Abstract
Finding synthetic pesticide alternatives for health and a healthy environment has become a crucial issue for scientific research. A number of studies have reported efficacy of Bacillus species on promoting plant development, as well as protecting plants against pathogen invasion, especially pathogenic fungi and bacteria. However, little was known about Bacillus species in controlling viral diseases. In this study, Bacillus atrophaeus strain HAB-5, isolated from cotton field, Xinjiang, China efficiently promoted the growth of tobacco plants. According to the results, the treatment with the strain HAB-5 increased the expression of NtEXP1 and NtEXP2. Then the Tobacco mosaic virus (TMV)/ Nicotiana tobacco system was employed to evaluate virus resistance induced by strain HAB-5. Tobacco leaves were treated with antimicrobial metabolites of strain HAB-5 (1 mg/mL), and 12 h later the treated leaves were challenged with TMV via rub-inoculation. The results showed that disease symptoms were obviously compromised by tobacco leaves treated with strain HAB-5, and the viral accumulation level was reduced extensively. Moreover, it was found that the signaling regulatory gene (NPR1), defense genes (PR-1a, PR-1b, Chia5), and hypertensive response related genes (Hsr203J, Hin1) were up-regulated in plants treated with the metabolites. Altogether, these accumulated results strongly support strain HAB-5 to be a biological controlling agent against TMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth promoting rhizobacteria (PGPR), the beneficial bacteria having ability to enhance plant growth and control plant diseases by colonizing the roots have been well recognized for various beneficial effects in crop plants (Bodelier et al. 2000; Eric and Donohoe 2003; Nie et al. 2015; Ge et al. 2015). Their usage as biological control agent increases health and productivity of different plant species under various edaphic conditions (Kloepper et al. 2009; Chaudhry et al. 2012; Bharti et al. 2016; Devendra et al. 2016; Bharti et al. 2016); PGPR often resists phytopathogenic negative effects by inhibiting them (Leeman et al. 1996; Wang et al. 2009; Khan et al. 2012).Various studies has been carried out focusing on the identification of Bacillus spp. for their ability to promote plant growth and disease control (Emmert and Handelsman 1999; Kloepper et al. 2004; Niu et al. 2011; Piromyou et al. 2011).Various strains of B. amyloliquefaciens, B. subtilis, B. pumilus, elicit significant reductions in the incidence severity of various diseases on a diversity of hosts (Zhang et al. 2004; Han et al. 2006; Raj et al. 2008; Raza et al. 2016). Therefore, several PGPR based products are recently available on commercial basis mostly containing Bacillus spp. Strains (Emmert and Handelsman 1999).
These PGPRs, commonly known biocontrol agents, are famous for producing variety of metabolites that can improve plant resistance against different crop pathogens, including lipopeptides, lytic enzymes, etc. (Worasatit et al. 1994; Ongena et al. 2007). It is interesting to note that different studies focused on harvesting these secreted compounds from culture suspension, cell filtrate and crude extract have been applied directly on plant as foliar treatment. For example, culture suspension of Trichoderma. harzianum sprayed on leaves successfully reduced gray mold on fruits and foliage (Lo et al. 1997; Luca et al. 2016). Leaves sprayed with the spore suspension of Bacillus subtilis reduced different crop disease (Batchimeg and Dondov 2015). Zhang et al reported the efficacy of cell free supernatant of B. cereus on plant leaves to control Sclerotinia sclerotiorum (Zhang and Xue 2010). The foliar spray treatment of Streptomyces spp. was reported efficiently to control rice sheath blight pathogen (Yang et al. 2017). The crude extract metabolites from different strains of B. amyloliquefaciens were capable of reducing the severity of different diseases on plant (Ji et al. 2013).
Despite the successful use of Bacillus species, reports about Bacillus atrophaeus application are rare. We have reported that B. atrophaeus strain HAB-5 can control a broad spectrum of crop disease-causing agents (Rajaofera et al. 2017, 2019). B. atrophaeus are diverse in terms of plant protection playing important role in crop yield reducing risks. Furthermore, keeping in view the growing concerns over global food safety and environmental quality, efforts in increasing practical use of natural products need a better understanding and research.
The current investigation is an effort to elucidate the concept of B. atrophaeus strain HAB-5 as a biocontrol resource. We have investigated strain HAB-5’s ability to promote plant growth and evaluate the potential of crude metabolites extracts to control TMV on tobacco plant.
Materials and methods
Bacterial strain
The isolate designed as strain HAB-5 was obtained from a healthy cotton field in Xinjiang province, P.R China. After isolation, strain HAB-5 was examined for morphological, physiological and biochemical characteristics (Rajaofera et al. 2017, 2019).
Plant material, growth condition and TMV culture
Tobacco seeds were surface sterilized (2 min 70% ethanol, 5 min 5% calcium hypochlorite), rinsed several times with sterile water, and placed on Petri dishes containing half strength Murashige–Skoog (MS) medium containing 0.8% agar and 1.5% sucrose. The seeds were vernalized in dark for 72 h at 4 °C to break dormancy, and then germinated to pot culture containing autoclaved soil (vermiculite and Pindstrup substrate, in the ratio of 1:1). TMV was maintained by sap inoculations on N. tabacum cv. Samsun NN plants.
Assessment of plant growth promotion and plant inoculation
The ability of strain HAB-5 to promote plant growth was assessed. Tobacco seeds were surface sterilized as described above. The seedlings were grown till 3–4 leaves stage. Biological replicates of each treatment were used to minimize the influence of an individual plant results. Seedlings were transplanted individually into pots (with the same soil on which tobacco seeds were germinated) and grown.
