Abstract
To determine the occurrence and distribution of prevalent viruses in commercially important vineyards, a survey was carried out in all thirteen wine-growing regions in Germany. Results reveal that the recently emerged Grapevine pinot gris virus (GPGV) was the most abundant virus with a percentage of 18% prevalence, followed by 13% Grapevine fleck virus (GFkV), 9% Grapevine leafroll-associated virus 1 (GLRaV-1), 4% Grapevine fanleaf virus (GFLV), 2% Raspberry ringspot virus (RpRSV), 2% Arabis mosaic virus (ArMV) and 2% Grapevine leafroll-associated virus 3 (GLRaV-3). Distribution of some viruses varies greatly between individual regions, thus regional hotspots or gradients were detected. GPGV for example is mostly found in southeastern Germany, while its incidence decreases to the north along the river Rhine. The findings of this survey provide an overview of the allocation of the most prevalent grapevine viruses in Germany and can support regional virus management and national risk assessment especially GPGV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grapevine is an important crop in Germany, particularly in the southwest, where it shapes the agricultural landscape and has a major impact on the tourism industry. At the same time, it is the perennial crop with the most adapted pathogens, 86 of which are viruses (Fuchs 2020). In order to support wine production and enable sustainable viticulture, the Wine Growing Ordinance (WGO) was introduced in Germany in 1986 (FAO 2022; BmZ 1986). It contains clear regulations about the production of pathogen-free planting material and is supplemented and adapted according to the current state of research. To date, the WGO regulates five viruses for propagation material, namely the Nepoviruses Arabis mosaic virus (ArMV) and Grapevine fanleaf virus (GFLV), the Ampeloviruses Grapevine leafroll-associated virus 1 (GLRaV-1) and Grapevine leafroll-associated virus 3 (GLRaV-3), and the Macularvirus Grapevine fleck virus (GFkV) which must exclusively be tested in rootstock material. While ArMV and GFLV are transmitted relatively slowly via two nematodes Xiphinema index and Xiphinema diversicaudatum, GLRaV-1 and -3 can be rapidly spread by various mealybug and soft scale insects (Jha et al. 1961; Raski et al. 1983; Martelli and Boudon-Padieu Martelli Boudon-Padieu 2006). All four viruses are known to cause severe yield losses and biological damage on grapevine (Fuchs 2020). GFkV, on the other hand, does not cause any symptoms itself. It rather intensifies symptoms of other viruses in case of a co-infection (Spring et al. 2012). In addition, no vector of GFkV is known so far, although natural spread in the vineyard has been reported (Martelli and Boudon-Padieu 2006).
The Nepovirus Raspberry ringspot virus (RpRSV) is not regulated within the WGO in Germany, but in other countries such as Slovenia, plant material must be mandatorily tested for the virus (Miljanić et al. 2022). RpRSV causes severe symptoms of the grapevine fanleaf disease, especially in co-infections with ArMV and GFLV (Bercks 1968). Besides grapevine, RpRSV infects berry crops and ornamental plants. It can potentially be transmitted by the three nematodes Paralongidorus maximus, Longidorus elongatus and Longidorus macrosoma, depending on host plant and virus strain (Wetzel et al. 2006).
In addition to the above-mentioned viruses, which have been known for a long time, new viruses have emerged in recent years, such as the Trichovirus Grapevine pinot gris virus (GPGV), posing possible threats to viticulture. After its characterization in 2012 in Italy, it was found in over 30 countries around the world in many different grapevine cultivars (Giampetruzzi et al. 2012; Reynard et al. 2016; Abou Kubaa et al. 2020; Debat et al. 2020; Abe and Nabeshima 2021). GPGV is associated with the grapevine leaf mottling and deformation (GLMD) disease which is marked by stunted shoots, chlorotic and deformed leaves and yield losses (Saldarelli et al. 2014). However, because the virus is frequently present in non-symptomatic plants, the correlation between GPGV and GLMD is questioned (Morán et al. 2018). The monophagous erineum mite Colomerus vitis was able to transmit GPGV under controlled conditions and in semi-field studies and is currently the only known vector (Malagnini et al. 2016). Nevertheless, GPGV was found in several herbaceous plants suggesting at least one additional vector (Gualandri et al. 2017). The extent to which GPGV is a threat to viticulture is currently still being assessed.
A recent publication presented data about the presence of grapevine viruses in propagation material from Baden-Wuerttemberg, a southern region of German viticulture, between the years 2009–2020 (Messmer et al. 2021). It revealed that virus incidences were significantly less present in pre-basic and basic planting material tested every 5–6 years than in certified material tested for viruses only every 10 years. The conclusion was the longer the official test periods were apart, the more the virus tended to be introduced into a propagation plot. Additionally, the study showed that GPGV was the most abundant virus in samples from 2018. The focus of this work was therefore placed on virus occurrence and distribution of commercially cultivated vineyards in all thirteen wine-growing regions in Germany. Therefore, all five regulated viruses ArMV, GFLV, GLRaV-1, GLRaV-3 and GFkV as well as the unregulated viruses RpRSV and GPGV were monitored. To our knowledge, this is the most comprehensive study regarding grapevine viruses in Germany. The results give an overview about the present infestation status and provide information about the local distribution of viruses in the German wine-growing areas. Since this is the first nation-wide survey including the new emerged Grapevine pinot gris virus, the data presented can help to assess the risk potential of this widespread virus.
Materials and methods
The virus survey was conducted over three consecutive years from 2019 to 2021 in different wine-growing regions of Germany. Samples were collected during spring from April to June. Due to the early stage of development of the plants, no virus symptoms were visible during sampling.
Vineyard selection
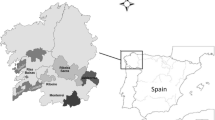
There are 13 wine-growing regions in Germany, which differ in terms of cultivation area and variety selection. All regions were included in the virus monitoring of this study (Fig. 1).
Variety selection for virus monitoring in all 13 German wine-growing regions. The three varieties with the greatest viticultural relevance of each region are listed with the respective number of plants collected for virus tests (#). In total, 4620 single plants were sampled and tested within the 3 years of the survey
The selected vineyards are exclusively used for wine production by full-time or part-time wine growers as well as state or foundation wineries. In order to obtain the most meaningful results for virus distribution in German viticulture, the three most relevant varieties of each region were used for monitoring. The number of samples was determined by the size of the regions and the willingness of the winegrowers to participate in the survey. The areas were randomly selected either by the authors themselves or by employees of the local vineyard registers. However, during selection, the widest possible range of plant age and spatial distribution was aimed for.
In most regions, Pinot noir and Dornfelder were sampled as red wine varieties, and Riesling, Mueller-Thurgau, and Pinot gris as white wine varieties. The white variety Pinot blanc, on the other hand, was only tested in Saale-Unstrut. In Wuerttemberg, regional varieties have a higher significance. Most frequently cultivated here are the two red wine varieties Lemberger and Trollinger.
