant defence pathways. Trends Plant Sci 1:233–240. doi:10.1016/1360-1385(96)86901-9
Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang XL, Sabatino A, Plourde GL (1999) Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol 121:135–146. doi:10.1104/pp. 121.1.135
Bhaskara Reddy MV, Arul J, Angers P, Couture L (1999) Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J Agric Food Chem 47:1208–1216
Bhatnagar A, Sillanpää M (2009) Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—a short review. Adv Colloid Interfac 152:26–38. doi:10.1016/j.cis.2009.09.003
Blume B, Nürnberger T, Nass N, Scheel D (2000) Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12:1425–1440. doi:10.2307/3871140
Bol JF, Linthorst HJM, Cornelissen BJC (1990) Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol 28:113–138. doi:10.1146/annurev.py.28.090190.000553
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126:1024–1030. doi:10.1104/pp. 126.3.1024
Chatterjee S, Chatterjee BP, Guha AK (2014) A study an antifungal activity of water-soluble chitosan against Macrophomina phaseolina. Int J Biol Macromol 67:452–457. doi:10.1016/j.ijbiomac.2014.04.008
Chen YP (2008) Isatis indigotica seedlings derived from laser stimulated seeds showed improved resistance to elevated UV-B. Plant Growth Regul 55:73–79. doi:10.1007/s10725-008-9258-7
Chen C, Belanger RR, Benhamou N, Paulitz TC (2000) Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol Mol Plant P 56:13–23. doi:10.1006/pmpp.1999.0243
Chirkov SN (2002) The antiviral activity of chitosan. Appl Biochem Micro 38:1–8
Chirkov SN, Il’ina AV, Surgucheva NA, Letunova EV, Varitsev YA, Tatarinova NY, Varlamov VP (2001) Effect of chitosan on systemic viral infection and some defense responses in potato plants. Russ J Plant Physl 48:774–779. doi:10.1023/A:1012508625017
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814. doi:10.1016/j.cell.2006.02.008
Chung YC, Chen CY (2008) Antibacterial characteristics and activity of acid-soluble chitosan. Bioresource Technol 99:2806–2814. doi:10.1016/j.biortech.2007.06.044
Chung YC, Su YP, Chen CC, Jia G, Wang HL, Wu JC, Lin JG (2004) Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin 25:932–936
Come V, Deschamps A, Mertial A (2003) Bioactive packaging materials from edible chitosan polymer-antimicrobial activity assessment on dairy-related contaminants. J Food Sci 68:2788–2792. doi:10.1111/j.1365-2621.2003.tb05806.x
Cota-Arriola O, Cortez-Rocha MO, Burgos-Hernández A, Ezquerra-Brauer JM, Plascencia-Jatomea M (2013) Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: development of new strategies for microbial control in agriculture. J Sci Food Agric 93:1525–1536. doi:10.1002/jsfa.6060
Dang QF, Yan JQ, Li Y, Cheng XJ, Liu CS, Chen XG (2010) Chitosan acetate as an active coating material and its effects on the storing of Prunus avium L. J Food Sci 75:S125–S131. doi:10.1111/j.1750-3841.2009.01483.x
Davydova VN, Naqorskaia VP, Gorbach VI, Kalitnik AA, Reunov AV, Solov’eva TF, Ermak IM (2011) Chitosan antiviral activity: dependence on structure and depolymerization method. Prikl Biokhim Mikrobiol 47:113–118. doi:10.1134/S0003683811010042
Djioua T, Charles F, Freire M, Filgueiras H, Ducamp-Collin MN, Sallanon H (2010) Combined effects of postharvest heat treatment and chitosan coating on quality of fresh-cut mangoes (Mangifera indica L.). Int J Food Sci Tech 45:849–855. doi:10.1111/j.1365-2621.2010.02209.x
Dodane V, Vilivalam VD (1998) Pharmaceutical applications of chitosan. Pharm Sci Technol To 1:246–253. doi:10.1016/S1461-5347(98)00059-5
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym 75:385–389. doi:10.1016/j.carbpol.2008.07.039
