Abstract
A new approach to modelling epidemics of brown rot caused by Monilinia spp. in Ebro Valley peach orchards has been developed. This compartmental model was subdivided according to the phenological stages in which the disease can develop (blossom, immature fruit, and ripe fruit). Information host susceptibility, primary and secondary inoculum sources and latent infections in immature fruit was taken into account. The compartmental model is described by a system of differential equations, and is simple enough to allow an analytical study of the main epidemiological factors that determine the rate of disease progress during a single growing season. The proposed model fits well to the epidemic pattern of brown rot observed in north-eastern Spain. The transmission of the disease as a non-linear term implied that small changes in the infection rate had a large effect on the development of the disease. The model has confirmed the usefulness of removing mummies (infected fruit that remains in the crop during winter) from the field to reduce the final incidence of the disease. In addition, all control measures that reduce the rate of secondary infection in ripe fruit, either through the use of more resistant varieties or the use of fungicides, are effective in reducing brown rot incidence. The proposed epidemic model is flexible and allows to add complexities to the system and evaluate the effectiveness of different control strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monilinia. laxa, M. fructigena and M. fructicola are the species of the genus Monilinia that cause blossom blight, twig blight and brown rot of peach fruit in Spain (Villarino et al., 2013). Economic losses can reach 80% in years with suitable environmental conditions for disease development, especially in late harvested peach and nectarine varieties (Gell et al., 2008). The risk of infection of peaches and nectarines with brown rot depends largely on the ripeness stage of the fruit (Bevacqua et al., 2018; Gell et al., 2008; Lee & Bostock 2007; Villarino et al., 2011), the amount of primary and secondary inoculum in the orchards, and climatic conditions, especially temperature and wetness duration (Villarino et al., 2010; Villarino et al., 2012). In general, immature peach fruit are less susceptible to Monilinia infection than mature fruit (Gell et al., 2008; Villarino et al., 2011), although fruit at the pit hardening stage may also be susceptible (Lee & Bostock, 2007).
The primary inoculum is capable of infecting blossoms and immature fruit during early spring (Byrde & Willetts, 1977). The primary inoculum consists mainly of mycelium and conidia of the pathogen on mummies remaining on trees during winter. In the Ebro Valley (Spain) a positive relationship was found between the number of mummified fruit and the incidence of post-harvest brown rot (Villarino et al., 2010). These infections can remain latent in blossoms and immature fruit when climatic conditions are unfavorable, and persist as latent infections during the fruit-growing season until climatic conditions become conducive to disease expression (Byrde & Willetts, 1977; Gell et al., 2008). The secondary inoculum may appear anywhere on infected tissue with adequate moisture content to allow pathogen sporulation (Villarino et al., 2012). The secondary inoculum consists mainly of mycelium and conidia of the pathogen on flowers and fruit. In the Ebro Valley, a positive relationship was found between the times of first appearance of airborne conidia, first appearance of conidia on the surface of flowers and fruit, first latent infection, number of conidia on the fruit surface and incidence of post-harvest brown rot (Villarino et al., 2012).
Understanding the spread of crop diseases is important to develop methods to reduce crop losses (Madden et al., 2007). The spread of crop diseases over distances ranging from a few metres to thousands of kilometres is due to the long-term dispersal of pathogenic fungi (Brown & Hovmøller, 2002). Predictive disease modelling has mainly focused on the timing and level of risk, as well as their frequency to help choose appropriate treatments (Segarra et al., 2001).
Holb’s review (2013) discusses the models developed to date on brown rot caused by the three main species of Monilinia spp. Luo et al. (2001) developed a risk analysis system for blossom blight of prune caused by M. fructicola. Luo and Michailides (2001) developed a similar risk analysis system for latent infection of prune caused by M. fructicola. For diseases caused by Monilinia spp. regression models have been developed to quantify the incidence of blighted blossom (Luo et al., 2001b) and fruit brown rot (Biggs & Northover, 1988) caused by M. fructicola on cherry and peach trees as a function of environmental factors. Another study on the phenological analysis of blossom blight of sweet cherry brown rot caused by M. laxa, was carried out by Tamm et al. (1995). Cherry brown rot caused by M. laxa in the Jerte Valley (Cáceres, Spain) could also be predicted by the number of consecutive days with relative humidity above 80% during March and April (Larena et al., 2021). Luo and Michailides (2003) developed a preliminary decision support model to guide fungicide application to reduce risk of prune fruit rot caused by M. fructicola. Holb et al. (2011) developed a brown rot forecasting and disease management model for M. fructigena on organic apple orchards. Huang (2003) developed a time and space-based model of brown rot incidence caused by M. laxa in a peach orchard in southern France, using a compartmental model. Although this model constitutes a good starting point it is limited due to the large number of parameters, which have to be estimated (Huang, 2003). Bevacqua et al. (2018) modified classical compartmental models to describe the temporal dynamics of brown rot spread only in the last stage of the fruit growth (from the end of pit hardening to harvest time) using field data from a peach orchard infected by M. laxa and M. fructicola in southern France, but without including the blossom stage or latent infections. This epidemiological model was recently coupled with a fruit-tree growth model that permitted to evaluate different agronomic practices such as fruit thinning and regulated deficit irrigation on expected yield quantity and quality (Bevacqua et al., 2019).