Since Baden was one of the first regions to be sampled and the distances from the State Institute of Viticulture and Enology in Freiburg were much shorter than to other regions, most samples were collected from there.
Plant material and sampling
As previous internal experiments have shown that shoots younger than the phenological stage 16 (according to the BBCH scheme) give the best results concerning quality and clarity of double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) signals for detection of viruses, sampling was carried out in spring (results not shown). Therefore, freshly emerged shoots between BBCH stages 10–16 were collected. Regardless of vineyard size, ten individual vines were randomly selected for sampling, with three shoots taken from each vine near the trunk. These were collected directly into labeled ELISA sample bags (Bioreba AG, Reinach, Switzerland). Two shoots were intended for DAS-ELISA, and the third shoot served as material for GPGV isolate analysis by reverse transcription polymerase chain reaction (RT-PCR). The filled sample bags were immediately transferred to a cold box and stored for a maximum of 2 days. Samples were sorted and either processed immediately or stored at 4 °C until the following day. All samples were processed within 3 days of collection. Shoots for isolate analysis were retained at − 20 °C until further processing for RT-PCR.
DAS-ELISA
All virus tests were performed with DAS-ELISA as described previously by Messmer et al. (2021). Assays were done utilizing the equipment and reagents of Bioreba AG. Briefly, approximately, 9 ml of a 1:10 (w/v) customized “Grapevine” extraction buffer was added to each sample and homogenized with a semi-automated bead grinder (HOMEX 5). Each sample was tested for ArMV, GFLV, GLRaV-1, GLRaV-3, GFkV, GPGV and RpRSV following the protocols provided by the manufacturer. Positive and negative controls originate from the virus collection of the State Institute of Viticulture and Enology in Freiburg, Germany. Photometric evaluations of ELISA plates were conducted after 30 and 60 min, utilizing an Infinite F50 reader and Magellan™ software (Tecan Trading AG, Maennedorf, Switzerland). Samples were counted positive if the absorbance value was twice the value of the negative control. To prevent false results, each sample was retested with one replicate. In the case of inconclusive results, samples were retested.
Determination of GPGV isolates
A selection of GPGV-infected grapevines was analyzed in more detail to determine the underlying GPGV isolates. Samples were selected to cover all German wine-growing regions and to represent plants from different varieties and of different ages (suppl. Table 2).
Chosen samples were individually ground in liquid nitrogen to a fine powder. Total RNA was extracted from 80 to 100 mg of plant material by using the Universal RNA Kit (RoboKlon GmbH, Berlin, Germany) following the manufacturer’s instructions. Purified RNA was transcribed into cDNA using Moloney Murine Leukemia Virus reverse transcriptase (M-MLV RT; Lucigen, Middleton, WI, USA) and oligo(dT) as primer as recommended by the manufacturer. GPGV specific PCR was performed using primers designed by Saldarelli et al. (2014) (DetF 5′-TGGTCTGCAGCCAGGGGACA-3′; DetR 5′-TCACGACCGGCAGGGAAGGA-3′). 1 µl of cDNA served as template for the amplification by 1 U proofreading S7 fusion polymerase (Mobydiag, Espoo, Finland) in a final volume of 50 µl. Thus, a 588 bp amplicon representing a partial sequence of the movement (MP) and the coat protein (CP) of GPGV was obtained. The following PCR parameters were chosen: initial denaturation at 95 °C for 3 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 60 °C and 40 s at 72 C and a final elongation step for 5 min at 72 °C. PCR success was verified by loading 5 µl of the reaction mix on an 1% (w/v) agarose gel. The remaining PCR reaction was directly purified using the NucleoSpin® Gel and PCR Clean-up Kit (Macherey–Nagel GmbH & Co KG, Dueren, Germany). The quality of the purified product was measured photometrically using a NanoDrop™ One (Thermo Fisher Scientific Inc, Waltham, MA, USA). Only samples with A260/A230 and A260/A280 ratios between 2.0–2.2 and ~ 1.8, respectively, were sent for Sanger sequencing to Microsynth SeqLab (Goettingen, Germany). PCR products from Rheingau and Hessische Bergstrasse did not pass this quality test and were discarded. Samples from 2019 were additionally cloned into pMiniT 2.0 plasmids and transformed into 10-beta competent E. coli using the NEB® PCR Cloning Kit (New England Biolabs Inc., Ipswich, MA, USA) to study mixed infections with different isolates. Plasmids of 5–10 single colonies were purified and sent for sequencing. Since none of the samples showed mixed infection with different isolates, this step was skipped for most samples collected in 2020 and 2021.
Phylogenetic analysis
GPGV isolates are currently assigned into three clades—A, B and C (Saldarelli et al. 2014; Bertazzon et al. 2017). In order to determine the genetic diversity of GPGV in Germany, sequences obtained from monitoring samples were analyzed in more detail. Therefore, sequences were assembled to single contigs using Clone Manager 9, Professional Edition software (Sci Ed Software LLC, Westminster, CO, USA). Constructed contigs were then aligned with reference genomes using the ClustalW algorithm of MEGA X Version 10.1.8 (Kumar et al. 2018). Reference genomes with the following GenBank accession numbers were selected from NCBI for clade A: LN606703.1 (MOLA 6), KU845348.1 (PIA-G44), MH019203.1 (RQ25), MH019204.1 (RQ30), 543,887,400 (SK30), KF134125.1 (SK13) and KX522755.1 (Riesling 25-3); for clade B: KU845367.1 (ORM-G40) and LN606705.1 (MOLA 14); for clade C: KU845372.1 (SUS-G49); LN606739.1 (ALA-P4) and FR877530.2 (ZA505-1A). Since the monitoring samples lack information on symptom expression, three further internal isolates were added, which were isolated from highly symptomatic plants from Baden and Wuerttemberg in 2018. Afterward, a phylogenetic tree was constructed following the maximum likelihood method and Tamura-3 parameter model and a bootstrap of 2,000.
Alternative GPGV host screening
To identify alternative host plants of GPGV, samples were taken from the accompanying flora in a selection of vineyards. Typical plants of the sub-vegetation in vineyards, but also fruit trees and shrubs in adjacent marginal areas, were sampled. The collection was done simultaneously with the sampling of grapevine material. Plant material was transferred to a cooling bag directly after sampling, brought to laboratory and stored at − 20 °C until further processing. For homogenization, each sample was individually ground to a fine powder using liquid nitrogen, pestle, and mortar. Total RNA was extracted using the Universal RNA Kit (RoboKlon GmbH, Berlin, Germany). M-MLV reverse transcriptase (Lucigen, Middleton, WI, USA) and oligo(dT)s were used for cDNA synthesis. This was followed by PCR with Det or CP-2 primer pairs (Saldarelli et al. 2014; Bertazzon et al. 2016) with the same settings as described in 2.4. To check whether the RNA extraction and cDNA from the potential alternative host plants have worked, an additional PCR was conducted using primers amplifying the mitochondrial NADH dehydrogenase subunit 5 of plants (Nad5-F 5′-GATGCTTCTTGGGGCTTCTTGTT-3′; Nad5-R 5′-CTCCAGTCACCAACATTGGCATAA-3′) (Kato et al. 1995). The following PCR settings were chosen: initial denaturation at 95 °C for 3 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 50 °C and 30 s at 72 °C. A final elongation step at 72 °C for 1 min was performed. Amplicons were loaded on a 1% (w/v) agarose gel to verify the PCR success.
