Abstract
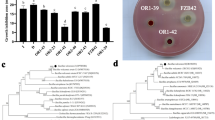
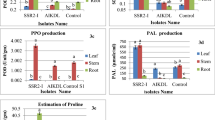
Tobacco black shank caused by Phytophthora nicotianae is a very important oomycete disease of tobacco, and it is widely distributed around the world. In order to develop effective prevention techniques, this study examined the effects of an antagonistic bacterium, Bacillus subtilis Tpb55 strain, on the prevention of tobacco black shank in vitro and in vivo. Dual culture test results showed B. subtilis Tpb55 strain have a strong antagonism to P. nicotianae, inhibit the growth of its hyphae, and produce significant inhibition zones. Scanning electron microscopy showed that the Tpb55 strain can damage the structure of P. nicotianae hyphae, cause hyphae deformity, hyphae rupture, and protoplasm leakage. Control effects of Tpb55 strains on tobacco black shank in pot and field experiment can reach up to 70.66 and 59.34 %, respectively. In this study, Tpb55 strain was also labeled with green fluorescent protein (GFP) in order to monitor their rhizosphere colonization of tobacco. The Tpb55 strain’s colonization on tobacco roots showed a diffused distribution, largely in the root meristem and elongation zone areas. They can gather focally into microcolonies, forming a biofilm like structure. A small number of these bacteria can colonize at the intercellular space and among vascular bundles. After inoculation, Tpb55-GFP was found to colonize tobacco roots for 30 days or more. The number of bacteria peaked on the 4th day at 1.51 × 107 cfu g−1. By the 12th day, it had dropped to 1.1 × 106 cfu g−1. This study shows that the effect of Tpb55 strains on controlling of tobacco black shank is correlated to their ability to inhibit mycelia growth and ability to successfully colonize tobacco roots.




Similar content being viewed by others
References
Cao G, Zhang X, Zhong L, Lu Z (2011a) A modified electro-transformation method for Bacillus subtilis and its application in the production of antimicrobial lipopeptides. Biotechnol Lett 33(5):1047–1051
Cao Y, Zhang Z, Ling N, Yuan Y, Zheng X, Shen B, Shen Q (2011b) Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47:495–506
Cartwright DK, Spurr HW (1998) Biological control of Phytophthora parasitica var. nicotianae on tabacco seedlings with non-pathogenic binucleate Rhizoctonia fungi. Soil Biol Biochem 30:1879–1884
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15(3):848–864
Chowdappa P, Kumar SM, Lakshmi MJ, Upreti KK (2013) Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol Control 65:109–117
Compant S, Clément C, Sessitsch A (2010) A Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
De Curtis F, Lima G, Vitullo D, De Cicco V (2010) Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a dripirrigation system. Crop Prot 29(7):663–670
Dietel K, Beator B, Budiharjo A, Fan B, Borriss R (2013) Bacterial traits involved in colonization of Arabidopsis thaliana roots by Bacillus amyloliquefaciens FZB42. Plant Pathol J 29(1):59
Eberl L, von Bodman SB, Fuqua C (2007) Biofilms on plant surfaces. The biofilm mode of life: mechanisms and adaptation. Environ Microbiol 12:215–234
Filho RL, Souza RMD, Ferreira A, Quecine MC, Alves E, Azevedo JLD (2013) Biocontrol activity of Bacillus against a GFP-marked Pseudomonas syringae pv. tomato on tomato phylloplane. Australas Plant Path 42(6):643–651
Gilbertson AW, Fitch MW, Burken JG, Wood TK (2007) Transport and survival of GFP-tagged root-colonizing microbes: implications for rhizodegradation. Eur J Soil Biol 43:224–232
Hale L, Luth M, Crowley D (2015) Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biol Biochem 81:228–235
Handelsman J, Stabb EV (1996) Biocontrol of soilborne plant pathogens. Plant Cell 8:1855–1869
Hiddink GA, van Bruggen AH, Termorshuizen AJ, Raaijmakers JM, Semenov AV (2005) Effect of organic management of soils on suppressiveness to Gaeumannomyces graminis var. tritici, its antagonist, Pseudomonas fluorescens. Eur J Plant Pathol 113:417–435