In theoretical epidemiology, compartmental models are often used with individuals classified as healthy (H), latent (L), infectious (S), and post-infectious (R) (Madden et al., 2007; Segarra et al., 2001). The aim of this paper is to develop a model of the brown rot disease in the three physiological host stages (blossom, immature and mature fruit) that explains the pattern of the epidemics in Ebro Valley and takes into account the host susceptibility, sources of primary and secondary inoculum, and latent infection of immature fruit.
Materials and methods
Experimental data
Brown rot incidence was documented through field surveys in three different commercial peach orchards (Prunus persica (L.) Batsch and P. persica (L.) Batsch var. nucipersica) over three growing seasons (from February to October) in the Ebro Valley (Lleida, Spain). Three consecutive surveys were conducted in two orchards located in Sudanell and Alfarrás, and two consecutive surveys were conducted in one orchard located in Albesa (Table 1). Within each of the orchards of approximately 150 trees, a disease study zone of 80 trees was delimited, among which 30 trees were randomly marked as observation zone, for the quantification of the primary inoculum (mummies) (Villarino et al., 2010) and secondary inoculum (diseased flowers and fruit) and to study the temporal evolution of the disease (Villarino et al., 2012). The remaining trees (approximately 50 trees), which were not marked within the study area were sampled to estimate latent infections on different dates throughout the crop season each year. The management of the orchards was the standard in the area, except for the absence of fungicide treatments. The Albesa orchard was used for one growing season for epidemic adjustment.
In these three orchards the dynamics of the disease along with the development of the crop was analysed determining the wintering inoculum of Monilinia spp., airborne conidia in the orchards, conidia on the surface of flowers and fruit, latent infections, disease incidence and host susceptibility to brown rot. These values were used in the development of a compartmental model of the disease, in the estimation of the different parameters of the model and in the determination of the per capita rate of disease transmission by nonlinear regression using the ordinary least squares method (Table 2).
Model description
The brown rot model was subjected to the following three main conditions (Kosman & Levy, 1994): i) the variables and parameters of the model must be biologically realistic and adequately reflect the corresponding biology of the system, ii) the model should have a high similarity between the dynamics of the simulated and observed disease, compared to the experimental data, and iii) the model must be simple enough for analytical study.
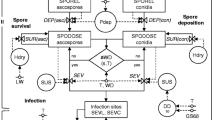
The model was used to show the dynamics of brown rot disease during one growing season in Albesa peach orchards. A “closed population” was assumed, with no migration to the pathogen population. It was also assumed that the host and pathogen populations were genetically and spatially homogenous. The individual in this model was the peach, which, during its development, passed through three physiological stages: blossom, immature fruit and mature fruit. Therefore, the dynamics of the disease would vary in each of the three stages of peach development and thus three sub-models were formulated, one for each stage. A representation of how the model works is shown in Fig. 1 and abbreviations are explained in Table 2.
Schematic representation of the epidemic model of peach brown rot. Boxes represent state variables; arrows show the flow of the infectivity status of individuals from one state variable to another. All variables and parameters are listed in Table 2
Blossom
In spring, during flowering, if the weather conditions were suitable, the mummies (X) left in the orchard the previous winter produce conidia. These conidia were dispersed through the air and when deposited on blossom were able to infect. In our experimental conditions, the mummies are the main source of primary inoculum (Villarino et al., 2010), but the state variable X includes all other sources of primary inoculum such as necrotic twigs and the cankers. Infected blossom produced spores and transmit the infection to new blossoms through secondary cycles. Flowers were classified as healthy (Sf), infectious (If) (if flowers showed symptoms of Monilinia spp. necrosis and sporulation) and dead or removed (Rf) (if flowers no longer sporulate).
The progress from Sf to If was due to the transmission of the disease through the primary inoculum X and/or through secondary inoculum. The probability of infection of a healthy or susceptible flower Sf was proportional to the number of mummies in the orchard βpX where βp was the per capita primary infection rate. The probability that a Sf flower was infected by an infected flower If was proportional to the number of If flowers in the orchard βsIf, where βs was the per capita secondary infection rate. The rate of transmission of the disease per unit of time was, therefore, equivalent to (βpX + βsIf)Sf. This S-I-R disease progression did not include latent infections in bloom stage, since the percentage of flowers with latent infections that become fruit is less than 1% (Luo & Michailides, 2003).