Statistics
Data were analyzed with the statistical software R (Version 1.2.5001, Boston, MA, USA). Chi-square tests with subsequent pairwise McNemar tests were used for analyses of hypotheses based on nominal data. The Cochran’s Q test was utilized for hypotheses based on dichotomous variables and multiple categories with paired responses. For all statistical tests, an alpha level of 0.05 was chosen.
Results
Between 2019 and 2021, 462 vineyards were tested as part of the present study, resulting in a total number of 4,620 individual samples. In order to obtain a high informative value about the virus distribution in German vineyards, the three most frequently cultivated varieties were monitored in each region (Fig. 1).
Frequency of viruses found in Germany
DAS-ELISA analysis revealed that 44% of the randomly collected samples showed an infection with one of the tested viruses (Fig. 2). GPGV was the most frequent virus in the monitoring, accounting for 18% prevalence. It is followed by GFkV with 13% and GLRaV-1 with 9%. In the case of GLRaV-1, it must be mentioned that the grape variety Lemberger has been excluded from the graph. Lemberger is obligatorily infected with leafroll-associated virus 1 and would lead to a biased result of 14% (Staudt et al. 1994). GFLV could be detected in 4% of the tested vines, RpRSV, ArMV and GLRaV-3 each in 2% of the samples. RpRSV was tested first within the second year of the survey. It was not tested in samples from Baden and Wuerttemberg.
Frequency of viruses in German vineyards. Results of 4,620 tested grapevines all over Germany. Each sample was tested for the following viruses: Grapevine pinot gris virus (GPGV), Grapevine fleck virus (GFkV), Grapevine leafroll-associated virus 1 (GLRaV-1), Grapevine fanleaf virus (GFLV), Raspberry ringspot virus (RpRSV), Arabis mosaic virus (ArMV) and Grapevine leafroll-associated virus 3 (GLRaV-3). According to a Cochran’s Q test, dissimilar letters indicate significant differences between viruses (Q (5, N = 4620) = 1467.289, p < 0.001). RpRSV was not included to statistical analysis, because it was not tested in all regions. Mixed infections were not considered. *Refers to a survey population excluding the grapevine variety Lemberger. **Refers to a survey population excluding the regions Baden and Wuerttemberg
Further analysis showed that nearly 80% of all virus infections in this monitoring were due to single infections (suppl. Table 1). Two viruses were detected in 18% of infected plants and three viruses in 2%. Quadruple and quintuple infections were found in only one vine each. No particular association between viruses was found in mixed infections. GFkV, although the second most prevalent virus in this survey, was found in the majority of vineyards (43.5%), directly followed by GPGV and GLRaV-1, each found in around 30% of the tested plots (Table 1). However, GPGV clearly has the highest percentage of vineyards where all samples tested positive (27.5%). GFkV and GLRaV-1 only infected all collected samples in 5.0% and 6.9% of the vineyards, respectively. GFLV was found in 15.4% of the vineyards, ArMV and RpRSV in around 11%, and GLRaV-3 was found in only 8.2% of the analyzed vineyards. None of those viruses was found in all samples collected in one vineyard.
Virus prevalence in vineyards of different ages
The oldest vineyard sampled was planted in 1944, while the youngest ones were planted in 2020. Samples were categorized into five groups: 1944–1980, 1981–1990, 1991–2000, 2001–2010 and 2011–2020 (Table 2).
GPGV was the only virus showing significantly more infections in the youngest vineyards than in the older ones. In the category with the oldest vines planted between 1944 and 1980, 9.7% of the plants show GPGV infections. In vines planted between 2011 and 2020, the percentage of GPGV positive plants increased to 28.2%. The incidences of GFkV, GLRaV-3 and RpRSV remain relatively constant between the categories. GFkV shows the highest incidences with values of 10% to 17% depending on the category. The incidences of GLRaV-3 and RpRSV were between 0 and 3%. GLRaV-1, GFLV and ArMV incidences, on the other hand, decrease between the categories from older to younger plants. While GLRaV-1 is found in 16.7% of the samples in the oldest plots, it is found in only 6.5% of the samples in the youngest group. The frequencies of GFLV and ArMV are 5.7% and 3.0%, respectively, in the oldest vineyards and 2.3% and 1.4%, respectively, in the youngest sites.
Spatial distribution of viruses in Germany
The distribution of the viruses in the German wine-growing regions varies greatly. For example, GPGV shows a high prevalence in Saxony and Saale-Unstrut, where it was found in almost 90% and 80% of the samples, respectively (Table 3). In Franconia, the virus has been detected in 40% of the samples. In Wuerttemberg and Baden, GPGV incidence is around 16%, and in Palatinate, Ahr and Rhine-Hesse, around 11%. Between 3% and 1.5%, GPGV-infected samples were found in the regions of Nahe, Hessische Bergstrasse, Mosel, Rheingau and Middle-Rhine. Mosel, Middle-Rhine and Rheingau showed statistically the fewest GPGV infections. The spatial distribution becomes particularly visible when the results are projected onto a map. GPGV is mainly found in the southwestern and eastern wine-growing regions (Fig. 3).
The occurrence of GFkV ranges from 20.8% in the Ahr region to 5.5% in Franconia. Despite the broad range of incidences, only the regions of Wuerttemberg, Mosel, Nahe and Franconia differ significantly from the regions Ahr, Rhine-Hesse and Baden. All other regions lay in between and are statistically indifferent.
Regarding GLRaV-1, the regions Nahe, Wuerttemberg and Rhine-Hesse show highest incidences with 37.5%, 34.6%, and 29.7%, respectively. In contrast, not a single infection was detected in the region Hessische Bergstrasse. The Mosel region, at 13.8%, has statistically fewer GLRaV-1 infections than the Nahe region, Württemberg, and Rhine-Hesse, but does not differ from Franconia (10.5%), Palatinate (7.4%), Baden (6.0%), Middle-Rhine (5.4%), and Saxony (5.0%). The remaining regions do not differ from each other with GLRaV-1 occurrences between 3.3% (Rheingau) and 2.3% (Ahr).
Most GFLV infections were detected in Wuerttemberg (13.1%). However, GFLV occurrences in Franconia (9.5%), Rhine-Hesse (5.4%), Baden (3.2%), Ahr (2.3%) and Middle-Rhine (2.3%) showed no statistical differences. The Mosel region had the fewest GFLV infections in relation to the samples tested (0.3%). No GFLV infections were detected in the Hessische Bergstrasse, Saxony, and Saale-Unstrut regions. All GFLV occurrences in the remaining regions did not differ significantly among themselves.