Dumas-Gaudot E, Slezak S, Dassi B, Pozo MJ, Gianinazzi-Pearson V, Gianinazzi S (1996) Plant hydrolytic enzymes (chitinases and ß–1,3 glucanases) in root reactions to pathogenic and symbiotic microorganisms. Plant Soil 185:211–221. doi:10.1007/BF02257526
Dutta PK, Tripathi S, Mehrotra GK, Dutta J (2009) Perspectives for chitosan based antimicrobial films in food applications. Food Chem 114:1173–1182. doi:10.1016/j.foodchem.2008.11.047
Eilenberg H, Pnini-Cohen S, Rahamim Y, Sionov E, Segal E, Carmeli S, Zilberstein A (2010) Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J Exp Bot 61:911–922. doi:10.1093/jxb/erp359
El-Ghaouth A, Arul J, Grenier J, Asselin A (1992) Antifungal activity of chitosan on two post-harvest pathogens of strawberry fruits. Phytopathology 82:398–402
El-Ghaouth A, Arul J, Grenier J, Benhamou N, Asselin A, Belanger R (1994) Effect of chitosan on cucumber plants: suppression of Pythium aphinodermatum and induction of defense reactions. Phytopathology 84:313–320
El-Hadrami A, Adam LR, El-Hadrami I, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8:968–987. doi:10.3390/md8040968
El-Hassni M, El-Hadrami A, Daayf F, Barka EA, El-Hadrami I (2004) Chitosan, antifungal product against Fusarium oxysporum f. sp. albedinis and elicitor of defence reactions in date palm roots. Phytopathol Mediterr 43:195–204
Elsenhans B, Blume R, Lembcke B, Caspary WF (1983) A new class of inhibitors for in vitro small intestinal transport of sugars and amino acids in the rat. Biochim Biophys Acta 727:135–143. doi:10.1016/0005-2736(83)90377-2
Farouk S, Mosa AA, Taha AA, Ibrahim HM, EL-Gahmery AM (2011) Protective effect of humic acid and chitosan on radish (Raphanus sativus, L. var. sativus) plants subjected to cadmium stress. J Stress Physiol Bioch 7:99–116
Fitza KNE, Payn KG, Steenkamp ET, Myburg AA, Naidoo S (2013) Chitosan application improves resistance to Fusarium circinatum in Pinus patula. S Afr J Bot 85:70–78. doi:10.1016/j.sajb.2012.12.006
Fondevilla S, Rubiales D (2012) Powdery mildew control in pea. A review. Agron Sustain Dev 32:401–409
Galván MI, Akuaku J, Cruz I, Cheetham J, Golshani A, Smith ML (2013) Disruption of protein synthesis as antifungal mode of action by chitosan. Int J Food Microbiol 164:108–112. doi:10.1016/j.ijfoodmicro.2013.03.025
García-Rincóna J, Vega-Pérezb J, Guerra-Sánchezb MG, Hernández-Lauzardoa AN, Peña-Díazc A, Velázquez-Del Vallea MG (2010) Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pestic Biochem Phys 97:275–278. doi:10.1016/j.pestbp.2010.03.008
Geisberger G, Gyenge EB, Hinger D, Käch A, Maake C, Patzke GR (2013) Chitosan-thioglycolic acid as a versatile antimicrobial agent. Biomacromolecules 14:1010–1017. doi:10.1021/bm3018593
Goy RC, Britto D, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polímeros 19:241–247. doi:10.1590/S0104-14282009000300013
Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J (2000) The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J 23:441–450. doi:10.1046/j.1365-313x.2000.00804.x
Guan YJ, Hu J, Wang XJ, Shao CX (2009) Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B 10:427–433. doi:10.1631/jzus.B0820373
Guo ZY, Chen R, Xing RE, Liu S, Yu HH, Wang PB, Li CP, Li PC (2006) Novel derivatives of chitosan and their antifungal activities in vitro. Carbohyd Res 341:351–354. doi:10.1016/j.carres.2005.11.002
Hadwiger LA (1999) Host-parasite interactions: elicitation of defense responses in plants with chitosan. EXS 87:185–200. doi:10.1007/978-3-0348-8757-1_13