Ten days after transplantation, bacterial strain was suspended in culture medium and incubated at 28 °C, 180 rpm for 24 h. After incubation, bacterial cells were harvested by centrifugation and re-suspended in distilled water, adjusted to 1 × 106–107 CFU/mL to maintain uniform cell density. The cell suspension (50 mL) was drenched in soil around each plant twice at weekly intervals. Plants only drenched with water were used as control. Experiments were carried out in three replicates and every replication contained 12 plants.
The experiment was terminated 60 days after inoculation. Plant length, fresh, dry weight of roots, shoots and chlorophyll content were measured from all harvested plants. Percent decrease or increase in each parameter was calculated with respective controls. The data obtained was analyzed statistically for standard error.
Quantification of HAB-5 strain colonization in N. tabacum rhizosphere
The resistant of the strain HAB-5 to antibiotics was first determined. The strain was streaked onto LB agar plate containing different antibiotics including 50 mg/L of streptomycin, 100 mg/L of riphamycine, 100 mg/L of ampicyline and 200 mg/L of kanamycin. The antibiotic which had the positive result was used for further experimentation.
The abundance of the PGPR rhizosphere was then quantified. Total root systems and tightly-adhering soil were harvested at five time points 0, 15, 30, 45 and 60 dpt. The samples were thoroughly mixed and 1 g of mixture was suspended in 9 ml of sterile 0.85% NaCl and vortexed for 5 min. The suspensions of each sample were plated on LB agar medium with 50 mg/L of streptomycin and 200 mg/L of kanamycin according to a previous method (Zhou et al. 2015). In order to inhibit the fungal growth, 100 mg/L of cycloheximide was also used. Colony Forming Units (CFUs) counting (based on morphological shape of HAB-5) was carried out after incubation at 28 °C for 24 h.
Biological control of tobacco mosaic virus by HAB-5 treatment
First, the antimicrobial metabolite from strain HAB-5 was extracted. The organism was cultured in LB for 48 h at 28 °C in an incubator shaker at 180 rpm. The fermentation broth was centrifuged at 10.000 rpm for 10 min at 4 °C. petroleum ether were added into the cell free supernatant in 1:1 ratio and shacked vigorously. The organic phase was concentrated to dryness under vacuum using rotary evaporator. The crude extract was lyophilized and re-suspended with distilled water (1 mg/mL). The substance obtained from the non-inoculated culture medium with same procedure was used as a control.
The evaluation of strain HAB-5 efficacy against TMV reduction and infection ratio was investigated. Two different set of experiment were carried out in order to evaluate strain HAB-5 effect against TMV. In one set of experiment, plants drenched with the bacteria root inoculation were subjected of TMV inoculation. In the other set of experiment, the crude extract antimicrobial metabolites from the strain were applied for foliar treatment and 12 h later the treated leaves were challenged with TMV via rub-inoculation. The effective treatment was used for further experiments.
For disease severity evaluation, plant treatments were assigned into three groups included HAB-5 + TMV group (sprayed with the antimicrobial metabolite crude extract from the strain 12 h before inoculation with TMV), mock+ TMV group (sprayed with the extract products from LB 12 h before inoculation with TMV) and control group (only sprayed with water, and PBS was used in the place of TMV). All experiments were carried out with three replicates.
The virus inoculum was prepared in the tobacco juvenile. Leaf tissue was macerated in 10 mL of 50 mM potassium phosphate buffer (pH 7.0). The suspension was sprayed on 3–4 leaf stage tobacco seedlings. Disease severity symptoms (mosaic, leaf deformation and stunting) were observed at 7 days post inoculation (dpi) according previous method (Manidipa et al. 2013). The rating scale were 0 = no symptoms observed; 1 = light mottling and a few thin yellow veins; 2 = mottling and vein clearing unevenly distributed on the leaf; 3 = mottling, leaf distortion, and stunting; and 4 = severe mottling, leaf curling, and stunting.
Enzyme-linked immunosorbent assay (ELISA)
ELISA analysis was carried out to determine the relative level of coat protein (CP) in leaves. Samples were ground in a 50 mM carbonate buffer, loaded onto ELISA 96-well plates, coated with plastic wrap, and incubated at 40 °C overnight. After washing with a washing buffer (0.5% Tween 20 in PBS), the plates were blocked with 3% nonfat dry milk in PBS at 37 °C for 1 h, and washed five times. Next, the primary antibody rabbit anti-TMV immunoglobulin (Ig) (1.0 μg/mL) was incubated in the 96-well plates at 37 °C for 1 h and rinsed. The secondary antibody goat anti-rabbit IgG-HRP (1/10.000) was added to each well, then incubated at 37 °C for 1 h and washed. Finally 3, 3′, 5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide were used as the substrate for developing at 37 °C for 15 min. The reaction was stopped by the addition of 50 μg of 1 M H2SO4. The plates were read in micro plate photometer.
Detection of hydrogen peroxide accumulation
To detect accumulation of hydrogen peroxide, leaf tissues were stained with 1 mg of diaminobenzidine (DAB, pH 3.8). Leaves were washed in distilled water and cleared with 95% (v/v) for 24 h. DAB staining visualizes hydrogen peroxide as dark brown precipitate under the light microscope.