By far the most RpRSV infections are found in Saxony (15.6%). Statistically, however, the occurrence of RpRSV is not different in Palatinate with 7.4%. In the Middle-Rhine and Nahe regions, the virus was not found in any of the samples. All other regions do not differ statistically from each other, with RpRSV incidences between 2.6% (Franconia) and 0.5% (Saale-Unstrut).
ArMV was generally detected in very few samples (suppl. Fig. 1). There is no statistical difference for this virus for any of the regions. It was found most frequently in Wuerttemberg with 3.7%. In the regions Hessische Bergstrasse, Saxony and Saale-Unstrut, it was not detected in any of the samples.
Positive GLRaV-3 samples were also found very rarely. From a statistical point of view, only three regions differ. In the Mosel region (4.4%), the virus was detected more frequently than in the Ahr and Nahe regions, where the virus was present in none of the samples. All other regions show a GLRaV-3 incidence between 1.9% (Baden) and 0.3% (Franconia).
Occurrence of GPGV isolates
The GPGV isolates from German vineyards are distributed across all clades (Fig. 4). Most samples (40 out of 53 isolates) assigned to clade A, six to clade B and seven to clade C. As expected, the internal isolate from a highly symptomatic plant from Baden clustered to clade C, while, interestingly, both isolates from Wuerttemberg clustered to clade A.
Clade A isolates have been predominant in the northeastern and northwestern regions, whereas in eastern regions, isolates from all clades were present (Table 4). Compared to clade A isolates, isolates assigned to clade B and C were rather rarely found in this study. It is interesting that despite the small number of tested samples from Saxony, only clade C isolates were identified. Clade C isolates were otherwise only found in single samples from Palatinate and in Wuerttemberg.
Concerning polymorphisms at the end of the MP region of GPGV, the German isolates were highly homogeneous (suppl. Table 3). Almost all isolates had the stop codon at position 6,702 which results in a 375 amino acid long MP. However, four isolates showed a T/C polymorphism at site 6,684 as described by Saldarelli et al. (2014) which causes a stop codon creating a protein six amino acids shorter. All isolates were assigned to clade C. No isolate showed the T/C polymorphism at site 6,687 known from Spanish isolates causing a 370 amino acid long MP (Morán et al. 2018).
Alternative GPGV host plants
A total of 104 additional samples of the accompanying flora of individual vineyards were sampled and tested by RT-PCR for GPGV infection (suppl. Table 4). Besides, randomly collected symptomless plants, also plant material showing leaf deformations, and mottling or stunted shoots were analyzed. However, in none of the samples, GPGV was present (suppl. Fig. 2).
Discussion
Samples from all thirteen German wine regions were tested for the viruses ArMV, GFkV, GFLV, GLRaV-1 and GLRaV-3 listed in the WGO (FAO 2022) as well as for the currently unregulated viruses RpRSV and GPGV. In line with a study performed on propagation material (Messmer et al. 2021), the present study proofs GPGV as the most abundant virus in commercially cultivated vineyards. 18% of all tested samples in German vineyards were positive for this virus (Fig. 2). The high GPGV infection rate becomes even more significant when considering that the samples were collected in a triple blind procedure. Both the vineyards and the ten vines per vineyard were randomly selected. The third level of randomness resulted from the sample date early after budbreak, so that no symptoms were visible, avoiding subconscious selection of conspicuous vines. GFkV was with 13% the second most common virus in commercial vineyards, while GLRaV-1 was present in 9% of the samples (excluding samples from Lemberger). GFLV was found in 4% of samples followed by RpRSV, ArMV and GLRaV- 3, each found in 2% of the vines tested.
The different frequencies of viruses in the survey could be influenced by various parameters. Certainly, vector insects have a great influence on virus occurrence. GPGV and GLRaV-1 show significantly higher frequencies in the survey than the Nepoviruses GFLV, RpRSV and ArMV. Nepoviruses are transmitted by soilborne nematodes, which are rather slow vectors (Jha et al. 1961; Raski et al. 1983). GPGV and GLRaV-1 are transmitted by the Eriophyes mite Colomerus vitis and by various mealybugs and soft scale insects, respectively (Martelli et al. 2006; Malagnini et al. 2016). These are common insects in German vineyards and more mobile than nematodes (Huebschen et al. 2004; Steinmetz et al. 2017). Therefore, viral spread of GPGV and GLRaV-1 is more likely than that of Nepoviruses. From this perspective, the very low incidence of GLRaV-3 in Germany is remarkable. In many other countries, this virus is one of the most common in grapevine where it causes severe symptoms and economic damage (Maree et al. 2013; Xiao et al. 2018; Blaisdell et al. 2020; Crnogorac et al. 2021; Čarija et al. 2022). GLRaV-3 is spread mainly by mealybugs of the genera Heliococcus, Phenacoccus, Pseudococcus, and Planococcus. Most of them are present in German wine-growing regions and are also able to transmit GLRaV-1 (Tsai et al. 2010; Bertin et al. 2016). Since GLRaV-1 occurs more often in German vineyards, it could be assumed that GLRaV-3 never became widely established in Germany, and thus, no vector-based spread occurred. In contrast, the high incidence of GFkV (13%) is difficult to explain by vector-based transmission alone, as no vector is known to date (Sabanadzovic et al. 2017; Martelli and Boudon-Padieu 2006). Presumably, the high incidence is due to the lack of testing of GFkV in scions, since according to the WGO, the virus must only be tested in rootstocks. Therefore, it can be assumed that the virus is also already present to a considerable extent in shoot material from where it is spread. These concerns have been shared by ICVG members since 2003 (Maliogka et al. 2015). However, all regulated viruses were significantly less frequent in the survey than the unregulated GPGV. This unregulated state currently allows GPGV to multiply exponentially through infected planting material, ultimately increasing the likelihood of being transmitted by its vector, explaining its high occurrence. Although RpRSV is also unregulated, it has a very low incidence (2%). Probably, this circumstance can be attributed again to its rather slow vectors of soilborne nematodes. The probability of a suitable nematode feeding on an infected vine is quite low at this small frequency of RpRSV. And even if this were the case, transmission to neighboring plants would take a long time due to the low mobility of the nematodes in the soil.
The planting year of the vineyards also seems to have an influence on virus occurrences. The incidences of almost all regulated viruses decreased continuously, so that significantly fewer viruses were present in young vineyards than in older ones (Table 2). Since the WGO was enacted in 1986, there could be a link between the successive reduction in the incidence of viruses and the new phytosanitary measures introduced at that time. However, exceptions to this are GFkV and GLRaV-3 which show more or less stable incidences around 13% and 1.5% over the last 8 decades, respectively. The reason for stable GFkV incidences on such high levels is not known. The incidence of GLRaV-3 appears to be so low that vector-based transmission, as with RpRSV, is unlikely. Consequently, the amount of virus is kept constantly low. In contrast to the other viruses, GPGV is the only virus that shows an almost continuous increase over the past 5 decades. Incidences have risen from 9.7% between 1965–1980, to 18.3% between 1981–1990, to 16.9% between 1991–2000, to 17.5% between 2001–2010, and eventually reached 28.2% between the years 2011–2020. Similar observations were recorded in a survey in Ontario where GPGV increased from 13.3% between 1974–1990 to 25.7% between 2006–2016 (Xiao et al. 2018). According to phylogeographic analyses of Hily et al. (2019), GPGV entered Europe in the mid-twentieth century from Asia. The oldest GPGV-infected vines in this study were planted in 1965, what might suggest that GPGV became established in Germany later than other viruses. However, the number of old vines tested during this study was rather low, and the suitable vector could just as well have transmitted the virus to older vines in recent years.
