Abstract
The mealybug Planococcus ficus is one of the main vectors of Grapevine leafroll associated virus-3 (GLRaV-3), which was commonly detected in cv “Albariño” planting material before certified stock was available. Mealybug infestations were rare in vineyards in southern Galicia (NW Spain) during the 1990s (2.2% of the vineyards surveyed) and are still rare in inland zones. However, mealybug infestations have spread since 2000, with 15% of surveyed vineyards infested in 2004 and 80% of surveyed vineyards infested in 2016. The spatial and temporal distributions of plants infected with GLRaV-3 were quantified over a 30-year period in an experimental plot established in 1989. The disease progress curve (DPC) was linear for 25 years, with a slow constant rate of spread of less than one newly infected plant per year (0.6%). Since 1992, >82% of infected plants were located on the west side of the plot as were 84% of newly infected plants. Newly infected plants were in contact with infected plants, suggesting vector-mediated transmission, but no potential vectors were found. In 2013, a small mealybug infestation was detected and identified as Pl. ficus. Between 2014 and 2016, the infection rate increased to >21% per year, and in 2019 all plants tested positive for GLRaV-3. This is a valuable case study illustrating how changes to the vector fauna can increase the rate of spread of an economically important virus of grapevine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemics of viral diseases have been widely described in woody plants, and different transmission models have been proposed for predicting changes in disease progress curves (DPCs) (Madden et al. et al., 2017). The best described epidemics of grapevine virus diseases are those caused by grapevine leafroll disease associated viruses, which are transmitted by several species of hemipteran insects. The most widely studied epidemics of leafroll viruses are those involving Grapevine leafroll associated virus-3 (GLRaV-3), although many other Ampelovirus epidemics have been documented in grapevines (Bonfiglioli et al., 2002; Le Maguet et al., 2013; Naidu et al., 2014) and other crops such as pineapple (Selvarajan et al., 2016; Sether et al., 1998).
Insect vectors of ampeloviruses occur in only two families: Pseudococcidae and Coccidae (reviewed by Herrbach et al., 2017). Accordingly, transmission of ampeloviruses does not appear to be specific. The number of vector species in these two families increases each year, and currently includes 10 mealybug species of several genera (Pseudococcus, Planococcus, Phenacoccus, Heliococcus and Ferrisia) and 7 species of soft scale in the genera Pulvinaria, Neopulvinaria, Parthenolecanium, Coccus, Ceroplastes and Parasaissetia (Herrbach et al., 2017). All mealybugs and soft scales that colonize grapevines can be considered potential vectors of ampeloviruses, although dispersal ability and transmission efficiency may differ greatly among species (Almeida et al., 2013).
Several soft scale insects (family Coccidae) act as vectors of GLRaV-3 (Belli et al., 1994) but are considered less epidemiologically important than mealybugs (family Pseudococcidae) for two reasons. First, soft scales have only one (in cool climates) or two generations (in Mediterranean climates) per year (Belli et al., 1993; Maree et al., 2013; Robayo-Camacho & Chong, 2015). Second, soft scales are not very mobile, although crawlers can reach neighboring plants and can also be transported by ants and wind (Hommay et al., 2019). Information about transmission of grapevine-infecting ampeloviruses by soft scale insects is limited due to inherent challenges in handling these highly sedentary insects (Belli et al., 1994; Hommay et al., 2019; Naidu et al., 2014).
Planococcus ficus (Signoret) is an emerging vineyard pest in many countries and is the most widely studied GLRaV-3 vector. In Spain, Pl. ficus mainly occurs in table grapes in the Mediterranean coastal area, but nowadays also appears in wine grapes and in areas where it was previously an unknown pest. Historically, the mealybug species mentioned in Spanish reports was usually Pl. citri (Toledo, 2004). However, more recent reports such as the Integrated Pest Management (IPM) Guide for wine grapes mentions both mealybug species (Martín et al., 2014) and Pl. ficus is now assumed to be dominant (Lucas, 2015). Similarly, Pl. ficus has replaced Pl. citri as the most reported mealybug species in Portugal and Italy, (Godinho & Franco, 2001; Lentini et al., 2008). It is likely that misidentification may often have occurred as the species are difficult to distinguish and mixed infestations are not unusual. Kol-Maimon et al. (2014) found evidence of gene flow between the two sympatric, genetically-related mealybug species, further complicating the situation. Nonetheless, there is evidence that populations of Pl. ficus have established and spread in vineyards in Spain, as has occurred in other regions in Europe, America and North and South Africa (Daane et al., 2012; Daane et al., 2018; Mansour et al., 2017; Walton & Pringle, 2004). In the vineyards established in Galicia (NW Spain) in the 1990s, high prevalence of leafroll viruses was due to lack of available certified virus-free plant material. Very few cases of virus spread were confirmed (Cabaleiro & Segura, 1997) and mealybugs were rarely observed in the vineyards.
For grapevine ampeloviruses, some studies report DPCs for vineyards infected at planting (Arnold et al., 2017; Cabaleiro & Segura, 2006; Goussard & Underhay, 2004), although most studies were initiated when the disease was first detected or after it was clear that the pathogen had been spreading for years (Arnold et al., 2017; Cabaleiro & Segura, 2006; Golino et al., 2008; Jordan et al., 1993; Walker et al., 2004). In some studies, it was not possible to associate pathogen spread with any particular vector species (Dimitrijevic, 1973; Habili et al., 1995; Teliz et al., 1989), similar to the observations made during the first two decades of this study (Cabaleiro et al., 2008; Cabaleiro & Segura, 1997). The existence of vectors other than scale insects has been suggested as a possible cause of pathogen spread when none of the known virus vectors were found (Habili & Nutter, 1997). Another possible cause is natural root grafting between adjacent plants (Epstein, 1978), although this is a rare occurrence that has not been demonstrated in Vitis species. The rate of spread of GLRaV-3 is reported to vary from 0.9% to 16% annually, with an annual average increase of around 7 to 8% (Arnold et al., 2017; Cabaleiro, 2009).