Measurement of cell death
Experiment was carried out as describe earlier (Singh and Upadhyay 2014). Leaves incubated with TMV for 7 days were soaked in 0.05% Evans blue for 24 h, rinsed, dried, and then boiled in anhydrous alcohol and glycerol (9:1 V/V) for 30 min to remove the chlorophyll. A blue area represents cell death. The amount of cell death in tobacco leaves was also quantified. Stained leaves were added to 4 mL 1% SDS water solution for 3 days to extract Evans blue. The supernatant was collected after centrifugation at 10.000 rpm for 5 min and the absorbance was measured at 600 nm.
Nitric oxide production
According the previous method (Jia et al. 2016), the epidermis was peeled neatly from the surface of rosette leaves and then incubated in MES/KCl buffer (10 mM MES/KOH, 50 mM KCl, 100 μM CaCl2, pH 6.5) for 30 min in the light. Then, incubated in Tris/KCl buffer (10 mM Tris, 50 mM KCl, pH 7.2) containing 5 μmol/LDAF-2DA (sigma) for 30 min in the dark at room temperature. Excess DAF-2DA was washed three times with fresh Tris/KCl buffer, the epidermis was placed in Tris/KCl buffer containing strain HAB-5 for 15 min, Tris/KCl buffer as contrast. NO production was photographed under fluorescence microscopy. The NO content in leaves was determined using Griess reagent (Beyotime Biotech) according to the manufacturer’s instructions. Rosette leaves were sprayed with the antimicrobial metabolites from strain HAB-5 or products from inoculated LB, incubated for 15 min, weighed and quickly frozen in liquid nitrogen for subsequent NO content determination. The experiments were repeated at least three times for each treatment.
Evaluation of biophysical and biochemical parameters
The ion leakage of systemic leaf tissue was measured as described as earlier (Fan et al. 1997). Leaves were immersed in 10 mL and 0.4 mL mannitol solution at room temperature with 200 rpm shaking for 3 h, and the conductivity was measured. The total conductivity was determined after boiling leaves for 10 min. The conductivity due to leakage was expressed as the percentage of the initial conductivity versus the total conductivity.
The amount of chlorophyll present in the tobacco leaf was estimated. Fresh tissue (300 mg) was ground with 10 ml of 80% acetone. The homogenate was centrifuged at 3000 rpm for 15 min. Absorbance was measured at 645 nm and 663 nm. The chlorophyll content was determined according the previous method (Bashan et al. 2006).
Total sugar determined by improved procedure (Gerchacov and Hatcher 1972). 0.2 g of fresh tobacco leaf tissue was crushed in 80% methanol and centrifuged at 10.000 rpm for 10 min. The supernatant was incubated in water bath at 70 °C. Equal volume of 5% phenol and 5 ml of H2SO4 were added and absorbance was measured at 640 nm. A standard curve was prepared using different concentration of standard glucose solution. The estimation of total sugar content was done on background of the graph.
Total proline content was quantified by the acid-ninhydrin method (Bates et al. 1973). Leaf tissue (500 mg) was ground in 10 ml 3% sulfosalicylic acid. 2 mL of supernatant was mixed with equal volumes of acid-ninhydrin and acetic acid. The mixture was incubated at 100 °C for 1 h. Reaction was finished in an ice bath and extracted with 4 ml of toluene by using a vortex mixer for 15–20 s. Absorbance was measured at 520 nm.
The leaf malondialdehyde (MDA) content was determined following the protocol described previously (Qiu et al. 2008). 0.3 g of fresh leaf tissue was homogenized in 5 ml of 5% trichloroacetic acid and centrifuged at 8.000×g for 15 min. 1 mL supernatant was then mixed with 2.5 mL of thiobarbituric acid and heated at 100 °C in a water bath for 20 min and quick-chilled on ice. The mixture was centrifuged at 10.000 x g for 5 min. The absorbance was measured at 532 nm, 600 nm and 450 nm. The MDA content was determined as follow:
RNA extraction, cDNA synthesis and qRT- PCR analysis
Total RNA was isolated from 100 mg leaf tissue of tobacco plants using RNA prep Pure Plant Kit Tiangen Biotech (Beijing, China Co., LTD) following the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized with the Fast Quant RT Super Mix Kit (Tiangen). Primers sets targeting genes related to development, signaling regulatory genes, defense genes, and hypersensitive response related genes were used for expression analysis (Table 1). SYBR Green Master Mix (Agilent technologies, Santa Clara, CA, USA) was used for qRT-PCR. N. tabacum elongation factor 1-alpha (EF1α) was used as a constitutive reference transcript (Lu and Du 2012). The threshold (Ct) value for each gene was normalized against Ct for EF1α. Relative expression level was analyzed using the delta-delta-Ct method (Livak and Schmittgen 2001). The following protocol was used for amplification: 94 °C for 5 min followed by 35 cycles at 94 °C for 30 s, 58 °C for 40 s and 72 °C for 60 s.
Statistical analysis
The data were subjected to an analysis of variance using SPSS software (SPSS Inc., Illinois, U.S.A.). When a significant F test was obtained at p < 0.05, the separation of the treatment means was accomplished by Fisher’s protected LSD.
Results
The strain HAB-5 promotes growth of tobacco plants
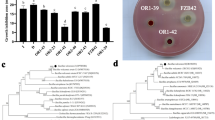
In greenhouse conditions up to 60 days, B. atrophaeus strain HAB-5 exposed remarkable ability to improve growth of tobacco plant. Inoculated plant exhibited significant increase in fresh shoot weight, dry shoot weight, fresh root weight and dry root weight by 76.47%, 80.58%, 71.71%, 82.10% respectively compared with non-inoculated control plant. Noticeable differences in plant vigour and dissimilarity of leaf area were observed (Fig. 1b). The inoculated plant was distinctly taller than the non-inoculated plants by 45.76% and displayed much darker green leaves.