After the infectious period, If became removed flowers Rf that did not transmit the disease. In order to facilitate analysis of the model, we assumed that the infectious period had an exponential distribution and that the rate of progression from If to Rf was μfIf, where the μf reciprocal (1/μf) was equivalent to the mean infectious period. Assuming that these epidemiological parameters were constant during flowering, the following SIRX equations were obtained:
It is assumed that on day 1 all individuals are flowers and that the blossoming period lasts 20 days. The graphical representation of blossoming epidemic, state variables and flows are shown at the top of Fig. 1.
Immature fruit
After blossoming, healthy flowers (Sf) developed into healthy immature fruit (Sg). Although the number of individuals in the population in this process was substantially reduced, the number was kept constant to facilitate the comparison of the parameters of the different sub-models. The development phase of the immature fruit was the longest, from the small-fruit stage until it began to ripen. Mummies from the previous year and infectious blighted blossoms are still present in the orchard. Both inoculum sources (primary and secondary) produced spores almost until summer, especially when environmental conditions were suitable. Due to the agro-climatic conditions of the Ebro Valley, no immature rotten peaches were observed. In our field experiments during the immature fruiting period there was no rotten fruit sporulating on the trees. During the immature fruit development stage, healthy fruit (Sg) that were infected remained latent (Lg). Disease transmission was modelled as in the flowering stage, although the lower susceptibility of the fruit meant that the parameters βp and βs were likely to be lower. The differential equations system that reflected the disease dynamics for the immature fruit development stage were as follows:
The epidemic on immature fruit develops from day 21 to day 140. At the end of blossoming period (day 20) all healthy flowers (Sf) are instantaneously become healthy immature fruit (Sg) as shown by the flow from Sf to Sg in Fig. 1. This flow is not explicitly modeled by an equation since the number of healthy flowers of day 20 is equal to the initial number of healthy immature fruit on day 21 of the epidemic (first day of the immature fruit epidemic). During this period, infectious flowers continue to sporulate and progressively die and become post-infectious flowers (Rf).
Mature fruit
When the fruit begins to ripen, the first rotten peaches appear on the trees. Latent infections in fruit (L) become progressively active depending on climatic conditions. Simultaneously, ripe fruit with brown rot symptoms sporulate immediately and abundantly and spread the disease to other fruit by contact or aerial spread of inoculum. When healthy fruit (S) becames infected, it develops an active latent infection (E), which after the latency period became infectious mature fruit (I). I fruit progress to class R (removed) when the infective period has elapsed. Some of these fruit will mummify and become a source of primary inoculum the following year.
During the mature fruit development stage, it was assumed that the fruit with inactive latent infections L become active latent infections E before becoming infectious I. Such progression could be made in two ways: i) by the evolution of the latent infection itself with a constant rate γ, or ii) by a new secondary infection with a rate βsl. The sub-model of disease dynamics during the mature fruit stage was formulated using the following SLEIR equations:
The mature fruit epidemic starts on day 141 and lasts until harvest on day 170. Healthy fruit (Sg) and latent immature fruit (Lg) at the end of day 140 instantly become healthy (S) and latent mature fruit (L), respectively. These flows are reflected in Fig. 1 with the arrows going from Sg to S and from Lg to L. The graphical representation of the whole epidemic model is shown in Fig. 1.
The simulation model for each of the three physiological stages of crop development was performed separately using the BERKELEY MADONNA version 8.0 program (Modelling and Analysis of Dynamic Systems, University of California, Department of Molecular and Cellular Biology, Berkeley, CA, USA http://www.berkeleymadonna.com).
Model parameter estimation
Variables and parameters used in the model are presented in Table 2. The size of the crop population was assumed to be constant throughout the growing season. The number of individuals N was estimated at 105 peaches/ha. Although the number of blossoms was much higher, the same number was used in order to facilitate comparison of rates of infection in blossom and fruit. The two basic components in the plant x pathogen interaction of an individual’s disease were the latent period and the infectious period. Both values depend on the development stage of the peach. The latent period of ripe fruit was very short (1/σ = 3 days). Under optimal laboratory conditions, symptoms and sporulation was observed after 2 days of inoculation. M. fructicola also sporulates at high temperatures on both peaches and nectarines 2–3 days after inoculation (Bernat et al., 2017). The model considered a mean latency period (1/σ) of 3 days, since conditions were not as optimal as in the laboratory and it was a mean value of the last 30 days before harvest.
In nectarines with visible brown rot caused by M. fructicola, the disease usually appeared after 24 h on the surface and in the uppermost layers of epidermal cells, which began to collapse after 48 h (Garcia-Benitez et al., 2016). Under optimal conditions, the decay of infected ripe peaches and nectarines may be visible 48–72 h after infection (Byrde & Willetts, 1977).
No information is available about the duration of the infectious period of Monilinia spp. in orchards. Our field observations showed that infected flowers had the ability to sporulate for several months if it rained. Based on that observation, so a mean infectious period (1/μf) of 45 days was assumed. In mature fruit, brown rot progressed rapidly and this fruit remained infectious for several weeks. A mean infectious period (1/μ) of 30 days was used in the model (Table 2).