Hadwiger LA (2008) Pea-Fusarium solani interactions contributions of a system toward understanding disease resistance. Phytopathology 98:372–379. doi:10.1094/PHYTO-98-4-0372
Hadwiger LA (2013) Multiple effects of chitosan on plant systems: solid science or hype. Plant Sci 208:42–49. doi:10.1016/j.plantsci.2013.03.007
Hadwiger LA. Klosterman SK. Chang MM. Friel P. Hosick HL (1997) Chitosan heptamer alters DNA, induces defense genes in plants and induces the accumulation of gene p53 product in animal cells. In: Domard A, Roberts GAF, Varum KM (eds.) In Advances in Chitin Science. Jacques Andre Publisher, Lyon, vol. II, pp102-109
Hadwiger LA, Beckman JM (1980) Chtosan as a component of Pea-Fusarium solani interactions. Plant Physiol 66:205–211. doi:10.1104/pp. 66.2.205
Hadwiger LA, Chiang C, Victory S, Horovitz D (1989) Chitin and chitosan: sources, chemistry, biochemistry, physical properties and applications. Elsevier Applied Science, London
Halim VA, Vess A, Scheel D, Rosahl S (2006) The role of salicylic acid and jasmonic acid in pathogen defence. Plant Biol 8:307–313. doi:10.1055/s-2006-924025
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int J Food Microbiol 71:235–244. doi:10.1016/S0168-1605(01)00609-2
Hemaiswarya S, Soudaminikkutty R, Narasumani ML, Doble M (2011) Phenylpropanoids inhibit protofilament formation of Escherichia coli cell division protein FtsZ. J Med Microbiol 60:1317–1325. doi:10.1099/jmm.0.030536-0
Hoat TX, Nakayashiki H, Yang Q, Tosa Y, Mayama S (2013) Molecular cloning of the apoptosis-related calcium-binding protein AsALG-2 in Avena sativa. Mol Plant Pathol 14:222–229. doi:10.1111/j.1364-3703.2012.00844.x
Hsu SH, Chang YB, Tsai CL, Fu KY, Wang SH, Tseng HJ (2011) Characterization and biocompatibility of chitosan nanocomposites. Colloid Surface B 85:198–206. doi:10.1016/j.colsurfb.2011.02.029
Hu Y, Cai J, Du YM, Lin JG, Wang CG, Xiong KJ (2009) Preparation and anti-TMV activity of guanidinylated chitosan hydrochloride. J Appl Polym Sci 112:3522–3528. doi:10.1002/app.29959
Iriti M, Faoro F (2008) Abscisic acid is involved in chitosan-induced resistance to tobacco necrosis virus (TNV). Plant Physiol Biochem 46:1106–1111. doi:10.1016/j.plaphy.2008.08.002
Iriti M, Faoro F (2009) Chitosan as a MAMP, searching for a PRR. Plant Signal Behav 4:66–68. doi:10.4161/psb.4.1.7408
Iriti M, Sironi M, Gomarasca S, Casazza AP, Soave C, Faoro F (2006) Cell death-mediated antiviral effect of chitosan in tobacco. Plant Physiol Biochem 44:893–900. doi:10.1016/j.plaphy.2006.10.009
Isaac J, Hartney SL, Druffel K, Hadwiger LA (2009) The non-host disease resistance response in peas; alterations in phosphorylation and ubiquitination of HMG A and histones H2A/H2B. Plant Sci 177:439–449. doi:10.1016/j.plantsci.2009.07.007
Issam ST, Adele MG, Adele CP, Stephane G, Veronique C (2005) Chitosan polymer as bioactive coating and film against Aspergillus niger contamination. J Food Sci 70:100–104
Jabeen N, Ahmad R (2013) The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J Sci Food Agric 93:1699–1705. doi:10.1002/jsfa.5953
Je JY, Kim SK (2006) Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J Agric Food Chem 54:6629–6633. doi:10.1021/jf061310p
Karthikeyan M, Jayakumar V, Radhika K, Bhaskaran R, Velazhahan R, Alice D (2005) Induction of resistance in host against the infection of leaf blight pathogen (Alternaria palandui) in onion (Allium cepa var aggregatum). Indian J Biochem Biophys 42:371–377
Khan W, Prithiviraj B, Smith DL (2003) Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J Plant Physiol 160:859–863. doi:10.1078/0176-1617-00905
Kim HJ, Chen F, Wang X, Rajapakse NC (2005) Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J Agric Food Chem 53:3696–3701. doi:10.1021/jf0480804