Effectiveness of B. atrophaeus HAB-5 to promote tobacco plant growth. a Measurement of growth parameter on each treatment (b) Inoculation effect of HAB-5 on tobacco plant comparing with non-inoculated plant. (c) Effect of HAB-5 on plants root. Representative plants of each treatment were photographed 45 days post inoculation. (d) Rhizosphere colonization of HAB-5 in N. tabacum. Data represent the numbers of CFU/g of plants rhizosphere with standard deviation from three replicates. Experiments was carried with three replicates and all replication contained twelve plants
To clarify the mechanism of variation between non-inoculated and those inoculated with the strain HAB-5, gene expression analysis was performed. We have selected two effectors, EXP1, EXP2 that are tobacco expansions encoded by NtEXP1, NtEXP2 which function for loosen cell walls, regulating plant development, promoting cell division and extension, thereby promoting plant growth mediated by ethylene (Kuluev et al. 2016). High expression levels were noticed in the strain HAB-5-inoculated plant. NtEXP1 and NtEXP2 genes showed parallel tendencies that expressed high level at 60 dpi in inoculated plant. In contrast both two genes showed a significant lower expression in non-inoculated plants (Fig. 2a and b).
To be an effective PGPR, bacteria must be able to colonize roots because they need to establish themselves in an adequate environment to produce beneficial effects. It was found that the strain HAB-5 was resistant to streptomycin and kanamycin. The colonization of strain HAB-5 of the tobacco plant soil was measured at different time points (Fig. 1d). The result showed that the amount of strain HAB-5 in soil was increased from 105 to 107 CFU per gram (0 h to 60 h) of rhizosphere soil in inoculated plants. In contrast, no HAB-5 strain was observed in non-inoculated soil. This indicates that strain HAB-5 successfully colonized the tobacco rhizosphere.
Metabolites of HAB-5 alleviate the virulence of TMV on tobacco plant
Two different applications were evaluated. Plants were amended with the bacteria suspension before virus inoculation. It was found at few days after virus inoculation; the bacteria non-inoculated plant was weak, faded and dead. The bacteria inoculated plant had vitality and did not display any disease symptom. Four weeks later, the bacteria inoculated plant showed disease symptom. Another set of experiment consist the application of crude extract metabolites from the strain as a foliar spray treatment 12 h before TMV inoculation. The antimicrobial-pretreated plant did not show any disease symptom except when the leaves were rubbed with the virus. The secretion metabolites from strain HAB-5 were found to inhibit systematic disease on tobacco plants.
Disease severity was quantified 7 days after TMV inoculation. Mock treated plants showed severe mosaic symptoms on the surface area of systemic leaves as compared to the healthy control plant which had a 0-scale disease rating. Infected plants showed severe mosaic symptom on surface area of systemic leaves. Plants pretreated with the antimicrobial metabolites exhibited significant tolerance to TMV infection, only showed 0–1 scale (Fig. 3).
Intense reduction of chl a, chl b and total chlorophyll content were noticed in TMV mock treated plants by 63.15%, 62.91% and 63% respectively, which was ameliorated by 41.84%, 41.43% and 41.59% respectively in pretreated leaves (Fig. 4e and f). Pretreated leaves showed a significant (50.94%) reduction in membrane ion leakage (Fig. 4b), MDA by 50.75% and proline by 27.63% as compared with mock treated leaves (Fig. 4a and c). An increased sugar content of 36.59% was noticed in pretreated leaves comparing to the control, while it was reduced by 94.89% in TMV mock treated leaves compared to those pretreated with the antimicrobial metabolites (Fig. 4d).
Biophysical and biochemical characters response in systemic leaves of N. tabacum cv. Samsun-NN. Revealing the improved plant efficiency in treated laves comparing with untreated leaves. The data was calculated at 7 day post TMV inoculation. Different letters indicate significant differences between treatments (Duncan’s multiple range test P < 0.05)
Both ROS accumulation and cell death are greatly attenuated in tobacco leaves treated with metabolites of HAB-5/TMV
The relationship between systemic resistance, cell death and H2O2 in the tobacco leaves was investigated. DAB staining revealed that H2O2 production was concentrated in the whole leaf of mock treated plants; it was nil in healthy control plant leaves and slightly appeared in pretreated-with-antimicrobial leaves (Fig. 5a). Leaves were also stained with Evan blue for cell death determination (Fig. 5b). No visible cell death was observed in control healthy leaves. A slight effect was found in pretreated leaves. While mock treated leaves had a 14.9-fold increase in stained cells (Fig. 5c). This data indicate that strain HAB-5 could inhibit ROS accumulation and cell death in leaves during TMV infection. It can be concluded that there is a mechanistic connection between H2O2 accumulation and cell death. Low H2O2 accumulation was fundamental and adequate for inhibiting cell death in tobacco leaves.