In this paper, we report on the spread of Pl. ficus in southern Galicia. In addition, results from a 30-year-long case study of a GLRaV-3 epidemic in a small plot established in 1989 and monitored since 1991 (Cabaleiro et al., 2008; Cabaleiro & Segura, 1997) is updated. The study findings illustrate the difficulties associated with early detection of vectors and the dramatic increase in GLD since the appearance of Pl. ficus.
Materials and methods
Survey of insect vectors
Vineyards supplying grapes to the five wine appellations in Galicia were surveyed in three phases (Fig. 1). In the first phase (1990–1993) 92 vineyards in the Rías Baixas and Valdeorras areas were surveyed. In the second phase (2003–2004), 126 vineyards in the Rías Baixas, Ribeiro, Ribeira Sacra and Monterrei areas were surveyed. Finally, in the most recent phase (2014–2016) 84 vineyards in the Rías Baixas, Ribeiro, Ribeira Sacra, Monterrei and Valdeorras areas were surveyed. The main objective of the surveys was to determine presence or absence of mealybugs and soft scale insects. Surveys were conducted by examining at least 20 vines for the presence of ants (known to obtain honeydew by tending mealybugs). Each vine was examined for one minute and if no ant movement was detected, the search on that plant was stopped. If ants were observed, the vine was then searched for mealybugs for an additional 1–3 minutes (depending on the age/size of the grapevine and the type of trellis system used).
Map showing the five wine growing areas with appellation of origin status in NW of Spain. Downloaded from https://portalgalicia.com/galicia/vinos-gallegos-5-denominaciones-de-origen/
Mealybug identification
In 1992, the species identity of collected mealybugs was determined by experts using morphological characteristics. In 2014, mealybug samples from several vineyards were identified by targeted DNA analysis using multiplex-polymerase chain reaction (PCR) (ten insects from each population). DNA was extracted from insect samples following the method described by Koekemoer et al. (2002). The multiplex-PCR method was based on the protocol and primers described by Saccaggi et al. (2008): C1-J-2427-F 5’-TAATTATTGCTATTCCTACAAGAATTAAAATC-3′ which produces a 587 base pair (bp) amplicon for Pl. citri, C1-J-2260-F 5’-TCAAATTATAAATCAAGAAAGGGGAAAAC-3′ which produces a 757 bp amplicon for Pl. ficus, and TL2-N-3014-R 5’-TCCAATGCACTAATCTGCCATATTA-3′ which is a common primer for both species (Simon et al., 1994).
A case study: 30 years of spread of GLRaV-3 in Meaño
Vineyard
The experimental plot was located in Meaño, Pontevedra, Galicia, NW Spain (42.433863°N, 8.772683°W) and occupies about 1500 m2 inside the vineyard (2.206,62 m2). The plot has previously been described in detail (Cabaleiro et al., 2008; Cabaleiro & Segura, 1997). In 1990, 160 scions from 4 indexed virus-free Albariño clones obtained from the collection belonging to the CSIC (Consejo Superior de Investigaciones Científicas) were grafted onto 196.17C or SO4 certified virus-free rootstocks planted the previous year in 8 rows of 20 plants. After 2005, a number of plants were uprooted by the owner due to problems related to vigour of the Albariño cultivar; a total of 63 plants were removed, 19 of which were positive for GLRaV-3. In the following years, 5 more plants were confirmed infected with GLRaV-3 were removed: 2 in 2009, 2 in 2014 and 1 in 2016. During the first years after vine removal, rows with missing vines were clearly visible. However, vines grew to cover the empty spaces on the horizontal trellis, facilitated by yearly pruning practices.
Virus testing
Up to 2006, all 160 plants in the Meaño plot were tested for GLRaV-3 by DAS-ELISA. After 2006, plants which tested negative in previous analyses were tested by tissue printing ELISA using the protocol described by Cabaleiro et al. (2008). Vines were sampled in late summer by collecting two mature leaves with their petioles from each vine. Antibodies and buffers were obtained from Bioreba AG (Basel, Switzerland) and nitrocellulose membranes (0.45 μm pore size) from Sartorius (Goettingen, Germany). BCIP-NBT ready-to-use liquid substrate was obtained from SIGMA (B-1911). Virus testing was conducted in 1992–1996 / 2003–2007 / 2009 / 2014 /2016–2019.
Vector surveys
During sampling for viral testing, all vines were searched for ants as an indication of presence of hemipteran vectors. In 2016, the two mature leaves collected from each vine for ELISA testing were first examined under a stereomicroscope for presence of mealybug nymphs (crawlers). In June 2017, all vines were thoroughly checked (trunk, branches, leaves) for mealybugs and a map was drawn of the whole plot, including border plants showing the spatial distribution of mealybug infestations.