Several authors have analysed the correlation between latent infections and the brown rot incidence at harvest. Luo and Michailides (2003) conclude that activation of latent infections on immature fruit occurs with fruit ripening and depends mainly on the amount of latent infections, stage of fruit development, inoculum concentration, timing of infection and weather conditions. No signs of disease are observed on the surface of nectarines with a M. fructicola latent infection over the 288-h (12 days) incubation period (Garcia-Benitez et al., 2016). The model estimated an activation rate constant γ = 0.05 from day 30 before harvest time (Gell et al., 2008). At this rate, approximately 80% of the fruit with latent infections had developed brown rot at harvest time (Table 2).
In the field, viable conidia are detected in tree mummies until near summer (Casals et al., 2015). Based on this information, a mean survival period of the mummies of 3 months was estimated, equivalent to a mortality rate of the mummies of h = 1/90 day−1 (Table 2).
The primary and secondary infection rates are the most difficult parameters to estimate and their values were specific to each epidemic. For epidemics of a single growing season, these parameters are estimated on the basis of i) the exponential rate of the initial epidemic growth, ii) the final scale of the disease or iii) the adjustment of the model to the epidemic observed (Diekmann & Heesterbeek, 2000; Heffernan et al., 2005). The incidence of brown rot observed at harvest in the three orchards during the study years ranged from 0.1 to 15%, so the transmission parameters were variable depending on the year and field. Epidemics were adjusted to the model by least squares assuming that rates of primary and secondary infection were the same (βp = βs). An infection rate in flowers and immature fruit of βp = βs = 1.5 × 10−5, and a secondary infection rate in mature fruit of β = 3.0 × 10−5 were taken as default parameters (Table 2).
Adjustment of a brown rot epidemic
The second brown rot epidemic in the Albesa peach orchard fitted the compartmental model. Data on the incidence of blossom, latent infections and rotten fruit were recorded from the time of blossom (day 0) to the time of harvest (day 170) during one growing season (Table 3). The incidence of blighted blossom and brown rot were determined by visual detection of disease signs every 15 days on thirty trees selected randomly. The incidence of latent infection on the flowers or fruit was estimated from 100 healthy-looking surface flowers or 100 fruit used above that were collected at each growth stage using a previously described protocol (Villarino et al., 2010).
Results
Simulation of blossom blight model
The progress of the blossoms blight incidence is shown in Fig. 2. The dynamics of the number of infectious flowers appears to be exponential. At the end of flowering (20 days after flowering), the progress of the epidemic in bloom was interrupted by the lack of flowers and the incidence of blighted blossom remains constant throughout the growing season (Fig. 2). The flowers gradually ceased to be infectious and became post-infectious. With the default values of the parameters, the incidence on the flowers was close to 180/ha. This was a small amount but in agreement with field observations. The number of blighted blossoms observed in the field at the end of flowering ranged from 0 to 1 per tree, depending on the field and year.
Simulation of the epidemic in flowers caused by Monilinia spp. [number of infectious flowers per hectare (I flower), number of removed (post-infectious) flowers per hectare (Rf) and number of diseased flowers] throughout the growing season (170 days). The flowering period is from day 0 to day 20. The values of the parameters used in the simulation are shown in Table 2
Simulation of immature fruit model
The dynamics of the number of immature fruit with latent infections is shown in Fig. 3a for the period from day 20 to day 140. The simulated epidemic pattern appeared to resemble monomolecular growth. Although the blossom epidemic was abruptly interrupted, infectious blossoms and primary inoculum sources continued to produce spores and infect immature fruit. Infections of immature fruit remained latent until the fruit ripened, at which time pathogen growth was activated. The incidence of latent infections on immature fruit (Lg) increased steadily throughout the period, although the absolute growth rate was highest at the beginning and decreased progressively. This pattern conforms to what is expected throughout this period when secondary disease transmission does not occur (Madden et al., 2007). The latent infections were the result of the initial inoculum, which decreased over time. A more variable pattern was observed in the field. Low incidence levels varied between dates and fields and ranged from 0 to 10%.
Simulation of the incidence of latent infections and brown rot caused by Monilinia spp. on fruit. (a) Incidence of latent infections [inactive (Lg, L) plus active (E)], (b) number of infectious rotten fruit per hectare (I), and number of removed (post-infectious) fruit per hectare (R), and (c) brown rot incidence. The values of the parameters used in the simulation are shown in Table 2
Simulation of mature fruit model
At the beginning of the ripe fruit stage (day 141) the simulation showed a small reduction in the number of latent infections in immature fruit (Fig. 3a). After an initial period of increase (driven by primary infection), the increase in the proportion of latent infections slowed down. The slowdown is due to inoculum decay, which reduces the rate of development of primary infections before secondary infection begins to accumulate (Gilligan & Kleczkowski, 1997). However, as the number of infectious fruit increased, the number of secondary infections increased and the incidence of latent infections increased (Fig. 3a). Finally, at this stage, most of the fruit with latent infections did not come from infections of immature fruit in which the pathogen had stopped growing, but came from the latency period of infected mature fruit, i.e., the time elapsed from infection to the onset of sporulation (3 days).