Determination of H2O2 and cell death on N. tabacum cv. Samsun-NN leaves. a High accumulation of H2O2 (represented by brown color in leaf) was detected in untreated leaves, followed by TMV infection pretreated leaves. No H2O2 accumulation was observed in control leaves. b Evan blue staining of tobacco leaf tissues treated with HAB-5/TMV and Mock/TMV. At 12 h post-spray with HAB-5 at 1 mg/mL or water (Mock), the leaves were challenged with TMV via rub-inoculation. The blue areas on leaves represent cell death. c Quantification of cell death in leaf tissues treated with HAB-5/TMV and Mock/TMV via detecting the absorbance of Evans blue from treated leaves indicated above. Experiment were carried out 7 days after TMV inoculation. Values means ± SD from triplicate independent measurements
Metabolites of HAB-5 induced nitric oxide production in tobacco leaves
It has been shown that nitric oxide (NO) plays important roles in immune regulation and is recognized as important signaling messenger involved in plant immunity. After spraying the leaves with 1 mg/mL of antimicrobial metabolites, the NO content in leaves was increased up to 2.32 nmol/g, whereas it was 1.11 nmol/g in the mock treated leaves (Fig. 6). This result showed that strain HAB-5 induced NO production in epidermal cells of tobacco. It is suggested that the NO stimulation on pretreated leaves contributed to the activation of defense responses in tobacco plant against TMV.
Treatment with HAB-5 greatly increases the production of NO in tobacco leaves. a Microscopy observation of NO production (green) in tobacco leaves treated with HAB-5. b Determination of NO content in tobacco leaves treated with HAB-5 using Griess reagent. Mean values ± SD from three independent measurements
Gene expression on tobacco leaves and ELISA analysis
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was used to investigate the role of HAB-5 in patterns genes included PR-1a, PR-1b and Chia5 (Pontier et al. 1994); NPR1 gene (Pontier et al. 1994); Hypersensitive response (HR) markers, Hin1 and Hsr203J (Mou et al. 2003; Kim and Zhang 2004). Total RNA was isolated from the tobacco leaf tissues at different time intervals from 0 h to 96 h post treatment (hpt). The amount of the PR-1b and NPR1 genes transcript peaked at 96 hpt. Expression of PR-1awas increased at 72 h and overexpressed in pretreated leaves. Transcripts of the Chia5 gene reached its maximum at 48 h and gradually increased with time (Fig. 7c). Expression of Hsr203J gene was increased with the time and high level was found at 96 h (Fig. 7a). Similarly, the Hin1 gene also showed high levels of expression at 96 hpt (Fig. 7b). By contrast, in mock treated leaves, the transcription of PR-1a and PR-1b genes was not detected (Fig. 7e and f) and was very low in NPR1 and Chia5 (Fig. 7c, d). Increased and over-expression of these genes confirm the success of strain HAB-5 in enhancing TMV resistance.
Comparison of expression level of disease defense-related genes between tobacco leaves treated with HAB-5 + TMV and Mock+TMV at different time points. HAB-5 + TMV and Mock + TMV represent N. tabacum plants pretreated with 1 mg/mL of antimicrobial metabolites and extracted products from non-inoculated LB, respectively, and challenged with TMV at 12hpt. The samples were collected at a series of time points as indicated, and total RNA was extracted and subjected for qRT-PCR detection. EF1a was determined as internal reference gene to normalize the data. Values were presented as means ± SD from three independent replicates
ELISA analysis was performed to quantify the amount of TMV CP. The healthy control plants did not show any virus accumulation; while virus accumulation in TMV mock treated plants was found to be approximately 3-fold higher than antimicrobial metabolite treated plants (Table 2). Based on the plant symptoms and virus accumulation, this result confirms that strain HAB-5 reduced the severity of TMV disease.
Table 2 Enzyme linked immune sorbent assay (ELISA) detection of TMV accumulation in rub-inoculated leaves at 7 dpt.
Discussion
In this study, the mechanism used by Bacillus spp. to stimulate plant growth is still elusive (Schmidt and Delaney 2010; Lyngwi et al. 2016). Strain HAB-5 cell suspension supported growth up to 60 days post inoculation under greenhouse conditions and increased tobacco plant growth parameters. Gene expression investigations on the interaction with Bacillus sp. still provide an avenue for understanding the molecular mechanisms convoluted in enhancing plant growth and increasing yield.
Biological control by using PGPR mechanisms emerged long time ago but unfortunately chemical control has proved to be irreplaceable. One of the major limitations to their success is because they are adapted for the soil ecological conditions and the mechanisms for disease control agents are found to be associated with plant roots. It is known that a wide range of soil microbial species secrete different kinds of antimicrobial compounds. Among these, surfactin and fengycin lipopeptides of Bacillus subtilis were reported as elicitors of induced systemic resistance in plants (Ongena et al. 2007, 2008). Research has shown that lipopeptides antibiotics of Bacillus spp. are involved in the suppression of Fusarium wilt in tomato (Abdallah et al. 2017).
As previously reported, except for root drenching, foliar application is one of the efficient methods for foliar disease control. Studies have focused on harvesting these compounds from the culture suspension, culture supernatant and crude extract sprayed on plants for disease control (Lo et al. 1997; Ji et al. 2013; Yang et al. 2017). The present study provides evidence that crude extract metabolites of efficacy from the PGPR strain HAB-5 in reducing the virulence of TMV on tobacco plant. Based on visible symptoms, the crude extract metabolites extracted from the strain protect plant from TMV. It was found that the crude extract metabolites can inhibit the systemic disease on tobacco plant. ELISA analysis also showed the ability of the strain to decrease (by approximately 3-fold) virus accumulation in pre-treated leaves. These data confirm successful protection by the strain HAB-5 against TMV and consolidate findings that crude extract can be used for diseases control (Ji et al. 2013).