Kim H, Tator CH, Shoichet MS (2011) Chitosan implants in the rat spinal cord: biocompatibility and biodegradation. J Biomed Mater Res A 97:395–404. doi:10.1002/jbm.a.33070
Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130:2152–2163
Koers S, Guzel-Deger A, Marten I, Roelfsema MR (2011) Barley mildew and its elicitor chitosan promote closed stomata by stimulating guard-cell S-type anion channels. Plant J 68:670–680. doi:10.1111/j.1365-313X.2011.04719.x
Köhle H, Jeblick W, Poten F, Blaschek W, Kauss H (1985) Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol 77:544–551
Komaraiah P, Ramakrishna SV, Reddanna P, Kavi Kishor PB (2003) Enhanced production of plumbagin in immobilized cells of Plumbago rosea by elicitation and in situ adsorption. J Biotechnol 101:181–187. doi:10.1016/S0168-1656(02)00338-3
Kong M, Chen XG, Liu CS, Liu CG, Meng XH, Yu LJ (2008) Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloid Surface B 65:197–202. doi:10.1016/j.colsurfb.2008.04.003
Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63. doi:10.1016/j.ijfoodmicro.2010.09.012
Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136. doi:10.1146/annurev.arplant.54.031902.134752
Lambert PA (2002) Cellular impermeability and uptake of biocides and antibiotics in gram-positive bacteria and mycobacteria. J Appl Microbiol 31:46S–54S. doi:10.1046/j.1365-2672.92.5s1.7.x
Lee DS, Je JY (2013) Gallic acid-grafted-chitosan inhibits foodborne pathogens by a membrane damage mechanism. J Agric Food Chem 61:6574–6579. doi:10.1021/jf401254g
Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247. doi:10.1007/s00425-002-0750-4
Li SJ, Zhu TH (2013) Biochemical response and induced resistance against anthracnose (Colletotrichum camelliae) of camellia (Camellia pitardii) by chitosan oligosaccharide application. For Path 43:67–76. doi:10.1111/j.1439-0329.2012.00797.x
Li B, Wang X, Chen RX, Huangfu WG, Xie GL (2008) Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohyd Polym 72:287–292. doi:10.1016/j.carbpol.2007.08.012
Li B, Liu BP, Shan CL, Ibrahim M, Lou YH, Wang YL, Xie GL, Li HY, Sun GC (2013a) Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag Sci 69:312–320. doi:10.1002/ps.3399
Li B, Shi Y, Shan CL, Zhou Q, Ibrahim M, Wang YL, Wu GX, Li HY, Xie GL, Sun GC (2013b) Effect of chitosan solution on the inhibition of Acidovorax citrulli causing bacterial fruit blotch of watermelon. J Sci Food Agric 93:1010–1015. doi:10.1002/jsfa.5812
Li XS, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR (2013c) Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol 2013:1–8. doi:10.1155/2013/387023
Liang C, Yuan F, Liu F, Wang Y, Gao Y (2014) Structure and antimicrobial mechanism of ε-polylysine-chitosan conjugates through Maillard reaction. Int J Biol Macromol. doi:10.1016/j.ijbiomac.2014.07.012
Liénart Y, Gautier C, Domard A (1991) Isolation from Rubus cell-suspension cultures of a lectin specific for glucosamine oligomers. Planta 184:8–13. doi:10.1007/BF00208229
Lin W, Hu X, Zhang W, Rogers WJ, Cai W (2005) Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J Plant Physiol 162:937–944. doi:10.1016/j.jplph.2004.10.003
Liu XF, Guan YL, Yang DZ, Li Z, Yao KD (2001) Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci 79:1324–1335. doi:10.1002/1097-4628(20010214)79:7<1324::AID-APP210>3.0.CO;2-L
Liu H, Du YM, Wang XH, Sun LP (2004) Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol 95:147–155. doi:10.1016/j.ijfoodmicro.2004.01.022
Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW, Luk KD (2005) An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials 26:2705–2711. doi:10.1016/j.biomaterials.2004.07.038