Since the survey has shown significant temporal differences between virus abundances, it is interesting to see if there are also spatial differences in virus occurrence in Germany. All viruses, except ArMV, show significant differences in abundance between German regions. GFkV is widespread in the country and fairly regularly distributed (Fig. 3). It was mostly found in Baden (20.3%), Rhine-Hesse (20.6%) and the Ahr region (20.8%). Individual hotspots could be identified for GLRaV-1 and GFLV. While GFLV is particularly prevalent in Wuerttemberg (13.1%), GLRaV-1 hotspots are present in the Nahe region (37.5%), Rhine-Hesse (29.7%) and in Wuerttemberg (34.6%). Hotspots were also found for RpRSV and GLRaV- 3. RpRSV has shown elevated incidences in Saxony (15.6%) and Palatinate (7.4%), whereas GLRaV- 3 has been found most in the Mosel region (4.4%). With GPGV incidences of 88% and 76%, respectively, the regions Saxony and Saale-Unstrut clearly stand out from the others. In Franconia, Baden and Wuerttemberg, GPGV could be detected in about 40% and 20% of the samples, respectively. In contrast, the occurrence of GPGV decreases toward the northern regions, along the river Rhine.
Spatial distribution of the four most frequently detected viruses in the Germany-wide survey. The maps show the percentage of positive samples in the probing districts for the viruses Grapevine pinot gris virus (GPGV, a, top left), Grapevine fleck virus (GFkV, b, top right), Grapevine leafroll-associated virus 1 (GLRaV-1, c, bottom left) and Grapevine fanleaf virus (GFLV, d, bottom right). The areas are color coded from red (100% virus occurrence) to yellow (50% virus occurrence) to white (0% virus occurrence)
Why some viruses are more frequently present in some regions might again depend on various influences. One reason could be the origin and particularly the quality of planting material. As observed in a previous study, there are significant differences in virus incidence between various propagation categories in Germany, depending on the extent and frequency of testing (Messmer et al. 2021). The category of standard propagation material was not considered in this study at that time. However, it can be assumed that plants of standard material have far more virus infections as the controls are based solely on visual assessment without any serological tests (FAO 2022). If plants, which have not been tested at all or have been tested only infrequently, also come from areas with high virus incidences, the probability of obtaining infected planting material is high.
Another influencing factor is again the presence of vector insects. The corresponding vectors of GLRaV-1, GLRaV-3 and GFLV may occur throughout Germany, so that the observed regional hotspots of these three viruses may indicate that the vectors are also more common in the affected regions (Huebschen et al. 2004; Hoffmann et al. 2022). In regions where both the virus and its appropriate vector are present, it is quite difficult to re-contain viral infection. Effective measures are limited to controlling the vector insect and consistently introducing healthy planting material over very large areas. Both require a high financial commitment and joint efforts of all winegrowers in the hotspot regions. These efforts are further challenged by the decreasing number of agents approved for vector insect control. Preventive measures that stop the formation of hotspots in the first place are consequently the best protection against viral infections. According to the survey results, special attention should be paid to GLRaV-3 as it remained very stable at a low level in Germany for almost 8 decades. This very low occurrence should be maintained in the future as long as possible, especially when it is seen to be much more abundant and damaging to vines in many countries (Caruso et al. 2022). The highest GLRaV-3 occurrence was found in the Mosel region. Thus, especially in this region, the occurrence of the corresponding vector insects should be monitored, and a high phytosanitary quality of the planting material should be aimed for.
For GPGV a clustering became visible in the eastern and southwestern wine regions of Germany (Fig. 3). The reason for such a distribution is not yet clear. The fact that GPGV was found in 27.5% of infected vineyards in ten out of ten samples (Table 1) suggests either that the virus was introduced through infected planting material or that the vector transmission of GPGV is highly efficient within vineyards. That both assumptions could be possible has already been demonstrated in other studies (Demian et al. 2020; Messmer et al. 2021; Bertazzon et al. 2020; Hily et al. 2021). The high proportion of completely contaminated vineyards is mainly due to the elevated incidence in the regions of Saxony and Saale-Unstrut (Table 3). The tested vines in the eastern regions were almost exclusively sourced from two nurseries in Palatinate, both with a wide sales area in Germany. Thus, highly elevated GPGV incidences should also occur in northwestern areas of German viticulture. Since this is not the case, the high infection rates in the eastern wine-growing regions are at least not exclusively triggered by the planting material from Palatinate. In addition, isolate analyses suggest that the high GPGV infestation in East Germany can possibly be attributed to planting material from other European countries. Between 1987 and 1989, vines were purchased in Saxony from many different countries, including Italy, France, and the Czech Republic (Höhne, personal communication). NCBI Blast results show that the Saxon isolates have the highest homologies (98.93–98.22%) with an Italian and a Slovenian isolate (GeneBank Acc.Nr.: LN606714.1; MW026696.1). That is a higher degree of identity than with the other clade C samples Mon P 271 from Palatinate (97.71–97.35%) and Mon WU 14.1 from Wuerttemberg (97.70–96.99%) is analyzed in this survey (suppl. Table 5). Similar blast results were achieved for clade B isolates from Saale-Unstrut. Since highly elevated GPGV incidences only occur in East Germany and the isolate composition there is by no means congruent with those in Palatinate, one has to assume that the vines were infected at a later date in Saxony and Saale-Unstrut through the suitable vector(s).
Also the fast spread of GPGV within vineyards indicates vectorial transmission (Bertazzon et al. 2020; Hily et al. 2021). So far, there is still no verification for the existence of another GPGV vector insect than the Eriophyes mite Colomerus vitis. However, the presence of another vector is very likely due to the detection of GPGV in non-Vitis plants. GPGV has already been detected in Ailanthus sp., Asclepias syriaca, Chenopodium album, Crataegus sp., Fraxinus sp., Rosa canina, Rubus sp., Sambucus sp., and Silene latifolia subsp. Alba (Gualandri et al. 2017; Demian et al. 2022). Since Colomerus vitis is monophagous, another insect must be able to transmit GPGV from grapevines to those plants or vice versa (Malagnini et al. 2016). To determine the situation of alternative host plants in Germany, samples of herbaceous plants from the vineyards as well as shrubs and fruit trees from their surroundings were analyzed for GPGV. Although some samples showed leaf mottling or stunted growth, the virus was not detected in any of them (suppl. Table 4). Since the sampling on vines and other plants was carried out simultaneously right at the beginning of the season, there was no knowledge of the phytosanitary situation in the vineyards. Thus, many samples were taken in vineyards without GPGV infestation and therefore with no virus pressure. It is also possible that the corresponding vector, which is able to transmit GPGV between vines and alternative hosts, is not yet established in Germany or the sampling was done too early in the growing season, and the vector was not yet active.