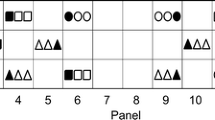
In 2016 a Delta trap containing pheromone specific for Pl. ficus (Suterra® Europe) was placed in the centre of the plot (for subsequent comparison of captures with those obtained in traps in other vineyards in southern Galicia). In 2017, six Delta traps were used to monitor the male flight activity weekly during the whole season (Fig. 2). In addition, a Delta trap was placed in a neighbouring plot with young vines (200 m away) belonging to the same vineyard owner, and another trap was placed in a small century-old vineyard that was >1 km away from the experimental plot. Pheromone capsules were replaced every 6 weeks.
Temporal analysis of the epidemic
Disease progress was quantified 16 times between 1989 and 2019, and disease incidence data were used to construct a disease progress curve (DPC). For DPC modelling, we fitted linear (untransformed data), exponential, logistic and Gompertz models (Madden et al., 2017). The goodness of fit of the linearized form of each model was assessed using the coefficient of determination (R2) and the standard error for the estimation of y (EEEy). Models were fit for: a) the whole period (1992 to 2019), b) the period between 1992 and 2014 (prior to observation of Pl. ficus in the experimental vineyard), and the period between 2014 and 2019 (after Pl. ficus was observed in the experimental vineyard). The removal of a number of healthy and infected plants after 2005 caused some interference in data collection, but the DPC was not significantly affected. Rates of disease spread were estimated from the slope of the regression lines of the model that provided the best fit for the DPC.
Spatial distribution of diseased plants
Maps of the spatial distribution of vines infected with GLRaV-3 were drawn based on the results of the surveys described above. Analysis of the spatial distribution of infected vines was affected by vine removal. Canopies quickly filled in after vines were removed, making it difficult to determine which canes were associated with which vine as the canopies were interlocking. Due to the continuous canopy, unidimensional analyses were inappropriate. Instead, the occurrence of newly infected vines in direct contact with known infected vines was determined for each year. Bidimensional analysis was performed by successively dividing the vineyard into subplots of increasing size, calculating the mean number of infected vines in each subplot and using these data to calculate Morisita’s index of dispersion (Campbell and Madden, 1990). The statistical significance of deviations in Morisita’s index from unity was estimated with the WinPatchy program (M. Maixner, version 3.2.9). The size of the units tested for clustering ranged from 2 × 2 to 8 × 8 m. However, because of the large number of missing vines and empty quadrats, the tolerance was changed to 25% and the “minimum number of units required for statistics” to 6 (M. Maixner, personal communication), the results of this analysis should be considered an approximation for comparison with previous published data in Cabaleiro & Segura, 1997 (1992–1995) and Cabaleiro et al., 2008 (1995–2005).
To determine if infected vines are more common on the edge or interior of vineyards, the GLRaV-3 status of each plant, for a given year, was subjected to regression analysis with column (1–20) or row (1–8) number as an independent variable (x) and the incidence of disease in that column or row as the dependent variable (y). Significant deviation of the slope from 0 was checked by a t-test and a test for linearity, performed by calculating the coefficient of correlation and conducting an F-test. Regression lines with slopes significantly different from 0 were considered to indicate the existence of a disease gradient in the direction corresponding to the independent variable. New positives in west or east zones of the plot were also counted since 1992 and in three phases: one before plant removal (1993–2005), and two after removal (2006–2014 and 2015–2019).
Results
Vector surveys
In the first survey, mealybugs were found at 2 locations in the Rías Baixas: Beluso (5 vineyards from the same winery) and one in O Rosal (one vineyard). In O Rosal, a large population was found with honeydew and sooty mould on some leaves in a vineyard near the Miño river on the border with Portugal. In Beluso, the populations were small, but the insect was widely distributed across all vineyards on a single property. The infested vineyards were isolated from other vineyards and surrounded by eucalyptus plantations.
In the second survey, mealybugs and soft scale insects (Parthenolecanium spp., Pulvinaria spp.) were found in 6.1 and 14% of surveyed vineyards, respectively. In the most recent survey, the inland wine-growing areas, least strongly influenced by the Atlantic climate (Ribeira Sacra, Monterrei and Valdeorras), appeared to be free of mealybugs, and soft scale insects were only found in 2 vineyards. By contrast, in the Ribeiro and Rías Baixas areas, the number of vineyards with mealybugs has increased to the point that in some locations, such as O Condado, it is now difficult to find mealybug-free vineyards, and some growers are expressing concern about the presence of ants, honeydew and sooty mould on the grapes (Tables 1 and 2).
Vector identification
Prior to the development of molecular techniques, identification of the mealybugs was uncertain (Table 3). It is difficult to distinguish Pl. ficus from Pl. citri using morphological characters and even when the species are reared with care, contamination easily occurs. The mealybugs detected in Beluso in the 1990s were identified as Pl. citri by several authors (Cabaleiro & Segura, 1997; Cid et al., 2010). The molecular analysis conducted in the third phase of the survey (2014) revealed that almost 100% of the mealybugs were Pl. ficus (Table 3). Pseudococcus viburni was identified, in mixed populations with Pl. ficus, in two vineyards. The citrus mealybug was identified only in one vineyard, also in mixed populations with Pl. ficus. In all the vineyards with Pl. ficus, leafroll disease symptoms were widely distributed, and GLRaV-3 was detected (Table 3). Ants (Hymenoptera: Formicidae) from several locations were sent to an expert (K. Gómez) for identification. The following species were detected: Lasius niger, L. grandis, Crematogaster scutellaris, Tetramonium caespitum_species group, Formica cunicularia, and of concern, Linepithema humile, the Argentine ant, a known invasive species, was found in O Ribeiro and O Rosal.