Liu H, Tian WX, Li B, Wu GX, Ibrahim M, Tao ZY, Wang YL, Xie GL, Li HY, Sun GC (2012) Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol Lett 34:2291–2298. doi:10.1007/s10529-012-1035-z
Lou MM, Zhu B, Muhammad I, Li B, Xie GL, Wang YL, Li HY, Sun GC (2011) Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohyd Res 346:1294–1301. doi:10.1016/j.carres.2011.04.042
Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J (2011) Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24:183–193. doi:10.1094/MPMI-07-10-0149
Ma ZX, Yang LY, Yan HX, Kennedy JF, Meng XH (2013) Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr Polym 94:272–277. doi:10.1016/j.carbpol.2013.01.012
MacDonald MJ, D’Cunha GB (2007) A modern view of phenylalanine ammonia lyase. Biochem Cell Biol 85:273–282. doi:10.1139/O07-018
Madihally SV, Matthew HW (1999) Porous chitosan scaffolds for tissue engineering. Biomaterials 20:1133–1142. doi:10.1016/S0142-9612(99)00011-3
Malerba M, Crosti P, Cerana R (2012) Defense/stress responses activated by chitosan in sycamore cultured cells. Protoplasma 249:89–98. doi:10.1007/s00709-011-0264-7
Manjunatha G, Roopa KS, Prashanth GN, Shetty HS (2008) Chitosan enhances disease resistance in pearl millet against downy mildew caused by Sclerospora graminicola and defence-related enzyme activation. Pest Manag Sci 64:1250–1257. doi:10.1002/ps.1626
Manjunatha G, Niranjan-Raj S, Prashanth GN, Deepak S, Amruthesh KN, Shetty HS (2009) Nitric oxide is involved in chitosan-induced systemic resistance in pearl millet against downy mildew disease. Pest Manag Sci 65:737–743. doi:10.1002/ps.1710
Mansilla AY, Albertengo L, Rodríguez MS, Debbaudt A, Zúñiga A, Casalongué CA (2013) Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl Microbiol Biotechnol 97:6957–6966. doi:10.1007/s00253-013-4993-8
Mejía-Teniente L, Duran-Flores FD, Chapa-Oliver AM, Torres-Pacheco I, Cruz-Hernández A, González-Chavira MM, Ocampo-Velázquez RV, Guevara-González RG (2013) Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. Int J Mol Sci 14:10178–10196. doi:10.3390/ijms140510178
Meng XH, Yang LY, Kennedy JF, Tian SP (2010) Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr Polym 81:70–75. doi:10.1016/j.carbpol.2010.01.057
Mi FL, Tan YC, Liang HF, Sung HW (2002) In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 23:181–191. doi:10.1016/S0142-9612(01)00094-1
Mitchell HJ, Hall JL, Barber MS (1994) Elicitor-induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol 104:551–556
Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci U S A 104:19613–19618. doi:10.1073/pans.0705147104
Ndimba BK, Chivasa S, Hamilton JM, Simon WJ, Slabas AR (2003) Proteomic analysis of changes in the extracellular matrix of Arabidopsis cell suspension cultures induced by fungal elicitors. Proteomics 3:1047–1059. doi:10.1002/pmic.200300413
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35. doi:10.1046/j.1469-8137.2003.00804.x
Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389. doi:10.1146/annurev.py.30.090192.002101
No HK, Park NY, Lee SH, Meyers SP (2002) Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74:65–72. doi:10.1016/S0168-1605(01)00717-6
Nurnberger T, Brunner F, Kemmerking B, Piater L (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198:249–266. doi:10.1111/j.0105-2896.2004.0119.x
Orgaz B, Lobete MM, Puga CH, Jose CS (2011) Effectiveness of chitosan against mature biofilms formed by food related bacteria. Int J Mol Sci 12:817–828. doi:10.3390/ijms12010817