Since no symptoms were visible on grapevine at the time of sample collection, winegrowers and the respective state offices who had collected the samples were asked about GLMD symptom development in the corresponding vineyards. Information on symptomatology is also of high interest in connection with isolate analysis. Over the years, the theory has been established that isolates assigned to clade A are mostly latent, whereas clade B and C isolates are mostly virulent and can elicit GLMD symptoms in grapevine (Bertazzon et al. 2017). Isolate analysis of this study determined relatively low genetic diversity in Germany. Mainly isolates of clade A were found (72%), while isolates of clade B (11%) and C (13%) were less abundant and preferably located in eastern Germany (Fig. 4). Especially in Saxony, where only clade C isolates were verified within the small subset of tested plants, severe symptoms within the analyzed vineyards were expected (Table 4). However, no symptomatic vines have been reported from there, so far. Strong symptoms have been reported solely from Baden, Wuerttemberg and Franconia (Reynard et al. 2016, Hofmann, personal communication). This inconsistency of the correlation between genetic variability of GPGV and symptom development was already stated by diverse groups (Bertazzon et al. 2017, 2020; Morán et al. 2018; Marra et al. 2020). Also, two of the three WBI internal controls from highly symptomatic grapevines were clustered to clade A, suggesting that GPGV symptoms are not only influenced by the polymorphism at the movement protein. Hily et al. (2021) pointed out that mixed isolate infections are difficult to reveal using simple Sanger sequencing which might also be a possible reason for the lack of correlation between tested isolate and GLMD expression. Even with an intermediated cloning step, only the predominant isolate will be detected. High throughput sequencing (HTS) is to date the only reliable method to get precise results on mixed infections. Furthermore, Hily et al. (2021) designed universal GPGV primer on the basis of HTS results able to detect all previously known GPGV isolates. That was not possible with the primer pair used for isolate analysis in this study (Saldarelli et al. 2014). Another explanation for the discrepancy of isolate and symptom expression may lie in cross-protection, a common phenomenon of various viruses (Bertazzon et al. 2020). In this process, an avirulent virus strain primes the plant and confers a certain resistance to more virulent strains due to the activation of the host-induced RNA silencing machinery. Tarquini et al. (2019) observed this priming effect under controlled conditions, and Bertazzon et al. (2020) collected field data in which symptomless plants over consecutive years indicate such a mechanism. It would be interesting to pursue this line of thought in future. For ArMV and GFLV, this method was not suitable due to negative influences on growth performances of the vine and yield losses (Komar et al. 2008). If it appears that GPGV is widely latent in vines in a stable manner, cross-protection could well be an opportunity.
Phylogeny of selected GPGV isolates. The tree was constructed by using the Maximum Likelihood method and Tamura-3 model of MEGA X software. The tree with the highest log likelihood (-1911.70) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Reference isolates are tagged with green icons (clade A dots, clade B squares, clade C triangles). Internal reference isolates of strongly symptomatic vines are marked with red rhombs. More details on the different samples can be found in suppl. Table 2
Conclusion
The data of this study demonstrated that the overall level of regulated viruses in commercial vineyards in Germany is relatively low. Few regional hotspots for some viruses were identified in which virus incidences are above the German-wide average. Even with suitable phytosanitary and agronomic measures, such local virus accumulations cannot be completely avoided. However, the results must be seen as a small and momentary excerpt of the entire phytosanitary situation of German viticulture. Other working groups for example have shown a general increase in GLRaV-1 in Germany which is probably due to scale insect habitat expanding supported by global warming (Hoffmann et al. 2022).
Regarding GPGV, the survey confirmed that the newly emerged Trichovirus is already present to a large extent in German commercial vineyards and is continuously increasing. This was less surprising after it could already be shown that the virus is strongly represented in propagation material (Demian et al. 2020; Messmer et al. 2021). Virus surveys in other countries have also identified GPGV as one of the most prevalent viruses (Xiao et al. 2018; Spilmont et al. 2018; Čarija et al. 2022). GPGV is obviously benefiting from its unregulated status as well as from its mobile vector insect(s). That it is mostly latent in grapevines additionally support its spread. The study again showed that GPGV symptomatology has not yet been fully elucidated. Contrary to the isolate distribution found in Germany, strong GLMD symptoms were only sighted in the regions of Baden, Wuerttemberg and Franconia, where this virus can cause high yield losses and, in the worst case, permanently impairs the growth of the vines so that clearing the area becomes inevitable (Messmer et al. unpublished data).
As already mentioned, prevention is currently the best protection against virus infections. This includes phytosanitary monitoring, reliable diagnostics and comprehensive phytopathological research. The high GPGV incidence in German vineyards and the rapid increase in the virus within the last decade are evidence of the need to look for prevention measures in time. Since the viruses regulated in the WGO in this study showed stable or even declining incidences over the last 8 decades, the inclusion of GPGV in the European-wide regulation should be considered. Also as part of an European and Mediterranean Plant Protection Organization (EPPO) project, Picard et al. (2018) proposed listing GPGV as a new regulated non-quarantine pest (RNQP) for grapevine.
Data availability
Sequences from this study were submitted to GenBank and assigned the accession numbers OP244966–OP245021.