Vectors in Meaño
During the successive sampling carried out at the end of the summer between 1990 until 2009, no ants or insects known to act as GLRaV-3 vectors were observed on vine trunks or leaves. During the unplanned visit in summer 2013 (the first since 2009), ants and mealybugs were observed on a small group of vines on the west side of the plot. Mealybugs were collected and identified by multiplex-PCR as Pl. ficus. The same species was subsequently also found in several other vineyards belonging to the same owner within a radius of 1 km of the Meaño vineyard.
In 2016, evaluation of leaf samples determined that 15% of vines had nymphs on at least one of the collected leaves. The full survey of the plot in July 2017 (plot and borders) detected the presence of Pl. ficus on 43 vines (33%), most of which were located on the east and south sides of the plot (Fig. 3). Fewer than 10 mealybugs were observed on 63% of infested vines. The majority of mealybugs (95%) were observed on trunks and branches, with only 5% found on leaves. Ants were observed on 39% of vines and on 100% of vines infested with mealybugs. The ant species observed during the surveys were identified as Lasius grandis and F. cunicularia. In the survey conducted during the summer of 2017, some adult females (with ovisacs) of the scale Pulvinaria vitis
In 2016, Delta traps containing Pl. ficus pheromone captured a large number of male Pl. ficus, with >1600 captured between June and September. In 2017, rates of pheromone trap capture were high, with more than 2600 males captured per trap for a total of 15,673 males captured in the study plot. The flight curve suggested 3–4 overlapping generations over a long season, with captures occurring through November (Fig. 4). In 2017, additional traps were monitored through October, one at a vineyard that was 200 m from the study plot and one in a century-old vineyard. The trap at the nearby vineyard captured 3357 males and the trap in the century-old vineyard captured 1951 males.
Temporal analysis of virus spread
The epidemic was characterised by two phases: a phase of long, slow increase occurring from 1992 to 2014 and a phase of rapid increase occurring between 2014 and 2019 (Fig. 5). Of the models tested, the exponential model best described disease progress from 1992 until 2019 (Table 4) but the explanatory power of the model was poor, with high EEEy and significant lack of fit due to bias that gives a not random residual pattern. Accordingly, evaluating the two distinct phases of the epidemic (1992–2014 and 2014–2019) provides a better description of events. The DPCs for both phases were almost linear (Table 4), with an annual rate of increase of 0.6% during the first phase (1992–2014) and an annual rate of increase of 14.1% during the second (2014–2019). The rate of spread peaked between 2014 and 2016 (21.7%). The DPC was not affected by the uprooting of plants at the end of 2005, as indicated by the similar proportion of healthy and infected plants before and after this event.
Spatial analysis of virus spread
Between 1992 and 2014, newly positive vines were most commonly observed on the west side of the plot around the area where infected vines were first detected in 1992 (Fig. 6 and Table 5). The observation of a west to east gradient was supported by the significantly negative slope of the regression lines for position and incidence per column and with intercepts of those lines increasing almost until the end of the epidemic (Table 6). Similar tests found no evidence for a north to south gradient (data not shown).
Although some vines on the east side tested positive for GLRaV-3 in 2005, in subsequent tests (2006 and later), these vines were always negative for the virus until 2016. Accordingly, detections on the east side in 2005 were assumed to be false positives and that the first detection of a positive plant on the east side of the plot occurred in 2009. Between 2006 and 2014 the number of newly infected vines on the west (4) and the east (3) sides of the plot were similar. After 2015, transmission had almost stopped on the west side of the plot, whereas spread was rapid on the east side of the plot (Fig. 5, Table 5). Throughout the epidemic, most new infections (84% of infections averaged over all years) were found contiguous to known positive vines. However, new infections detected between 2014 and 2016, when the rate of spread of the virus was highest (21.7%), had the lowest association with known infected vines. Specifically, from 2014 to 2016 only 74.2% of newly infected vines were in contact with a known infected vine (Table 5). Morista’s index of dispersion indicated that infected vines were aggregated (> 1) from 1992 to 2015, until the final phase of the epidemic (2015–2019) when nearly all vines on the west side were infected and number of infected vines on the east side was increasingly rapidly (Table 5).
Discussion
Pl. ficus is no longer an occasional or secondary pest in the south of Galicia. In fact, Pl. ficus has become the dominant mealybug species, with Pl. citri now absent in vineyards even when it is known to occur in nearby lemon and orange trees (captured in pheromone traps). PCR-based methods for identifying mealybug species were useful for preventing misidentification and determining if populations were mixed. Mixed populations were observed in two vineyards, one in Beluso (Cid et al., 2010) and the other in Ribeiro. In Ribeiro, Pl. ficus was the more abundant species and was observed in grape bunches and being tended by the Argentine ant. The first observations of Pl. ficus in Galicia were reported during the first decade of the twenty-first century, possibly in association with climate change, similar to reports from other grape growing regions in Spain and other countries (Daane et al., 2008; Lucas, 2015; Walton & Pringle, 2004). Molecular studies evaluating Pl. ficus populations from grape growing countries outside of Europe found that populations belonged to two major divisions: a European grouping (Europe, Tunisia, Turkey) and a Middle Eastern grouping (Israel and Egypt) (Daane et al., 2018). The existence of distinct populations suggests there may also be differences in behaviour and vector competency. Movement of Pl. ficus may be facilitated by inadvertent shipping of infested plant material from nurseries to vineyards or if plant material is moved within vineyards.