Orlita A, Sidwa-Gorycka M, Paszkiewicz M, Malinski E, Kumirska J, Siedlecka EM, Łojkowska E, Stepnowski P (2008) Application of chitin and chitosan as elicitors of coumarins and fluoroquinolone alkaloids in Ruta graveolens L. (common rue). Biotechnol Appl Biochem 51:91–96. doi:10.1042/BA20070200
Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci U S A 96:6553–6557. doi:10.1073/pnas.96.11.6553
Ozeretskovskaia OL, Vasiukova NI, Chalenko GI, Gerasimova NG, Revina TA, Valueva TA (2009) Wound healing and induced resistance in potato tubers. Prikl Biokhim Mikrobiol 45:220–224. doi:10.1134/S0003683809020148
Palma-Guerrero J, Huang IC, Jansson HB, Salinas J, Lopez-Llorca LV, Read ND (2009) Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genet Biol 46:585–594. doi:10.1016/j.fgb.2009.02.010
Palma-Guerrero J, Lopez-Jimenez JA, Pérez-Berná AJ, Huang IC, Jansson HB, Salinas J, Villalaín J, Read ND, Lopez-Llorca LV (2010) Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol Microbiol 75:1021–1032. doi:10.1111/j.1365-2958.2009.07039.x
Park S-I, Daeschel MA, Zhao Y (2004) Functional properties of antimicrobial lysozyme–chitosan composite films. J Food Sci 69:215–221. doi:10.1111/j.1365-2621.2004.tb09890.x
Park Y, Kim MH, Park SC, Cheong H, Jang MK, Nah JW, Hahm KS (2008) Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. J Microbiol Biotechn 18:1729–1734
Petutschnig EK, Jones AM, Serazetdinova L, Lipka U, Lipka V (2010) The lysin motif receptor-like kinase (Lysm-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J Bio Chem 285:28902–28911. doi:10.1074/jbc.M110.116657
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678. doi:10.1016/j.progpolymsci.2009.04.001
Pizzo P, Giurisato E, Tassi M, Benedetti A, Pozzan T, Viola A (2002) Lipid rafts and T cell receptor signaling: a critical re-evaluation. Eur J Immunol 32:3082–3091. doi:10.1002/1521-4141(200211)32:11<3082::AID-IMMU3082>3.0.CO;2-2
Povero G, Loreti E, Pucciariello C, Santaniello A, Di Tommaso D, Di Tommaso G, Kapetis D, Zolezzi F, Piaggesi A, Perata P (2011) Transcript profiling of chitosan-treated Arabidopsis seedlings. J Plant Res 124:619–629. doi:10.1007/s10265-010-0399-1
Qiu M, Wu C, Ren G, Liang X, Wang X, Huang J (2014) Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem 155:105–111. doi:10.1016/j.foodchem.2014.01.026
Raafat D, Bargen KV, Haas A, Sahl H-G (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microb 74:3764–3773. doi:10.1128/AEM.00453-08
Rabea EI, Steurbaut W (2010) Chemically modified chitosans as antimicrobial agents against some plant pathogenic bacteria and fungi. Plant Protect Sci 4:149–158
Rabea EI, Badawy ME-T, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465. doi:10.1021/bm034130m
Rabea EI, Badawy MEI, Steurbaut W, Stevens CV (2009) In vitro assessment of N-(benzyl)chitosan derivatives against some plant pathogenic bacteria and fungi. Eur Polym J 45:237–245. doi:10.1016/j.eurpolymj.2008.10.021
Rahman M, Punja ZK (2005) Biochemistry of ginseng root tissues affected by rusty root symptoms. Plant Physiol Biochem 43:1103–1114. doi:10.1016/j.plaphy.2005.09.004
Raho N, Ramirez L, Lanteri ML, Gonorazky G, Lamattina L, ten Have A, Laxalt AM (2011) Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J Plant Physiol 168:534–539. doi:10.1016/j.jplph.2010.09.004
Rajan A, Kurup JG, Abraham TE (2005) Biosoftening of arecanut fiber for value added products. Biochem Eng J 25:237–242. doi:10.1016/j.bej.2005.05.011
Rakwal R, Tamogami S, Agrawal GK, Iwahashi H (2002) Octadecanoid signaling component “burst” in rice (Oryza sativa L.) seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochem Biophys Res Commun 295:1041–1045. doi:10.1016/S0006-291X(02)00779-9