In the epidemic development of fruit rot, all initial inoculum came from latent infections of immature fruit, as blighted blossoms infected in spring and mummies from the previous year no longer produced inoculum. These latent infections gradually became activated, transforming into infectious fruit capable of transmitting the disease to other fruit. The progression of brown rot incidence in ripe fruit appeared to be exponential (Fig. 3b, c) and followed the pattern of the epidemic observed in the field. The incidence of brown rot at harvest, with the default values of the parameters used (Table 2) was approximately 15% (Fig. 3b, c). This incidence roughly corresponds to the average incidence observed in the field, depending on pre-harvest weather conditions for a range between less than 1% and up to 50%.
Adjustment of a brown rot parameters
The model was fitted by ordinary least squares regression to the epidemic observed in the Albesa peach orchard as a function of the disease transmission parameters βp, βs and β, using the default values (Table 2) for the remaining parameters. The best adjustment was obtained for values βp = β s = 1.5 × 10−6 and β = 2.7 × 10−6 in the Albesa peach orchard. Figure 4 shows the goodness of fit of the regression to the epidemic pattern in ripe fruit, although the predicted blossom incidence was lower than observed.
Sensitivity of disease dynamics to parameters
The sensitivity of the disease dynamics to changes in the parameter values used was analysed. In this way parameters can be found that affect the epidemic severity. Model simulations showed a small decrease in latent infections at the beginning of the fruit ripening period due to their activation, but they quickly increased due to new secondary infections leading to active latent infections (E) (Supplementary Fig. S1a). Model also showed that reducing the number of mummies in the orchard significantly reduced the brown rot incidence at harvest time (Supplementary Fig. S1b) but not to eradicate the disease.
Other recommended control measures like fungicides applications during last weeks before harvest or the use of resistant varieties reduce the rate of secondary fruit infection β (Gilligan, 2002). Model sensitivity is shown using different values of β (Fig. 5a). The brown rot incidence at harvest was approximately 2%, 14% and 52% for β values of 1 × 10−6, 3 × 10−6 and 5 × 10−6 day−1, respectively. These results showed that the model was very sensitive to β. Small changes in the econdary infection rate β have a proportionately greater effect on the brown rot incidence at harvest due to the non-linear effect of disease transmission (Gilligan, 2002). Therefore, favourable meteorological conditions for infection also have important effect on disease development by increasing the value of the β parameter.
Simulation of the brown rot incidence caused by Monilinia spp. for (a) different per capita secondary infection rates in ripe fruit (β = 1 × 10−6, β = 3 × 10−6, and β = 5 × 10−6), and (b) different activation rates of latent infections (γ) in immature fruit (γ = 0.02, γ = 0.06, and γ = 0.1). The remaining values of the parameters used in the simulation are shown in Table 2
Under our climatic conditions, we did not observe immature fruit with rot symptoms on the tree. However, during this period there were infections that remain latent in the fruit from the primary inoculum and blighted blossoms. This means that at the beginning of the ripe fruit stage there may potentially be a large amount of secondary inoculum. This will depend on the percentage of latent infections that are activated. If only a small percentage of the latent infections are activated, there would be a large amount of fruit capable of transmitting the disease. Several numerical simulations were carried out to compare the effect of the activation rate of latent infections in immature fruit γ on the development of brown rot (Fig. 5b). Figure 5b shows how the higher the activation rate of latent infections, the higher the incidence of rot at harvest.
Discussion
A compartmentalised epidemiological model of brown rot was developed for the Ebro Valley incorporating three physiological stages of the crop: blossom, immature fruit and mature fruit. Other authors have identified only two phases in brown rot development (Bevacqua et al., 2018; Luo et al., 2001b). The model takes into account, host susceptibility, primary and secondary sources of inoculum and latent infections in immature fruit.
The proposed model described well the epidemics observed in the Ebro Valley. The dynamics of the number of infectious flowers appeared to be exponential in the model, although the incidence of blighted blossoms on peach and nectarine trees observed in the Ebro Valley was very low and only sporadically detected. In this model, LIs were not included in the flower stage as they do not affect its dynamics. Generally only 15–20% of peach flowers set to fruit. The average LI at bloom stage is less than 10% and only 10% of these become fruit (Luo & Michailides, 2003). Infections of immature fruit remain latent until the fruit ripens, and pathogen growth is activated (Gell et al., 2008; Villarino et al., 2012). The model simulation shows a monomolecular pattern of latent infection incidence during the immature fruit stage. The incidence of latently infected fruit is low, with an average ranging from 0 to 5% (Gell et al., 2008) and varies according to phenological stage and climatic conditions. Brown fruit rot was detected 3 to 5 weeks before harvest. As soon as the first fruit appears, the epidemic spreads exponentially and the incidence of brown rot at harvest can exceed 50% if climatic conditions are favourable.