Although Pl. ficus is a common pest, the damage is not perceived as harmful in Galicia because sooty mould does not appear in grape bunches until after harvest. However, winegrowers with vineyards close to the river Miño, near the border with Portugal, are becoming concerned as issues such as early defoliation and poor vine vigour appear to be increasing. In addition, infestation of wine grapes with mealybugs has been shown to be associated with negative descriptors and lower quality in sensory analyses (Bordeu et al., 2012).
Delta traps containing pheromones specific for Pl. ficus are valuable tools for monitoring mealybug populations in and around vineyards. The large numbers of males captured in the small vineyard in Meaño (>15,000) indicated that many insects are arriving from outside the vineyard and therefore that the insect is likely widespread within the zone. In some new and replanted vineyards, pheromone traps are already being used for the early detection of mealybugs (Vilas, 2020), as done in other countries (Daane et al., 2008, 2012, 2020; Mansour et al., 2017).
Although mealybugs have been recognized as a pest for the past decade, control measures are only now being implemented. Few insecticides are authorized, and their efficacy is questionable (Mansour et al., 2018). Further, application of insecticides can disrupt management of other pests. Several countries are facing similar problems regarding the active fight against mealybugs to prevent infestation of the new plantations established with certified leafroll free vines (Daane et al., 2020; Le-Vieux & Malan, 2013). As alternatives to insecticides, biological control, mating disruption and several cultural measures each have little effect alone, although they can be effective when used as part of in an integrated pest management (IPM) approach (Daane et al., 2008; Mansour et al., 2017; Vilas, 2020).
The grapevine growing area now colonized by Pl. ficus in Galicia was established in the 1990s with plant material mostly obtained from vineyards in O Salnés (Fig. 1), where the “Albariño” expansion had begun earlier, in the 1970s. In small vineyards it was common to plant rootstocks and graft them in the field but more often buds taken randomly from pruning wood were sent to nurseries to be grafted onto certified rootstocks. According to our surveys in the 1990s, GLRaV-3 was present in most vineyards in O Salnés, with an average of 25–35% of the plants infected; meaning that the new vineyards would have had high incidence at establishment (Segura et al., 1993), a level now considered economically damaging (Bell et al., 2018). Currently, leafroll is sufficiently common that many growers considered it a cultivar characteristic, as we were able to verify during the field sessions of the PAThOGEN course on grapevine viruses (Di Gaspero et al., 2018). Awareness of the risk posed by mealybugs and GLD is increasing, particularly as high levels of GLD could lead to the uprooting of young vines (< 25 years old) and additional losses due to delays in ripening associated with infection (Song et al., 2021).
The virus was presumed to be spread by vectors even during the long first phase of the epidemic (1992–2014). Initially, most infected plants were located on the west side of the plot, only 3 years after grafting. It is possible that infections were present in the rootstocks on which healthy clones were grafted in 1990. (Cabaleiro & Segura, 1997). During the interval from 1993 to 2014 infected plants continued to appear mainly on the west side. Finally, during the last phase (2014–2019), infections rose rapidly on the east side of the plot. Consistent with vector transmission, 84% of newly infected plants were adjacent to at least one known infected plant across the whole study period. In contrast, Sokolsky et al. (2013) found that with large insect populations similar numbers of distant and adjacent plants were infected. In the present case, infection of distant plants was rare, possibly due to the small vector population and horizontal trellis, which reduced the likelihood of crawler spread being aided by wind or wind generated by the air-blast sprayer equipment regularly used in vineyards.
On the east side of the plot, the first new positive plant was not detected until 2009 (Fig. 6). The rate of virus spread was greatest between 2014 and 2016 (21.7%) and the average rate of spread was 14.14% between 2014 and 2019 (Fig. 5). These rates of spread are faster than those reported for other leafroll epidemics (Cabaleiro, 2009) except for Jordan et al. (1993) which reported a rate of spread of 16.8% during a 5-year epidemic. The shape of the DPC described in this study is unusual as it shows two phases with different transmission rates (Fig. 5). High rates of spread at the end of the epidemic were likely due to mealybugs becoming widespread throughout the plot.
We do not know when Pl. ficus first appeared in the plot, despite exhaustive searches when the disease was first suspected. The initial colonization of the study plot by Pl. ficus likely occurred between the last assessment of field collected leaves in 2009 and the discovery of Pl. ficus in 2013. However, as Pl. ficus is not easy to detect, small populations may have persisted hidden under the bark for some time.
The initial slow spread typical of the sigmoid type DPC that usually describes this kind of plant virus epidemic may be longer than expected due to the previously mentioned uprooting of plants between 2005 and 2007. However, even without that disturbance, the initial phase was already unusually long (between 1992 and 2005), with an annual increase of only 0.9% of plants infected each year. In a vineyard in South Australia with GLD incidence at the time of planting of 23.1%, Habili and Nutter (1997) reported no transmission at all for 6 years and then transmission apparently began in the absence of vectors. In the present study, the early phase of the epidemic was well described by a linear model until 2014 (R2 = 0.997). The logistic and Gompertz models did not perform well for describing the epidemic, even though they have performed well in most GLD epidemics described (Arnold et al., 2017; Cabaleiro, 2009; Habili & Nutter, 1997). In our previous papers reporting field data obtained up to 2005, we speculated that the slow plant to plant transmission indicated either occasional root grafting (Epstein, 1978) or vector transmission by soft scale insects rather than by mealybugs. Although we did not observe the soft scale insect Pulvinaria vitis until 2017, very small (undetected) populations may have been responsible for the slow spread of the virus from 1992 through 2014.