To further clarify antimicrobial-metabolite mechanisms which will benefit in controlling plant diseases, physiological, biochemical, anatomical changes between the treatments were assessed. First, chlorophyll is important in photosynthesis for plant bioproductivity. In the present study, significant increases in chlorophyll content were observed in strain HAB-5 treatment compared to the control. Similar to other biocontrol methods (Mou et al. 2003), foliar treatment helped plants to maintain chlorophyll during TMV infection. Then, the content of MDA and ion leakage were reduced in pretreated leaves. It has been reported that MDA was induced during stress on plants leading to enhanced membrane peroxidation disease tolerance mechanisms (Bashan et al. 2006; Li et al. 2013). In addition, the molecular mechanisms of sugar in disease remain largely unknown. We found that the sugar content was increased in treated plants. The possible explanation is high sugar content helps to reduce disease in plants. This finding suggested the ex vivo treatment maneuvered the ion leakage sugar, proline, chlorophyll, and malondialdehyde to protect plant cells integrity leading to induced TMV tolerance.
The hypersensitive response is a mechanism used by plants to prevent the propagation of pathogen infection, and commonly results in the formation of necrotic lesions (Qiu et al. 2008). It is characterized by the appearance of localized cell death (Pontier et al. 2004; Wang et al. 2012). In this study, cell death in leaves were correlated with the amount of virus present in leaves (Fig. 6). The antimicrobial pretreated leaves were able to maintain the viability of host cells against TMV. In addition, reduction of H2O2 triggered by strain HAB-5 treatment may play a role in the enhancement of plant resistance. This suggests that the low level of H2O2 accumulation in tobacco leaves may insufficiently cause cell death. This finding provide new information on incompatible interactions between TMV infection and plant hosts.
Depressed proline and higher ROS accumulation in plants usually contributes to the ability of the bacteria to maintain ROS balance in accordance with plant requirements (Elsayed et al. 2013). The need for proline in maintaining the elevated ROS level has been reported earlier (Apostol and Heinstein 1989; Kumar et al. 2016). Interestingly, our results showed lower proline and ROS accumulation in pretreated plants. It suggests that the antimicrobial-metabolite induced systemic resistance via proline and ROS in epidermal plant cells was activated in an independent manner.
NO is an important second messenger involved in plant immunity, acting as a signaling molecule in plant/pathogen interactions (Savicka and Škute 2010). It was observed that NO content in rosette leaves of tobacco increased following strain HAB-5 antimicrobial treatment (Fig. 7). This finding suggests that NO may contribute to the induction of defense response in plant leaves.
The expression of plant defense-related genes usually occurs quickly after plant treatments. PR proteins were found in genotypes of tobacco infected with tobacco mosaic virus (Hare and Cress 1997; Singh et al. 2017). NPR1 is known to regulate SAR (Systemic acquired resistance) and Induced systemic resistance (ISR) resistance pathways (Pontier et al. 2004). We pretreated the leaves with antimicrobial metabolites 12 h before TMV inoculation, and we noticed that plant immune associated genes were stimulated. Hsr203J and Hin1 reported as HR-related genes exhibit rapid, high level, specific activation in response to HR-inducing bacteria (Pontier et al. 1994; Delledonne et al. 1998). Unexpectedly, evidence was not presented in the present study that expressions of those genes were noticed at late time point (96 hpt). The use of different pathogen species, different plant cultivar, different bacteria, different treatments from a different genetic background, or plant encoded functions can modulate their action. Similarly, recent reports also indicate the induction of Hsr203J in response to hrp mutants of Pseudomonas. syringae pv. tabaci WF4 and P. syringae pv. syringae at late time points (Park and Kloepper 2000; Raupach et al. 1996; Pontier et al. 1994).
Overall, results conclude that effective biological control not only depends on the organisms but also on the control strategy. We found that foliar application of a crude extract for controlling diseases was effective for foliar treatment and proved to be potential biocontrol treatment against TMV used as a bio-pesticide. This aspect will be a new instrument in improving the application of bioactive natural products for plant protection.
References
Abdallah, R. A. B., Stedel, C., Garagounis, C., Nefzi, A., Jabnoun-Khiareddine, H., Papadopoulou, K. K., et al. (2017). Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of fusarium wilt by endophytic Bacillus spp. in tomato. Crop Protection, 99, 45–58.
Apostol, I., & Heinstein, P. F. (1989). Low P.S. rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiology, 90, 109–116.
Bashan, Y., Bustillos, J. J., Leyva, L. A., Hernandez, J. P., & Bacilio, M. (2006). Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biology and Fertility of Soils, 42, 279–285.
Batchimeg, T., & Dondov, B. (2015). Results of Bacillus subtilis against major diseases on greenhouse crops. Journal of Agriculture Science (Cambridge), 15, 134.
Bates, L., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Bharti, N., Pandey, S. S., Barnawal, D., Patel, V. K., & Kalra, A. (2016). Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Scientific Reports, 6, 34768.
Bodelier, P. L., Roslev, P., Henckel, T., & Frenzel, P. (2000). Stimulation by ammonium based fertilizers of methane oxidation in soil around rice roots. Nature, 403, 421–424.
Chaudhry, V., Dang, H. Q., Tran, N. Q., Mishra, A., Chauhan P. S., Gill, S. S, Nautiyal, C. S., & Tuteja, N. (2012). Impact of salinity-tolerant MCM6 transgenic tobacco on soil enzymatic activities and the functional diversity of rhizosphere microbial communities. Research in Microbiology, 163, 511-7.
Delledonne, M., Xia, Y., Dixon, R. A., & Lamb, C. (1998). Nitricoxide functions as a signaling plant disease resistance. Nature, 394, 585–588.
Devendra, K. C., Amrita, K., Shekhar, J., Anukool, V. S., Kumari, Kanti, P. S., & Ajit, V. (2016). Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. Journal of Plant Growth Regulation, 35, 276–300.