Kumar KVK, Yellareddygari SK, Reddy MS, Kloepper JW, Lawrence KS, Zhou XG, Sudini H, Groth DE, Raju SK, Miller ME (2012) Efficacy of Bacillus subtilis MBI 600 against sheath blight caused by Rhizoctonia solani and on growth and yield of rice. Rice Sci 19(1):55–63
Li S, Zhang N, Zhang Z, Luo J, Shen B, Zhang R, Shen Q (2013) Antagonist Bacillus subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation. Biol Fertil Soils 49:295–303
Lugtenberg BJJ, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490
Mercado-Flores Y, Cárdenas-Álvarez IO, Rojas-Olvera AV, Pérez-Camarillo JP, Leyva-Mir SG, Anducho-Reyes MA (2014) Application of Bacillus subtilis in the biological control of the phytopathogenic fungus Sporisorium reilianum. Biol Control 76:36–40
Neveu B, Labbé C, Bélanger RR (2007) GFP technology for the study of biocontrol agents in tritrophic interactions: a case study with Pseudozyma flocculosa. J Microbiol Methods 68:275–281
Park K, Park JW, Lee SW, Balaraju K (2013) Disease suppression and growth promotion in cucumbers induced by integrating PGPR agent Bacillus subtilis strain B4 and chemical elicitor ASM. Crop Prot 54:199–205
Perez-Garcia A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Ramos C, Mølbak L, Molin S (2000) Bacterial activity in the rhizosphere analyzed at the single-cell level by monitoring ribosome contents, synthesis rates. Appl Environ Microbiol 66:801–809
Ren X, Zhang N, Cao M, Wu K, Shen Q, Huang Q (2012) Biological control of tobacco black shank and colonization of tobacco roots by a Paenibacillus polynyxa strain C5. Biol Fertil Soils 48:613–620
Sang MK, Kim KD (2014) Biocontrol activity and root colonization by Pseudomonas corrugata strains CCR04 and CCR80 against Phytophthora blight of pepper. BioControl 59:437–448
Sansinenea E, Ortiz A (2011) Secondary metabolites of soil Bacillus spp. Biotechnol Lett 33(8):1523–1538
Su Z, Wang J, Zhou J, Li X, Zheng L, Li Y, Zhao B, Yang F, Wang Y (2011) Different conditions on the cultural characteristics of race 0 of Phytophthora parasitica var. nicotianae. Chinese Tobacco. Science 32(1):56–60 (in Chinese)
Sukhada M, Manjula R, Rawal RD (2011) Evaluation of arbuscular mycorrhiza and other biocontrol agents against Phytophthora parasitica var. nicotianae infecting papaya (Carica papaya cv. Surya) and enumeration of pathogen population using immunotechniques. Biol Control 58:22–29
Timmusk S, Grantcharova N, Wagner EGH (2005) Paenibacillus polymyxa invades plant roots and forms biofilm. Appl Environ Microb 71(11):7292–7300
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Widmer TL (2014) Screening Trichoderma species for biological control activity against Phytophthora ramorum in soil. Biol Control 79:43–48
Yin Y, Yuan X, Li Q, Wang Z (2010) Construction of green fluorescent protein gene tagged biocontrol bacteria Bacillus subtilis CQBS03 and its colonization on the citrus leaves. Sci Agric Sin 17:3555–3563 (in Chinese)
Yu X, Ai C, Xin L, Zhou G (2011) The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur J Soil Biol 47(2):138–145
Zhang C, Kong F, Guan X, Wang J, Li D (2008) Identification and antagonistic activity of tobacco phyllosphere bacteria strain Tpb55. Chin J Biol Control 24:63–68 (in Chinese)
Zhang N, Wu K, He X, Li S, Zhang Z, Shen B, Yang X, Zhang R, Huang Q, Shen Q (2011) A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil 344:87–97
Zhao Q, Ran W, Wang H, Li X, Shen Q, Shen S, Xu Y (2013) Biocontrol of Fusarium wilt disease in muskmelon with Bacillus subtilis Y-IVI. BioControl 58:283–292
Acknowledgments
This research was financially supported by National Natural Science Foundation of China (project no. 31000878).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Monica Hofte.
Rights and permissions
About this article
Cite this article
Han, T., You, C., Zhang, L. et al. Biocontrol potential of antagonist Bacillus subtilis Tpb55 against tobacco black shank. BioControl 61, 195–205 (2016). https://doi.org/10.1007/s10526-015-9705-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9705-0