Ray SD (2011) Potential aspects of chitosan as pharmaceutical excipient. Acta Pol Pharm 68:619–622
Reglinski T, Elmer PAG, Taylor JT, Wood PN, Hoyte SM (2010) Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol 59:882–890. doi:10.1111/j.1365-3059.2010.02312.x
Romanazzi G, Nigro F, Ippolito A, Di Venere D, Salerno M (2002) Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J Food Sci 67:1862–1867. doi:10.1111/j.1365-2621.2002.tb08737.x
Romanazzi G, Mlikota Gabler F, Margosan D, Mackey BE, Smilanick JL (2009) Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopathology 99:1028–1036. doi:10.1094/PHYTO-99-9-1028
Sanford PA (2003) Commercial sources of chitin and chitosan and their utilization. In: Varum KM, Domard A, SmidsrØd O (eds) Advances in Chitin Science, vol 6. NTNU, Trondheim, pp 35–42
Selitrennikoff CL (2001) Antifungal proteins. Appl Environ Microbiol 67:2883–2894. doi:10.1128/AEM.67.7.2883-2894.2001
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7:41–48. doi:10.1016/S1360-1385(01)02187-2
Seyfarth F, Schliemann S, Elsner P, Hipler U-C (2008) Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int J Pharm 353:139–148. doi:10.1016/j.ijpharm.2007.11.029
Shi ZL, Neoh KG, Kang ET, Wang W (2006) Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 27:2440–2449. doi:10.1016/j.biomaterials.2005.11.036
Shibuya N, Minami E (2001) Oligosaccharide signalling for defence responses in plant. Physiol Mol Plant P 59:223–233. doi:10.1006/pmpp.2001.0364
Silva TH, Alves A, Ferreira BM, Oliveira JM, Reys LL, Ferreira RJF, Sousa RA, Silva SS, Mano JF, Reis RL (2012) Materials of marine origin: a review on polymers and ceramics of biomedical interest. Int Mater Rev 57:276–306(31). doi:10.1179/1743280412Y.0000000002
Singla AK, Chawla M (2001) Chitosan: some pharmaceutical and biological aspects-an update. J Pharm Pharmacol 53:1047–1067. doi:10.1211/0022357011776441
Su XW, Zivanovic S, D’Souza DH (2009) Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J Food Protect 72:2623–2628
Sudarshan NR, Hoover DG, Knorr D (1992) Antibacterial action of chitosan. Food Biotechnol 6:257–272. doi:10.1080/08905439209549838
Sun B, Zhang L, Yang L, Zhang F, Norse D, Zhu Z (2012) Agricultural non-point source pollution in China: causes and mitigation measures. Ambio 41(4):370–379. doi:10.1007/s13280-012-0249-6
Sung WS, Lee DG (2010) Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl Chem 82:219–226. doi:10.1351/PAC-CON-09-01-08
Tan HL, Ma R, Lin CC, Liu ZW, Tang TT (2013) Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J Mol Sci 14:1854–1869. doi:10.3390/ijms14011854
Tang H, Zhang P, Kieft TL, Ryan SJ, Baker SM, Wiesmann WP, Rogelj S (2010) Antibacterial action of a novel functionalized chitosan-arginine against gram-negative bacteria. Acta Biomater 6:2562–2571. doi:10.1016/j.actbio.2010.01.002
Tayel AA, Moussa S, Opwis K, Knittel D, Schollmeyer E, Nickisch-Hartfiel A (2010) Inhibition of microbial pathogens by fungal chitosan. Int J Biol Macromol 47:10–14. doi:10.1016/j.ijbiomac.2010.04.005
Tocci N, Simonetti G, D’Auria FD, Panella S, Palamara AT, Valletta A, Pasqua G (2011) Root cultures of Hypericum perforatum subsp. angustifolium elicited with chitosan and production of xanthone-rich extracts with antifungal activity. Appl Microbiol Biotechnol 91:977–987. doi:10.1007/s00253-011-3303-6
Toshkova R, Manolova N, Gardeva E, Ignatova M, Yossifova L, Rashkov I, Alexandrov M (2010) Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int J Pharm 400:221–233. doi:10.1016/j.ijpharm.2010.08.039
Treutter D (2006) Significance of flavonoids in plant resistance: a review. Environ Chem Lett 4:147–157. doi:10.1007/s10311-006-0068-8