Sensitivity analysis of the model parameters has shown that the infection rate and, in particular, the secondary infection rate of ripe fruit β is a critical parameter in the incidence of brown rot. Disease transmission is non-linear term implying that a small change in infection rate can have a large influence on disease development. A key factor in epidemiological models is disease transmission, which in the present model has been described as a βIS transmission term. The incorporation of heterogeneousness in the interaction will require more complex terms related to the threshold for pathogen invasion and the criterion for disease persistence (Diekmann & Heesterbeek, 2000). Small changes in secondary infection rate β have a disproportionally larger effect on the incidence of brown rot at harvest as a result of the non-linear effect of disease transmission (Gilligan, 2002).
Compartmental epidemiological models are very flexible and allow for adding complexities to the system and evaluating different control strategies. When the epidemiological effect of a control measure on parameter values has been established, the effect of the control measure on disease dynamics can be analysed through simulation (Gilligan, 2002).
The model tested the usefulness of eradicating mummies from the field to reduce the final incidence of the disease. These results are similar to those observed by Huang (2003). Bevacqua et al. (2018) find that crop load has important consequences on the progression of brown rot epidemics. Bevacqua et al. (2018, 2019) recommend several measures against rot following an epidemiological study, e.g., applying fruit treatments in early summer rather than closer to harvest time, which will also be less detrimental to human health. A decrease in fruit growth rate would increase yield by decreasing the incidence of brown rot and is consistent with the observation of Mercier et al. (2008), who found that water deprivation decreased fruit size and thus brown rot incidence; removal of infectious fruit during the growing season could significantly help in brown rot control by decreasing the average infectious period; an intermediate level of fruit load would maximize yield in the presence of the disease and highlights the importance of evaluating the consequences of agricultural practices on disease control in a comprehensive epidemiological framework (Bevacqua et al., 2019). All control measures that reduce the rate of secondary infection in mature fruit, including integrated control measures such as the use of more resistant varieties and the combination of fungicides with non-chemical methods (Holb, 2019), have also been shown to be effective in reducing the incidence of brown rot.
These conclusions have been reached using the default values of the parameters in Table 2 or by modifying them. In the modelled system, there is an interaction between the parameters, so the effect of one parameter may vary depending on the value of the others. Therefore, we cannot draw general conclusions from specific numerical simulations.
Although the disease progress curve is a function of epidemic parameter values, the qualitative dynamics is characterised by the basic reproduction rate (R0). R0 is defined as the mean number of new infections produced by a single diseased individual when introduced into a fully susceptible population (Anderson & May, 1979). The R0 value of a disease shows whether an epidemic will develop, depending on whether it is greater or less than one. R0 also determines final disease incidence (Jeger & van den Bosch, 1994; Swinton & Anderson, 1995). The calculation of R0 also allows the effectiveness of control measures be assessed. For the simple SLEIR fruit rot model
and replacing the parameters by the default values in Table 2, R0 = 9. This calculated value is only an approximation of the true disease transmission capacity since, at the time of harvest, the vast majority of brown rot affected fruit are still infectious and have not completed their full disease transmission potential. The value obtained in this work is substantially higher than the maximum value of 4 obtained by Bevacqua et al. (2018) from a peach field near Avignon (France). The proposed model incorporates several complexities, such as two sources of inoculum, the passage through different stages and the existence of large infectious periods in relation to the duration of the epidemic. All these factors complicate the calculation of R0 and further analysis is required. In this regard, the approach taken by Bevacqua et al. (2018), to our knowledge, is new and interesting as they obtained the R0 of an infected individual as function of infection time and harvest time. Thus, the R0 value decreases as harvest approaches because the newly infected peach does not have enough time to go through its entire infectious period before harvest. The Bevacqua et al. (2018) approach for calculating R0 can be very useful for characterizing annual crop epidemics.
The proposed epidemiological model is an approximation to the dynamics of brown rot in peach trees. In the future, the model should be complemented by introducing the fruit incidence from thinning operations, the effect of the incidence of brown rot in the previous year, the survival rate of mummies, variability as a consequence of environmental factors, plant-pathogen interaction and the process by which the disease is transmitted. Finally, the introduction of space, which can be an important element in understanding how the disease spreads in the field (Madden et al., 2007). The prolific production of conidia, which are disseminated by wind and rain, allows for rapid epidemic development within an orchard or region (Byrde & Willetts, 1977). The form of inoculum dissemination, aerial and contact, confers certain characteristics relevant to the development of the epidemic, which would help to complete the understanding of the spatio-seasonal dynamics of brown rot. Rotten fruit are known to transmit the disease to all healthy fruit by contact with each other (Michailides & Morgan, 1997). The variability observed in plant disease epidemics has been modelled (Gibson et al., 1999; Kleczkowski et al., 1996). In addition to the above elements, fungicide resistance in the pathogen population and the role of host resistance should be included in future models (Holb, 2019). The formulation of a stochastic model would allow to explain the observed variability between epidemics, to estimate the evolution of the probability distribution of rot incidence and thus to predict the epidemic risk under different conditions (Gilligan, 2002).