Elsayed, B. B., Kamel, S. M. H., & Hassan, M. M. (2013). Production of antimicrobial metabolites by Bacillus subtilis and their applications. Journal of Biotechnology, 12, 14–24.
Emmert, E. A. B., & Handelsman, J. (1999). Biocontrol of plant disease: A gram-positive perspective. FEMS Microbiology Ecology, 171, 1–9.
Eric, H., & Donohoe, M. (2003). Health issues of migrant and seasonal farm workers. Journal Health Care Poor Underserved, 14, 153-7.
Fan, L., Zheng, S., & Wang, X. (1997). Antisense suppression of phospholipase D retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell, 9, 2183–2196.
Ge, X., He, C., Li, T., & Ouyang, Z. (2015). Effect of Bacillus subtilis and Pseudomonas fluorescens on growth of greenhouse tomato and rhizosphere microbial community. Journal of Northeast Agricultural University, 22, 32–42.
Gerchacov, S. M., & Hatcher, P. G. (1972). Improved technique for analysis of carbohydrates in sediments. Limnology and Oceanography: Methods, 17, 938–943.
Han, H. S., Supanjani, D., & Lee, K. D. (2006). Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant, Soil and Environment, 52, 130–136.
Hare, P. D., & Cress, W. A. (1997). Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regulation, 21, 79–102 JHCPU. 14, 153-164.
Ji, S. H., Chandra, P. N., Xin, D. J., Kim, Y. S., Yun, B.-S., & Yu, S. H. (2013). Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Microbiology, 41, 234–242.
Jia, X., Meng, Q., Zheng, H., Wang, W., & Yin, H. (2016). Chitosan oligosaccharide induces resistance to tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Scientific Reports, 6, 26144.
Khan, N., Mishra, A., & Nautiyal, C. S. (2012). Paenibacillus lentimorbus B-30488r controls early blight disease in tomato by inducing host resistance associated gene expression and inhibiting Alternaria solani. Biological Control, 62, 65–74.
Kim, C. Y., & Zhang, S. (2004). Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. The Plant Journal, 38, 142–151.
Kloepper, J. W., Ryu, C. M., & Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology, 94, 1259–1266.
Kloepper, J. W., Rodríguez-Ubana, R., Zehnder, G. W., Murphy, J. F., Sikora, E., & Fern-andez, C. (2009). Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australasian Plant Pathology, 28, 21–26.
Kuluev, B., Avalbaev, A., Mikhaylova, E., Nikonorov, Y., Berezhneva, Z., & Chemeris, A. (2016). Expression profiles and hormonal regulation of tobacco expansin genes and their involvement in abiotic stress response. Journal of Plant Physiology, 206, 1–12.
Kumar, S., Chauhan, P. S., Agrawal, L., Raj, R., Srivastava, A., Gupta, S., Mishra, S. K., Yadav, S., Singh, P. C., Raj, S. K., & Nautiyal, C. S. (2016). Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of cucumber mosaic virus. PLoS One, 11(3), e0149980.
Leeman, M., Den-ouden, F. M., Van-Pelt, J. A., Dirkx, F. P. M., Steijl, H., Bakker, P. A. H. M., & Schippers, B. (1996). Iron availability affects induction of systemic resistance to fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology, 86, 149–155.
Li, H., Li, X., Zhang, D., Liu, H., & Guan, K. (2013). Effects of drought stress on the seed germination and early seedling growth of the endemic desert plant Eremosparton songoricum (fabaceae). EXCLI Journal, 12, 89–101.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−ΔΔC) method. Methods, 25, 402–408.
Lo, C. T., Nelson, E. B., & Harman, G. E. (1997). Improved biocontrol efficacy of Trichoderma harzianum 1295-22 for foliar phases of turf diseases by use of spray applications. Plant Disease, 81, 1132–1138.
Lu, J., & Du, Z. X. (2012). Transcriptome analysis of Nicotiana tabacum infected by cucumber mosaic virus during systemic symptom development. PLoS One, 7(8), e43447.
Luca, P., Letizia, B., Timothy, S. B., Marco, T., & Luigi, L. (2016). Botanical and biological pesticides elicit a similar induced systemic response in tomato (Solanum lycopersicum) secondary metabolism. Phytochemistry, 130, 56–63.
Lyngwi, N. A., Nongkhlaw, M., Kalita, D., & Joshi, S. R. (2016). Bioprospecting of plant growth promoting bacilli and related genera prevalent in soils of pristine sacred groves: Biochemical and molecular approach. PLoS One, 11, e0152951.
Manidipa, R., Dutta, S. G., & Venkata, R. C. (2013). Pseudomonads: Potential biocontrol agents of rice diseases. Research Journal of Agriculture and Forestry Sciences, 1(9), 19–25.
Mou, Z., Fan, W., & Dong, X. (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell, 113, 815–826.
Nie, M., Bell, C., Wallenstein, M. D., & Pendall, E. (2015). Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Scientific Reports, 5, 9212.
Niu, D. D., Liu, H. X., Jiang, C. H., Wang, Y. P., Wang, Q. Y., Jin, H. L., & Guo, J. H. (2011). The plant growth promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. MPMI., 24, 533–542.
Ongena, M., & Jacques, P. (2007). Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends in Microbiology, 16, 115-25.
Ongena, M., & Jacques, P. (2008). Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends in Microbiology, 16, 115-25.
Park, K. S., & Kloepper, J. W. (2000). Activation of PR-1a promoter by rhizobacteria that induce systemic resistance in tobacco against Pseudomonas syringae pv. Tabaci. Biological Control, 18, 2–9.