References
Abe J, Nabeshima T (2021) First report of grapevine pinot gris virus in wild grapevines (Vitis Coignetiae) in Japan. J Plant Pathol 103(2):725–725. https://doi.org/10.1007/s42161-021-00800-w
Abou Kubaa R, Choueiri E, Jreijiri F, El Khoury Y, Saldarelli P (2020) First report of grapevine pinot gris virus in Lebanon and the middle east. J Plant Pathol 102(2):565–565. https://doi.org/10.1007/s42161-019-00453-w
Bercks VR (1968) Über den nachweis des himbeerringflecken-virus (Raspberry Ringspot Virus) in Reben. J Phytopathol 62(2):169–173. https://doi.org/10.1111/j.1439-0434.1968.tb03029.x
Bertazzon N, Filippin L, Forte V, Angelini E (2016) Grapevine pinot gris virus seems to have recently been introduced to vineyards in Veneto, Italy. Adv Virol 161(3):711–714. https://doi.org/10.1007/s00705-015-2718-2
Bertazzon N, Forte V, Filippin L, Causin R, Maixner M, Angelini E (2017) Association between genetic variability and titre of grapevine pinot gris virus with disease symptoms. Plant Pathol 66(6):949–959. https://doi.org/10.1111/ppa.12639
Bertazzon N, Forte V, Angelini E (2020) Fast Transmission of grapevine pinot gris virus (GPGV) in vineyard. VITIS: J Grapevine Res. https://doi.org/10.5073/VITIS.2020.59.29-34
Bertin S, Cavalieri V, Gribaudo I, Sacco D, Marzach C (2016) Transmission of grapevine virus A and grapevine leafroll-associated virus 1 and 3 by Heliococcus Bohemicus (Hemiptera: Pseudococcidae) nymphs from plants with mixed infections. J Econ Entomol 109(4):8
“BmZ 1986.” n.d. https://www.gesetze-im-internet.de/rebpflv_1986/BJNR002040986.html. Accessed July 12, 2022
Blaisdell GK, Zhang S, Rowhani A, Klaassen V, Cooper ML, Daane KM, Almeida RPP (2020) Trends in vector-borne transmission efficiency from coinfected hosts: grapevine leafroll-associated virus-3 and grapevine virus A. Eur J Plant Pathol 156(4):1163–1167. https://doi.org/10.1007/s10658-019-01916-7
Čarija M, Radić T, Černi S, Mucalo A, Zdunić G, Vončina D, Jagunić M, Hančević K (2022) Prevalence of virus infections and GLRaV-3 genetic diversity in selected clones of croatian indigenous grapevine cultivar plavac mali. Pathogens 11(2):176. https://doi.org/10.3390/pathogens11020176
Caruso AG, Bertacca S, Ragona A, Matić S, Davino S, Panno S (2022) Epidemiological survey of grapevine leafroll-associated virus 1 and 3 in sicily (Italy): genetic structure and molecular variability. Agriculture 12(5):647. https://doi.org/10.3390/agriculture12050647
Crnogorac A, Panno S, Mandić A, Gašpar M, Caruso AG, Noris E, Davino S, Matić S (2021) Survey of five major grapevine viruses infecting Blatina and Žilavka cultivars in Bosnia and Herzegovina. PLoS ONE 16(1):e0245959. https://doi.org/10.1371/journal.pone.0245959
Debat H, Luna F, Moyano S, Zavallo D, Asurmendi S, Gomez-Talquenca S (2020) First Report of grapevine pinot gris virus infecting grapevine in Argentina. J Plant Pathol 102(4):1321–1321. https://doi.org/10.1007/s42161-020-00608-0
Demian E, Jaksa-Czotter N, Molnar J, Tusnady GE, Kocsis L, Varallyay E (2020) Grapevine rootstocks can be a source of infection with non-regulated viruses. Eur J Plant Pathol 156(3):897–912. https://doi.org/10.1007/s10658-020-01942-w
Demian E, Jaksa-Czotter N, Varallyay E (2022) Grapevine pinot gris virus is present in different non-vitis hosts. Plants 11(14):1830. https://doi.org/10.3390/plants11141830
“FAO 2022.” n.d. https://www.fao.org/faolex/results/details/en/c/LEX-FAOC169933/. Accessed July 12, 2022
Fuchs M (2020) Grapevine viruses: a multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J Plant Pathol 102(3):643–653. https://doi.org/10.1007/s42161-020-00579-2
Giampetruzzi A, Roumi V, Roberto R, Malossini U, Yoshikawa N, La Notte P, Terlizzi F, Credi R, Saldarelli P (2012) A new grapevine virus discovered by deep sequencing of virus- and viroid-derived small RNAs in Cv pinot gris. Virus Res 163(1):262–268. https://doi.org/10.1016/j.virusres.2011.10.010
Gualandri V, Asquini E, Bianchedi P, Covelli L, Brilli M, Malossini U, Bragagna P, Saldarelli P, Si-Ammour A (2017) Identification of herbaceous hosts of the grapevine pinot gris virus (GPGV). Eur J Plant Pathol 147(1):21–25. https://doi.org/10.1007/s10658-016-0989-4
Hily J-M, Poulicard N, Candresse T, Vigne E, Beuve M, Renault L, Velt A, Spilmont A-S, Lemaire O (2019) Datamining, genetic diversity analyses, and phylogeographic reconstructions redefine the worldwide evolutionary history of grapevine pinot gris virus and grapevine berry inner necrosis virus. Phytobiomes J 4(2):165–177. https://doi.org/10.1094/PBIOMES-10-19-0061-R
Hily J-M, Komar V, Poulicard N, Vigne E, Jacquet O, Protet N, Spilmont A-S, Lemaire O (2021) Biological evidence and molecular modeling of a grapevine pinot gris virus outbreak in a vineyard. Phytobiomes J 5(4):464–472. https://doi.org/10.1094/PBIOMES-11-20-0079-R
Hoffmann C, Herrbach E, Fuchs R, Kamecke D, Winterhagen P, Schulze-Sylvester M, Trippel C, Kortekamp A (2022) Bericht vom Fachgespräch zur Rolle von Schild- und Schmierläusen als Virusvektoren im Weinbau am Oberrhein: management und beratungsempfehlung. J Kult. https://doi.org/10.5073/JFK.2022.07-08.04
Huebschen J, Kling L, Ipach U, Zinkernagel V, Bosselut N, Esmenjaud D, Brown DJF, Neilson R (2004) Validation of the specificity and sensitivity of species-specific primers that provide a reliable molecular diagnostic for Xiphinema Diversicaudatum, X. Index and X. Vuittenezi. Eur J Plant Pathol 110(8):779–788. https://doi.org/10.1007/s10658-004-0995-9
Jha A, Posnette AF (1961) Transmission of arabis mosaic virus by the nematode Xiphinema Diversicaudatum (Micol.). Virology 13(1):119–123. https://doi.org/10.1016/0042-6822(61)90038-1
Kato S, Shimamoto Y, Mikami T (1995) The apple mitochondrial Atp9 gene: RNA editing and co-transcription with exons a and b of the Nad5 gene. Physiol Plant 93(3):572–575. https://doi.org/10.1111/j.1399-3054.1995.tb06860.x
Komar V, Vigne E, Demangeat G, Lemaire O, Fuchs M (2008) Cross-protection as control strategy against grapevine fanleaf virus in naturally infected vineyards. Plant Dis 92(12):1689–1694. https://doi.org/10.1094/PDIS-92-12-1689
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Malagnini V, de Lillo E, Saldarelli P, Beber R, Duso C, Raiola A, Zanotelli L et al (2016) Transmission of grapevine pinot gris virus by colomerus vitis (Acari: Eriophyidae) to grapevine. Adv Virol 161(9):2595–2599. https://doi.org/10.1007/s00705-016-2935-3
Maliogka VI, Martelli GP, Fuchs M, Katis NI (2015) Control of viruses infecting grapevine. Adv Virus Res 91:175–227. https://doi.org/10.1016/bs.aivir.2014.11.002