Data availability
Not applicable.
Code availability
Not applicable.
References
Anderson, R. M., & May, R. M. (1979). Population biology of infectious-diseases, 1. Nature, 280(5721), 361–367.
Bernat, M., Segarra, J., Xu, X. M., Casals, C., & Usall, J. (2017). Influence of temperature on decay, mycelium development and sporodochia production caused by Monilinia fructicola and M. laxa on stone fruits. Food Microbiology, 64, 112–118. https://doi.org/10.1016/j.fm.2016.12.016
Bevacqua, D., Quilot-Turion, B., & Bolzoni, L. (2018). A model for temporal dynamics of Brown rot spreading in fruit orchards. Phytopathology, 108, 595–601.
Bevacqua, D., Génard, M., Lescourret, F., Martinetti, D., Vercambre, G., Valsesia, P., & Mirás-Avalos, J. M. (2019). Coupling epidemiological and tree growth models to control fungal diseases spread in fruit orchards. Scientific Reports, 9, 8519. https://doi.org/10.1038/s41598-019-44898-6
Biggs, A. R., & Northover, J. (1988). Early and late-season susceptibility of peach fruits to Monilinia fructicola. Plant Disease, 72, 1070–1074.
Brown, J., & Hovmøller, M. S. (2002). Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science, 297(5581), 537–541. https://doi.org/10.1126/science.1072678
Byrde, R. J., & Willetts, H. J. (1977). The brown rot fungi of fruit - their biology and control. Pergamon.
Casals, C., Segarra, J., De Cal, A., Lamarca, N., & Usall, J. (2015). Overwintering of Monilinia spp. on mummified stone fruit. Journal of Phytopathology, 163(3), 160–167.
Diekmann, O., & Heesterbeek, J. A. P. (2000). Mathematical epidemiology of infectious diseases: Model building, analysis and interpretation. Wiley.
Garcia-Benitez, C., Melgarejo, P., De Cal, A., & Fontaniella, B. (2016). Microscopic analyses of latent and visible Monilinia fructicola infections in nectarines. PLoS One, 11(8), e0160675. https://doi.org/10.1371/journal.pone.0160675
Gell, I., De Cal, A., Torres, R., Usall, J., & Melgarejo, P. (2008). Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. European Journal of Plant Pathology, 121, 487–498.
Gibson, G. J., Gilligan, C. A., & Kleczkowski, A. (1999). Predicting variability in biological control of a plant–pathogen system using stochastic models. Proceedings of the Royal Society B Biological Sciences, 266(1430), 1743–1753. https://doi.org/10.1098/rspb.1999.0841
Gilligan, C. A., & Kleczkowski, A. (1997). Population dynamics of botanical epidemics involving primary and secondary infection. Philosophical Transactions of The Royal Society B Biological Sciences, 352(1353), 591–608. https://doi.org/10.1098/rstb.1997.0040
Gilligan, C. A. (2002). An epidemiological framework for disease management. Advances in Botanical Research, 38, 1–64. https://doi.org/10.1016/S0065-2296(02)38027-3
Heffernan, J. M., Smith, R. J., & Wahl, L. M. (2005). Perspectives on the basic reproductive ratio. Journal of the Royal Society Interface, 2, 281–293.
Holb, I. (2013). Disease warning models for brown rot fungi of fruit crops. International Journal of Horticultural Science, 19, 1–2. https://doi.org/10.31421/IJHS/19/1-2/1078
Holb, I. J., Balla, B., Abonyi, F., Fazekas, M., Lakatos, P., & Gáll, J. M. (2011). Development and evaluation of a model for management of brown rot in organic apple orchards. European Journal of Plant Pathology, 129, 469–483. https://doi.org/10.1007/s10658-010-9710-1
Holb, I. (2019). Brown rot: Causes, detection and control of Monilinia spp. affecting tree fruit. In University of Debrecen and Hungarian Academy of Sciences, Hungary. Integrated management of diseases and insect pests of tree fruit (pp.103-150). https://doi.org/10.19103/AS.2019.0046.06.
Huang, Y. (2003). The interplay of migration and population dynamics in a patchy world. Utrecht University, Ph.D dissertation. Electronically available at http://www.library.uu.nl/digiarchief/dip/diss/2003-0709- 122256/inhoud.htm.
Jeger, M. J., & van den Bosch, F. (1994). Threshold criteria for model-plant disease epidemics.1. Asymptotic results. Phytopathology, 84, 24–27.