Piromyou, P., Buranabanyat, B., Tantasawat, P., Tittabutr, P., Boonkerd, N., & Teaumroong, N. (2011). Effect of plant growth promoting rhizobacteria (PGPR) inoculation non microbial community structure in rhizosphere of forage corn cultivated in Thailand. European Journal of Soil Biology, 47, 44–54.
Pontier, D., Godiard, L., Marco, Y., & Roby, D. (1994). Hsr203J, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. The Plant Journal, 5, 507–521.
Pontier, D., Del, P. O., & Lam, E. (2004). Cell death in plant disease. Plant Cell Death Processes, 37–50.
Qiu, Z. B., Liu, X., Tian, X. J., & Yue, M. (2008). Effects of CO2 laser preatreatment on drought stress resistance in wheat. Journal of Photochemistry and Photobiology B: Biology. 90, 17-25.
Raj, S. K., Kumar, S., Pratap, D., & Singh, B. P. (2008). Viruses affecting ornamental plants: Characterization, diagnosis and Management of Plant Viruses. Grain, Crops and Ornamental, 4, 1–29.
Rajaofera, M. J. N., Jin, P. F., Fan, Y. M., Sun, Q. Q., Huang, W. K., Wang, W. B., Shen, H. Y., Zhang, S., Lin, C. H., Liu, W. B., Zheng, F. C., & Miao, W. G. (2017). Antifungal activity of the bioactive substance from Bacillus atrophaeus, strain HAB-5 and its toxicity assessment on Danio rerio. Pesticide Biochemistry and Physiology, 147, 153.
Rajafaofera, M. J. N., Wang, Y., Ghulam, Y. D., Jin, P. F., Lixia, F., Liangxiang, X., Wenbo, L., & Weiguo, M. (2019). Volatile compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pesticide Biochemistry and Physiology, 156, 170-176.
Raupach, G. S., Liu, L., Murphy, J. F., Tuzun, S., & Kloepper, J. W. (1996). Induced systemic resistance in cucumber and tomato against cucumber mosaic cucumo virus using plant growth-promoting rhizobacteria (PGPR). Plant Disease, 80, 891–894.
Raza, W., Ling, N., Yang, L., Huang, Q., & Shen, Q. (2016). Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Scientific Reports, 6, 24856.
Savicka, M., & Škute, N. (2010). Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija, 56, 26–33.
Schmidt, G. W., & Delaney, S. K. (2010). Stable internal reference genes for normalization of real-time RTPCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Molecular Genetics and Genomics, 283, 233–241.
Singh, V. K., & Upadhyay, R. S. (2014). Fusaric acid induced cell death and changes in oxidative metabolism of Solanum lycopersicum L. Botanical Studies, 55, 66.
Singh, S. K., Reddy, V. R., Fleisher, D. H., & Timlin, D. J. (2017). Relationship between photosynthetic pigments and chlorophyll fluorescence in soybean under varying phosphorus nutrition at ambient and elevated CO2. Photosynthetica, 55, 421–433.
Wang, S., Wu, H., Qiao, J., Ma, L., Liu, J., Xia, Y., & Gao, X. (2009). Molecular mechanism of plant growth promotion and induced systemic resistance tobacco mosaic virus by Bacillus spp. Journal of Microbiology and Biotechnology, 19, 1250–1258.
Wang, C. J., Yang, W., Wang, C., Gu, C., Niu, D. D., Liu, H. X., Wang, Y. P., & Guo, J. H. (2012). Induction of drought tolerance in cucumber plants by a consortium of three plant growth promoting rhizobacterium strains. PLoS One, 7(12), e52565.
Worasatit, N., Sivasithamparam, K., Ghisalberti, E. L., & Rowland, C. (1994). Variation in pyrone production, lytic enzymes and control of rhizoctonia root rot of wheat among single-spore isolates of Trichoderma koningii. Mycological Research, 98, 1357–1363.
Yang, J. H., Zhang, W. W., & Zhuang, Y. Q. (2017). Biocontrol activities of bacteria from cowdung against the rice sheath blight pathogen. Journal of Plant Diseases and Protection, 124, 1–11.
Zhang, J. X., & Xue, A. G. (2010). Biocontrol of sclerotinia stem rot (Sclerotinia sclerotiorum) of soybean using novel Bacillus subtilis strain SB24 under control conditions. Plant Pathology, 59, 382–391.
Zhang, S., Reddy, M. S., & Kloepper, J. W. (2004). Tobacco growth enhancement and blue mold disease protection by rhizobacteria: Relationship between plant growth promotion and systemic disease protection by PGPR strain 90-166. Plant and Soil, 262, 277–288.
Zhou, D., Guo, J., Manter, D. K., Reardon, K. F., & Vivanco, J. M. (2015). Bacillus spp. from rainforest soil promote plant growth under limited nitrogen conditions. Journal of Applied Microbiology, 118, 672–684.
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (31160359, 31360029), China Agriculture Research System (No.CARS-33-BC1), China Agriculture Research System (No.CARS-34-BC1) and the Hainan Province Natural Science Foundation of China (No.20153131). The funder had no role in data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Human participants and/or animals
This article does not contain any studies with human or animals subjects performed by any of the authors.
Informed consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rajaofera, M.J.N., Wang, Y., Jatoi, Z.A. et al. Bacillus atrophaeus HAB-5 secretion metabolites preventing occurrence of systemic diseases in tobacco plant. Eur J Plant Pathol 156, 159–172 (2020). https://doi.org/10.1007/s10658-019-01873-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01873-1