Maree HJ, Almeida RPP, Bester R, Chooi KM, Cohen D, Dolja VV, Fuchs M et al (2013) Grapevine leafroll-associated virus 3. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00082
Marra M, Giampetruzzi A, Abou Kubaa R, de Lillo E, Saldarelli P (2020) Grapevine pinot gris virus variants in vines with chlorotic mottling and leaf deformation. J Plant Pathol 102(2):531–531. https://doi.org/10.1007/s42161-019-00418-z
Martelli GP, Boudon-Padieu E (eds) (2006) Directory of infectious diseases of grapevines and viroses and virus-like diseases of the grapevine: bibliographic report 1998–2004. In: Directory of infectious diseases of grapevines and viroses and virus-like diseases of the grapevine: bibliographic report 1998–2004. Vol. 55. options méditerranéennes : Série B. Etudes et Recherches. Bari : CIHEAM. http://om.ciheam.org/om/pdf/b55/00800521.pdf
Messmer N, Bohnert P, Schumacher S, Fuchs R (2021) Studies on the occurrence of viruses in planting material of grapevines in southwestern Germany. Viruses 13(2):248. https://doi.org/10.3390/v13020248
Miljanić V, Rusjan D, Škvarč A, Chatelet P, Štajner N (2022) Elimination of eight viruses and two viroids from preclonal candidates of six grapevine varieties (Vitis Vinifera L.) through in vivo thermotherapy and in vitro meristem tip micrografting. Plants 11(8):1064. https://doi.org/10.3390/plants11081064
Morán F, Olmos A, Lotos L, Predajňa L, Katis N, Glasa M, Maliogka V, Ruiz-García AB (2018) A novel specific duplex real-time RT-PCR method for absolute quantitation of grapevine pinot gris virus in plant material and single mites. PLoS ONE 13(5):e0197237. https://doi.org/10.1371/journal.pone.0197237
Picard C, Afonso T, Benko-Beloglavec A, Karadjova O, Matthews-Berry S, Paunovic SA, Pietsch M, Reed P, van der Gaag DJ, Ward M (2018) Recommended Regulated non-quarantine pests (RNQPs), associated thresholds and risk management measures in the European and Mediterranean Region. EPPO Bulletin 48(3):552–568. https://doi.org/10.1111/epp.12500
Raski DJ, Goheen AC, Lider LA, Meredith CP (1983) Strategies against grapevine fanleaf virus. Plant Dis 63(3):335–339. https://doi.org/10.1094/PD-67-335
Reynard J-S, Schumacher S, Menzel W, Fuchs J, Bohnert P, Glasa M, Wetzel T, Fuchs R (2016) First report of grapevine pinot gris virus in German vineyards. Plant Dis 100(12):2545–2545. https://doi.org/10.1094/PDIS-07-16-0966-PDN
Sabanadzovic S, Aboughanem-Sabanadzovic N, Martelli GP (2017) Grapevine fleck and similar viruses. In: Meng B, Martelli GP, Golino DA, Fuchs M (eds) Grapevine viruses molecular biology, diagnostics and management. Springer International Publishing, Cham, pp 331–349. https://doi.org/10.1007/978-3-319-57706-7_16
Saldarelli P, Giampetruzzi A, Morelli M, Malossini U, Pirolo C, Bianchedi P, Gualandri V (2014) Genetic variability of grapevine pinot gris virus and its association with grapevine leaf mottling and deformation. Phytopathology 105(4):555–563. https://doi.org/10.1094/PHYTO-09-14-0241-R
Spilmont A-S, Sevin A-F, Guinard J, Beuve M, Alliaume A, Marais A, Faure C, Candresse T, Lemaire O (2018) Occurrence of grapevine pinot gris virus (GPGV) and grapevine leaf mottling and deformation (GLMD) syndrome in france: genetic diversity and field monitoring in diverse viticulture areas. In: Proceedings of the 19th congress of the international council for the study of virus and virus-like diseases of the grapevine (ICVG), Santiago, Chile, 9–12. Santiago, Chile
Spring JL, Reynard J-S, Viret O, Maigre D, Gugerli P (2012) Effets du virus 1 associé à l’enroulement (GLRaV-1) et du virus de la marbrure (GFkV) sur le comportement agronomique et la qualité des vins de Gamay. Rev Suisse Vitic Arboric Hortic 44(3):180–188
Staudt G, Kassemeyer HH (1994) Elimination of grapevine leafroll associated virus type I in vitis Vinifera Cv. lemberger. Vitis 33:179–180
Steinmetz N, Maixner M, Hoffmann C (2017) The role of scale insects as vectors of grapevine viruses in German viticulture. In: 10th young scientists meeting, 73
Tarquini G, Zaina G, Ermacora P, De Amicis F, Franco-Orozco B, Loi N, Martini M et al (2019) Agroinoculation of grapevine pinot gris virus in tobacco and grapevine provides insights on viral pathogenesis. PLoS ONE 14(3):e0214010. https://doi.org/10.1371/journal.pone.0214010
Tsai C-W, Rowhani A, Golino DA, Daane KM, Almeida RPP (2010) Mealybug transmission of grapevine leafroll viruses: an analysis of virus-vector specificity. Phytopathology® 100(8):830–834. https://doi.org/10.1094/PHYTO-100-8-0830
Wetzel T, Ebel R, Moury B, Le Gall O, Endisch S, Reustle GM, Krczal G (2006) Sequence analysis of grapevine isolates of raspberry ringspot nepovirus. Adv Virol 151(3):599–606. https://doi.org/10.1007/s00705-005-0665-z
Xiao H, Shabanian M, Moore C, Li C, Meng B (2018) Survey for major viruses in commercial vitis vinifera wine grapes in Ontario. Virol J 15(1):127. https://doi.org/10.1186/s12985-018-1036-1
Acknowledgements
We thank Petra Ehrhardt for excellent technical assistance. We also thank Frieder Tränkner and Eike Harbrecht from the LfULG in Saxony, Ute Knauf from the ALFF in Saxony-Anhalt, Claudia Jung from the RP Darmstadt in Hesse and Heinrich Hofmann from the LWG in Bavaria for an excellent cooperation. We would also like to thank all the wine growers who agreed to the sampling.
Funding
This research was funded by the Forschungsring des Deutschen Weinbaus (FDW).
Author information
Authors and Affiliations
Contributions
Conceptualization was done by NM and RF; methodology was done by NM and RF; validation was done by NM, RF and SS; formal analysis was done by NM and LA; investigation was done by NM; resources were done by RF; data curation was done by NM; writing—original draft preparation were done by NM; writing—review and editing were done by RF, SS and RTV; visualization was done by NM; supervision was done by RF and RTV; project administration was done by RF; and funding acquisition was done by RF.
Corresponding author
Ethics declarations
Conflict of interest
N.M. is being inducted into the role of Editor in Chief of JPDP since 2023.
Informed consent
All authors have reviewed the manuscript and approved its submission to Journal of Plant Disease and Protection.
Human or animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Messmer, N., Bohnert, P., Askani, L. et al. Occurrence and distribution of Grapevine pinot gris virus and other grapevine viruses in German viticultural regions. J Plant Dis Prot 130, 1385–1399 (2023). https://doi.org/10.1007/s41348-023-00776-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00776-y