Kleczkowski, A., Bailey, D. J., & Gilligan, C. A. (1996). Dynamically generated variability in plant–pathogen systems with biological control. Proceedings of the Royal Society B Biological Sciences, 263(1371), 777–783. https://doi.org/10.1098/rspb.1996.0116
Kosman, E., & Levy, Y. (1994). Fungal foliar plant pathogen epidemic: Modeling and qualitative analysis. Plant Pathology, 44, 328–337.
Larena, I., Villarino, M., Melgarejo, P., & De Cal, A. (2021). Epidemiological studies of Brown rot in Spanish cherry orchards in the Jerte Valley. Journal of Fungi, 7, 203. https://doi.org/10.3390/jof7030203
Lee, M. H., & Bostock, R. M. (2007). Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox? Phytopathology, 97, 269–277. https://doi.org/10.1094/PHYTO-97-3-0269
Luo, Y., Morgan, D. P., & Michailides, T. J. (2001). Risk analysis of brown rot blossom blight of prune caused by Monilinia fructicola. Phytopathology, 91, 759–768.
Luo, Y., & Michailides, T. J. (2001). Factors affecting latent infection of prune fruit by Monilinia fructicola. Phytopathology, 91, 864–872.
Luo, Y., & Michailides, T. (2003). Threshold conditions that lead latent infection to prune fruit rot caused by Monilinia fructicola. Phytopathology, 93, 102–111.
Madden, L. V., Hughes, G., & Van den Bosch, F. (2007). The study of plant disease epidemics. St. APS Press.
Mercier, V., Bussi, C., Plenet, D., & Lescourret, F. (2008). Effects of limiting irrigation and of manual pruning on brown rot incidence in peach. Crop Protection, 27, 678–688.
Michailides, T. J., & Morgan, D. P. (1997). Influence of fruit-to-fruit contact on the susceptibility of French prune to infection by Monilinia fructicola. Plant Disease, 81, 1416–1424.
Segarra, J., Jeger, M. J., & van den Bosch, F. (2001). Epidemic dynamics and patterns of plant diseases. Phytopathology, 91, 1001–1010.
Swinton, J. & Anderson, R. M. (1995). Model frameworks for plant-pathogen interactions. Ecology of infectious diseases in natural populations, ed. BT Grenfell and AP Dobson, Cambridge University press, pages 280-294.
Tamm, L., Minder, C. E., & Fluckiger, W. (1995). Phenological analysis of brown-rot blossom blight of sweet cherry caused by Monilinia laxa. Phytopathology, 85, 401–408.
Villarino, M., Melgarejo, P., Usall, J., Segarra, J., & De Cal, A. (2010). Primary inoculum sources of Monilinia spp. in Spanish peach orchards and their relative importance in brown rot. Plant Disease, 94, 1048–1054.
Villarino, M., Sandin-España, P., Melgarejo, P., & De Cal, A. (2011). High Chlorogenic and Neochlorogenic acid levels in immature peach reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. Journal of Agricultural and Food Chemistry, 59, 3205–3213. https://doi.org/10.1021/jf104251z
Villarino, M., Melgarejo, P., Usall, J., Segarra, J., Lamarca, N., & De Cal, A. (2012). Secondary inoculum dynamics of Monilinia spp. and relationship to the incidence of postharvest brown rot in peaches and the weather conditions during the growing season. European Journal of Plant Pathology, 133, 585–598. https://doi.org/10.1007/s10658-011-9931-y
Villarino, M., Egüen, B., Lamarca, N., Segarra, J., Usall, J., Melgarejo, P., & De Cal, A. (2013). Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. European Journal of Plant Pathology, 137, 835–845. https://doi.org/10.1007/s10658-013-0292-6
Acknowledgments
This study was supported by grant PID2020-115702RB-C21 and AGL2017-84389-C2-2-R from the Ministry of Science and Innovation (Spain). We thank M.T. Morales Clemente and R. Castillo for technical support, and the peach growers for their support and collaboration.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by grant PID2020-115702RB-C21 and AGL2017–84389-C2–2-R from the Ministry of Science and Innovation (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The authors declare that ethical standards have been followed and that no human participants or animals were involved in this research.
Consent to participate
Not applicable.
Consent for publication
All authors consent to this submission.
Conflict of interest
This work was not submitted for publication to another journal. All authors have contributed to the work, have read the manuscript and declare that there are no potential conflicts of interest.
Supplementary information
Supplementary Fig. S1
Simulation of the incidence of (a) latent infections [inactive (Lg, L) plus active (E)], and (b) brown rot caused by Monilinia spp. in peach orchards for different amounts of initial inoculum (X = 1, X = 30, and X = 100 mummies per hectare). The remaining values of the parameters used in the simulation are shown in Table 2 (JPG 233 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Villarino, M., Usall, J., Casals, C. et al. Development of brown rot epidemics in Spanish peach orchards. Eur J Plant Pathol 163, 641–655 (2022). https://doi.org/10.1007/s10658-022-02504-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02504-y