In summary, prior to the detection of vectors, spread of GLRaV-3 was slow. However, once vectors were observed, pathogen spread was rapid. The initial slow phase of the epidemic was presumed to be caused by less efficient vectors such as scale insects, whereas the rapid phase of the epidemic was due to presence of Pl. ficus. Nonetheless, results from this study support the observation that all types of scale insect should be monitored, and that early detection of vector populations is crucial to limiting virus transmission (Almeida et al., 2013). As in most winegrowing regions, the economic margins in the Rías Baixas are increasingly narrow and it is essential to lower costs and guarantee, as far as possible, the stability of harvests, in terms of both quantity and quality (Cabaleiro et al., 2014). The presence of leafroll viruses and their vectors is a destabilising factor. New vineyards should be planted with certified virus-free vines and vineyards should be routinely surveyed for presence of vectors and diseased vines. To our knowledge this is the longest epidemic of GLD described to date. The findings of this long-term case study serve as a warning of how the dispersal of one or several GLRaV-3 vectors can exacerbate the economic impact of GLD.
Data availability
Not applicable.
References
Almeida, R., Daane, K. M., Bell, V., Blaisdell, G., Cooper, M., Herrbach, E., et al. (2013). Ecology and management of grapevine leafroll disease. Frontiers on Microbiology, 4, 94. https://doi.org/10.3389/fmicb.2013.00094
Arnold, K., Golino, D. A., & McRoberts, N. (2017). A synoptic analysis of the temporal and apatial aspects of grapevine Leafroll disease in a historic Napa vineyard and experimental vine blocks. Phytopathology, 107, 418–426. https://doi.org/10.1094/PHYTO-06-16-0235-R
Bell, V. A., Hedderley, D. I., Pietersen, G., & Lester, P. J. (2018). Vineyard-wide control of grapevine leafroll-associated virus 3 requires an integrated response. Journal of Plant Pathology, 100, 399–408.
Belli, G., Fortusini, A. & Prati, S. (1993). Natural spread of grapevine leafroll disease in a vineyard of Northern Italy. Extended Abstracts of the XI Meeting of the International Council for the Study of Viruses and Virus-like Disease of the Grapevine, Montreux (Suisse), 110–111.
Belli, G., Fortusini, A., Casati, P., Belli, L., Bianco, P. A., & Prati, S. (1994). Transmission of a grapevine leafroll associated closterovirus by the scale insect Pulvinaria vitis L. Rivista di Patologia Vegetale, 4, 105–108.
Bonfiglioli, R., Hoskins, N., & Edwards, F. (2002). Grapevine leafroll virus type 3 spreading in New Zealand. Australian and New Zealand Grapegrower and Winemaker, 457, 58–61.
Bordeu, E., Troncoso, D. O., & Zaviezo, T. (2012). Impact of mealybug (Pseudococcus spp.) infested bunches on wine quality in Carmenere and chardonnay grapes. International Journal of Food Science and Technology, 47, 232–239. https://doi.org/10.1111/j.1365-2621.2011.02830.x
Cabaleiro, C. (2009). Current advances on the epidemiology of grapevine leafroll disease. Extended Abstracts of the XVI Meeting of the International Council for the study of the virus and virus-like diseases of the grapevine, Dijon (France), 264–268.
Cabaleiro, C., & Segura, A. (1997). Field transmission of grapevine leafroll associated virus 3 by Planococcus citri Risso. Plant Disease, 81, 283–287.
Cabaleiro, C., & Segura, A. (2006). Temporal analysis of GLRaV-3 epidemics. European Journal of Plant Pathology, 114, 441–446.
Cabaleiro, C., Couceiro, C., Pereira, S., Cid, M., Barrasa, M., & Segura, A. (2008). Spatial analysis of epidemics of grapevine leafroll associated virus-3. European Journal of Plant Pathology, 121, 121–130. https://doi.org/10.1007/s10658-006-0006-4
Cabaleiro, C., Pesqueira, A. M., Barrasa, M., & García-Berrios, J. J. (2014). Analysis of the losses due to grapevine leafroll disease in Albariño vineyards in Rías Baixas (Spain). Ciência e Técnica Vitivinícola, 28(2), 43–50.
Campbell, C. L. & Madden, L. V. (1990). Introduction to plant disease epidemiology. John Wiley & Sons.
Cid, M., Pereira, S., Cabaleiro, C., & Segura, A. (2010). Citrus mealybug (Hemiptera: Pseudococcidae) movement and population dynamics in an arbor-trained vineyard. Journal of Economic Entomology, 102(3), 619–630. https://doi.org/10.1603/EC09234
Daane, K. M., Cooper, M., Triapitsyn, S., Walton, V., Yokota, G., Haviland, D., Bentley, W., Godfrey, K., & Wunderlich, L. (2008). Vineyard managers and researchers seek sustainable solutions for mealybugs, a changing pest complex. California Agriculture, 62(4), 167–176. https://doi.org/10.3733/ca.v062n04p167
Daane, K. M., Almeida, R. P., Bell, V. A., Walker, J. T. S., Botton, M., et al. (2012). Biology and Management of Mealybugs in vineyards. In: N.J. Bostanian, C. Vincent and R. Isaack (Eds.) arthropod Management in Vineyards: Pests, approaches, and future directions. Springer science+business media B.V. Chapter, 12, 271–307.