Upadhyaya L, Singh J, Agarwal V, Tewari RP (2013) Biomedical applications of carboxymethyl chitosans. Carbohydr Polym 91:452–466. doi:10.1016/j.carbpol.2012.07.076
Vaara M (1992) Agents that increase the permeability of the outer membrane. Microbiol Rev 56:395–411
Vallance J, Déniel F, Floch GL, Guérin-Dubrana L, Blancard D, Rey P (2011) Pathogenic and beneficial microorganisms in soilless cultures. Agron Sustain Dev 31:191–203. doi:10.1051/agro/2010018
Van Loon LC, Van Kammen A (1970) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiunu tubucum var. ‘Samsun’ and ‘Samsun NN’: II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 40:199–201
Vishu KAB, Varadaraj MC, Gowda LR, Tharanathan RN (2005) Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem J 391:167–175. doi:10.1042/BJ20050093
Wang W, Wang SX, Guan HS (2012) The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs 10:2795–2816. doi:10.3390/md10122795
Wiśniewska-Wrona M, Niekraszewicz A, Ciechańska D, Pospieszny H, Orlikowski LB (2007) Biological properties of chitosan degradation products. Polish Chitin Society, Monograph XII, pp 149–156
Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322:681–692
Xing K, Chen XG, Li YY, Liu CS, Liu CG, Cha DS, Park HJ (2008) Antibacterial activity of oleoyl-chitosan nanoparticles: a novel antibacterial dispersion system. Carbohydr Polym 74:114–120. doi:10.1016/j.carbpol.2008.01.024
Xing K, Chen XG, Kong M, Liu CS, Cha DS, Park HJ (2009a) Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Carbohydr Polym 76:17–22. doi:10.1016/j.carbpol.2008.09.016
Xing K, Chen XG, Liu CS, Cha DS, Park HJ (2009b) Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int J Food Microbiol 132:127–133. doi:10.1016/j.ijfoodmicro.2009.04.013
Yang T, Poovaiah BW (2002) Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci U S A 99:4097–4102. doi:10.1073/pnas.052564899
Ye XL, Li XG, Yuan LJ, Ge LH, Zhang BS, Zhou SB (2007) Interaction of houttuyfonate homologues with the cell membrane of gram-positive and gram-negative bacteria. Colloid Surface A 301:412–418. doi:10.1016/j.colsurfa.2007.01.012
Yin H, Li S, Zhao X, Du Y, Ma X (2006) cDNA microarray analysis of gene expression in Brassica napus treated with oligochitosan elicitor. Plant Physiol Biochem 44:910–916. doi:10.1016/j. plaphy.2006.10.002
Yin H, Fretté XC, Christensen LP, Grevsen K (2012) Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J Agric Food Chem 60:136–143. doi:10.1021/jf204376j
Younes I, Hajji S, Frachet V, Rinaudo M, Jellouli K, Nasri M (2014) Chitin extraction from shrimp shell using enzymatic treatment. antitumor, antioxidant and antimicrobial activities of chitosan. Int J Biol Macromol 69:489–498. doi:10.1016/j.ijbiomac.2014.06.013
Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW (2007) Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 25:2085–2094. doi:10.1016/j.vaccine.2006.11.034
Zakrzewska A, Boorsma A, Brul S, Hellingwerf KJ, Klis FM (2005) Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryot Cell 4:703–715. doi:10.1128/EC.4.4.703-715.2005
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31101502, 31370062), the Program of Natural Science Foundation of the Jiangsu Higher Education Institutions of China (11KJD210002), Qing Lan Project of Jiangsu Province (2014), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Xing, K., Zhu, X., Peng, X. et al. Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agron. Sustain. Dev. 35, 569–588 (2015). https://doi.org/10.1007/s13593-014-0252-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13593-014-0252-3