Daane, K. M., Middleton, M. C., Sforza, R. F. H., Kamps-Hughes, N., Watson, G. W., Almeida, R. P. P., Correa, M. C. G., Downie, D. A., & Walton, V. M. (2018). Determining the geographic origin of invasive populations of the mealybug Planococcus ficus based on molecular genetic analysis. PLoS One, 13(3), e0193852. https://doi.org/10.1371/journal.pone.0193852
Daane, K. M., Yokota, G. Y., Walton, V. M., Hogg, B. N., Cooper, M. L., Bentley, W. J., & Millar, J. G. (2020). Development of a mating disruption program for a mealybug, Planococcus ficus, in vineyards. Insects, 11, 635. https://doi.org/10.3390/insects11090635
Di Gaspero, M., Guinard, J., Pesqueira, A.M., Angelini, E. et al. (2018). The PAThOGEN project: A new approach to improve grapevine virus knowledge and management. Extended abstracts of the XIX meeting of the International Council for the Study of viruses and virus-like disease of the grapevine, Santiago (Chile),130-131.
Dimitrijevic, B. (1973). Some observations on natural spread of grapevine leafroll disease in Yugoslavia. Rivista di Patologia Vegetale, 9(S.IV), 114–119.
Epstein, A. H. (1978). Root graft transmission of tree pathogens. Annual Review of Phytopathology, 1, 181–192.
Godinho, M. A., & Franco, J. C. (2001). Survey on the pest status of mealybugs in Portuguese vineyards. Integrated Control in Viticulture IOBC wprs Bulletin, 24(7), 221–225.
Golino, D. A., Weber, E., Sim, S., & Rowhani, A. (2008). Leafroll disease is spreading rapidly in a Napa Valley vineyard. California Agriculture, 62, 156–160. https://doi.org/10.3733/ca.v062n04p156
Goussard, P. G., & Underhay, J. P. (2004). Leafroll transmission and characterisation of infected vines (cabernet sauvignon/Richter 99) at Welgevallen experimental farm. A case study. Wynboer, 176, 84–87.
Habili, N., & Nutter, F. W. (1997). Temporal and spatial analysis of grapevine leafroll-associated virus 3 in pinot noir grapevines in Australia. Plant Disease, 81, 625–628.
Habili, N., Fazeli, C. F., Ewart, A., Hamilton, R., Cirami, R., & Saldarelli, et al. (1995). Natural spread and molecular analysis of grapevine leafroll-associated virus 3 in Australia. Phytopathology, 85, 1418–1422.
Herrbach, E., Alliaume, A., Prator, C. A., Daane, K. M., Cooper, M. L., & Almeida, R. P. (2017). Vector transmission of grapevine Leafroll-sssociated viruses. In B. Meng et al. (Eds.), Grapevine viruses: Molecular biology, Diagnostics and Management (pp. 483–503). Springer International Publishing AG. https://doi.org/10.1007/978-3-319-57706-7_24
Hommay, G., Wiss, L., Chadoeuf, J., Le Maguet, J., Beuve, M., & Herrbach, E. (2019). Gone with the wind: Aerial dispersal of Parthenolecanium corni crawlers in a newly planted grapevine plot. Annals of Applied Biology, 174(3), 372–387. https://doi.org/10.3390/v12121447
Jordan, D., Petersen, C., Morgan, L., & Segaran, A. (1993). Spread of grapevine leafroll associated virus in New Zealand vineyards. Extended Abstracts of the XI Meeting of the International Council for the Study of Viruses and Virus-like Disease of the Grapevine, Montreux (Switzerland), 113–114.
Koekemoer, L., Kamau, L., Hunt, R. H., & Coetzee, M. (2002). A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. The American Journal of Tropical Medicine and Hygiene, 6, 804–811.
Kol-Maimon, H., Ghanim, M., Franco, J. C., & Mendel, Z. (2014). Evidence for gene flow between two sympatric mealybug species (Insecta; Coccoidea; Pseudococcidae). PLoS One, 9(2), e88433. https://doi.org/10.1371/journal.pone.0088433
Le Maguet, J., Fuchs, J. J., Chadoeuf, J., Beuve, M., Herrbach, E., & Lemaire, O. (2013). The role of the mealybug Phenacoccus aceris in the spread of grapevine leafroll-associated virus −1 (GLRaV-1) in two French vineyards. European Journal of Plant Pathology, 135, 415–427. https://doi.org/10.1007/s10658-012-0099-x
Lentini, A., Serra, G., Ortu, S., & Delrio, G. (2008). Seasonal abundance and distribution of Planococcus ficus on grapevine in Sardinia. Integrated Protection in Viticulture IOBC/wprs Bulletin, 36, 267–272.
Le-Vieux, P., & Malan, A. (2013). An overview of the vine mealybug (Planococcus ficus) in south African vineyards and the use of entomopathogenic nematodes as potential biocontrol agent. South African Journal for Enology and Viticulture, 34(1), 108. https://doi.org/10.21548/34-1-1086
Lucas, A. (2015). Plagas en expansión: araña amarilla, mosquito verde y melazo de la vid. Phytoma-España, 274, 32–35.
Madden, L. V., Hughes, G., & Van der Bosch, F. (2017). The study of plant disease epidemics. APS Press. https://doi.org/10.1094/9780890545058
Mansour, R., Kaouthar, G. L., Mounir, K., Imed, C., Imen, T., Ameur, S., & Marti, S. (2017). Pheromone-mediated mating disruption of Planococcus ficus (Hemiptera: Pseudococcidae) in Tunisian vineyards: Effect on insect population and dynamics. Biologia, 72(3), 333–341. https://doi.org/10.1515/biolog-2017-0034
Mansour, R., Belzunces, L. P., Suma, P., et al. (2018). Vine and citrus mealybug pest control based on synthetic chemicals. A review. Agronomy for Sustainable Development, 38(4), 1–20. https://doi.org/10.1007/s13593-018-0513-7
Maree, H. J., Almeida, R. P. P., Bester, R., Chooi, K., Cohen, D., Dolja, V. V., ... & Burger, J. (2013). Grapevine leafroll-associated virus 3. Frontiers in Microbiology, 4, 82–93. https://doi.org/10.3389/fmicb.2013.00082.
Martín, A., Ramos, J.L. & Rodríguez, M. (Eds). (2014). Guía De Gestión Integrada de Plagas: uva de vinificación. Ministerio de Agricultura, Alimentación y Medio ambiente, Madrid, 201 pp.
Naidu, R., Rowhani, A., Fuchs, M., Golino, D., & Martelli, G. P. (2014). Grapevine leafroll: A complex viral disease affecting a high-value fruit crop. Plant Disease, 98(9), 1172–1185. https://doi.org/10.1094/PDIS-08-13-0880-FE
Robayo-Camacho, E., & Chong, J. H. (2015). General biology and current management approaches of soft scale pests (Hemiptera: Coccidae). Journal of Integrated Pest Management, 6(1), 17. https://doi.org/10.1093/jipm/pmv016
Saccaggi, D. L., Kruger, K., & Pietersen, G. (2008). A multiplex PCR assay for the simultaneous identification of three mealybug species (Hemiptera: Pseudococcidae). Bulletin of Entomological Research, 98, 27–33. https://doi.org/10.1017/S000748530700538X
Segura, A., González, M.L. & Cabaleiro, C. (1993). Presence of grapevine leafroll in north West Spain. Extended Abstracts of the XI Meeting of the International Council for the Study of Viruses and Virus-like Disease of the Grapevine, Montreux (Switzerland), 125–126.
Selvarajan, R., Balasubramanian, V., & Padmanaban, B. (2016). Mealybugs as vectors. In M. Mani & C. Shivaraju (Eds.), Mealybugs and their Management in Agricultural and Horticultural crops. Springer.
Sether, D., Ullman, D. E., & Hu, J. S. (1998). Transmission of pineapple mealybug wilt – Associated virus by two species of mealybug (Dysmicoccus spp). Phytopathology, 88, 1224–1230.
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., & Flook, P. (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 87, 651–701.
Sokolsky, T., Cohen, Y., Zahavi, T., Sapir, G., & Sharon, R. (2013). Management efficiency of grapevine leafroll disease. Australian Journal of Grape and Wine Research, 19, 431–438. https://doi.org/10.1111/ajgw.12037
Song, Y., Hanner, R. & Meng, B. (2021). Probing into the effects of grapevine leafroll-associated viruses on the physiology, fruit quality and gene expression of grapes. Viruses, 13(4), 593. https://doi.org/10.3390/v13040593
Teliz, D., Gonsalves, D., Hu, J. & Hummer, D.K. (1989). Detection of grapevine leafroll-associated closteroviruses in recently infected tissues in New York and spread of the disease in Mexico. Extended Abstracts of the IX Meeting of the International Council for the Study of Viruses and Virus-like Disease of the Grapevine, Kiryat Anavim, 109–115.
Toledo, J. (2004). Melazo. In: Los Parásitos de la Vid. Mundi-Prensa, Madrid.
Vilas, R. (2020). Monitoring and control of Planococcus ficus sig. (Hemiptera: Pseudococcidae) in a vineyard of Albariño in the D.O. Rías Baixas in O Rosal: Study of epidemiological conditions. Master thesis for Agriculture Engineering, University of Santiago de Compostela.
Walker, J.T.S., Charles, J.G., Froud, K.J. & Connolly, P. (2004). Leafroll virus in vineyards, modelling the spread and economic impact. Report to New Zealand winegrowers limited. HortResearch, client report 12795, 19 pp.
Walton, V. M., & Pringle, K. L. (2004). Vine mealybug, "Planococcus ficus" (Signoret) (Hemiptera: Pseudococcidae), a key pest in south African vineyards: A review. South African Journal of Enology and Viticulture, 25(2), 54–62. https://doi.org/10.21548/25-2-2140
Acknowledgements
We particularly thank Gerardo Méndez, the vineyard owner, for helpful advice and maintenance of the vineyard, Kiko Gómez for identification of the ants, Marta Araujo for extra information about the mealybugs in vineyards in O Condado (Rías Baixas D.O.). Thanks to Michael Maixner for giving us his new version of Patchy and his help with the interpretation of results. And special thanks to R. Sharon and company because it was during their visit in 2013 that we returned to Meaño after 5 years and discovered the focus of mealybug infestation.
Code availability
Not applicable.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This long-term study was partly funded by grants obtained from diverse institutions already mentioned in several papers; in the last 12 years with public and private resources from the Vitis Research group (GI1988), USC. Suterra® provided the Delta traps and pheromone capsules in 2016 and 2017.
Author information
Authors and Affiliations
Contributions
Conceptualization: Cabaleiro and A. Segura; Methodology: C. Cabaleiro and A. Pesqueira. Formal analysis and investigation: C. Cabaleiro, A. Segura and A. Pesqueira. Writing - original draft preparation: C. Cabaleiro. Funding acquisition: C. Cabaleiro and A. Segura. All authors contributed to writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cabaleiro, C., Pesqueira, A.M. & Segura, A. Planococcus ficus and the spread of grapevine leafroll disease in vineyards: a 30-year-long case study in north-West Spain. Eur J Plant Pathol 163, 733–747 (2022). https://doi.org/10.1007/s10658-022-02513-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02513-x