Abstract
Hazelnut (Corylus avellana) is cultivated on 118 ha and ranks eighth in Slovenian fruit growing production, representing 2.8% of the total area of fruit plantations in the country. However, decline of some of the trees appeared in 2012 in two plantations located in eastern Slovenia. Together these orchards cover 5 ha, with around 1600 trees planted 12 to 15 years ago. By October 2018, ~12% of these trees had died, and an additional 12% showed decay symptoms. The dead and dying trees were scattered throughout both orchards, with no apparent pattern. The most affected cultivar was ‘Istrska dolgoplodna leska’. Using molecular diagnostic methods, we showed infection of symptomatic trees with three unrelated phytoplasmas: ‘Candidatus Phytoplasma fragariae’, of the 16SrXII-E phytoplasma subgroup, and phytoplasma of the 16SrV and 16SrIX groups. In 2018, the presence of ‘Ca. P. fragariae’ and/or phytoplasma of 16SrV group were confirmed in decayed hazelnut trees in eastern, north-eastern, central, south-eastern and western Slovenia. ‘Ca. P. fragariae’ has also been detected in a forest in south-western Slovenia, for Acer campestre, Carpinus betulus, Crataegus laevigata, Fraxinus ornus and Quercus petraea. All infected forest trees showed unusual dense proliferation of sprouts from roots and/or trunks. Molecular characterisations of partial 16S rRNA, secY, map and ribosomal protein genetic locus of hazelnut 16SrV phytoplasma isolates show that they are identical to isolates that can cause grapevine flavescence dorée disease. Here, the results of our recent study and the open questions on this burning issue for hazelnut production are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corylus avellana L. (European hazelnut, or common hazelnut) is a monoecious and wind-pollinated broadleaf species. It is typically a shrub, and is very common in naturally regenerated mixed-hardwood stands. It can be found throughout Europe, from Norway to the Iberian Peninsula, and East as far as the Urals. This species is very appreciated for its nuts, for which it is cultivated worldwide, and especially in European countries, and the United States and Canada. The hazelnut tree has been used extensively in breeding programmes, and there are now more than 400 described cultivars. Hazelnut trees can be propagated both in generative (i.e., by seeds) and vegetative means. It is commonly propagated by vegetative means using shoot and root suckers and cuttings (Enescu et al. 2016).
In Slovenia, hazelnut ranks eight in fruit production, and represents 2.8% (118 ha) of the total area of intensive fruit orchards (Solar 2019). The most common cultivar grown in Slovenian plantations is ‘Istrska dolgoplodna leska’ with pollenizers ‘Istrska okrogloplodna leska’ and ‘Hall’s giant’. Other cultivars, such as ‘Tonda di Giffoni’, ‘Tonda gentile Romana’ ‘Ennis’ and some others are widespread to a lesser extent (Solar and Stampar 2011). In last few years, severe damage in some Slovenian hazelnut plantations has been observed. This has included mainly dead branches, with many new dead or dying hazelnut trees found each year. Among the cultivars in these plantations, the most affected was ‘Istrska dolgoplodna leska’.
In the regions around the World where hazelnut is grown, its production can be negatively affected by several pathogens. Among these, for example, Pseudomonas avellana and Pseudomonas syringae pv. coryi are responsible for bacterial canker and decline of hazelnut in Europe (Scortichini 2002; Scortichini et al. 2005). This disease is characterised by a sudden wilting of the twigs and branches, especially at the end of spring and during summer. Xanthomonas arboricola pv. corylina causes severe damage in some hazelnut plantations in Italy (Lamichhane et al. 2012). Another pathogen that has caused heavy losses of hazelnut production in Italy is the fungus Spaceloma coryli (Minutolo et al. 2016). Hazelnuts can also be affected by Fusarium lateritium, which is the causal agent of nut grey necrosis and twig canker (Belisario et al. 2005; Santori et al. 2010). Alternaria from several distinct species can also cause diseases on hazelnut (Belisario et al. 2004). In North America, the main diseases of hazelnut are caused by Anisogramma anomala (Molnar et al. 2010), with cankering, branch dieback and tree death seen.
No bacteriological or mycological causes for the decline of these Slovenian hazel trees could be confirmed for most of the cases, although recently some of the trees from two plantations in eastern Slovenia tested positive for ‘Candidatus Phytoplasma fragariae’, which is the phytoplasma of the 16SrXII-E subgroup (Mehle et al. 2018). This phytoplasma has already been shown to cause high mortality rates in two plantations of hazelnut in the UK (DEFRA 2015). Decline and yellows disorders of hazelnut in Germany and Italy have been associated with infections by the 16SrX group phytoplasmas that usually cause apple proliferation, pear decline and European stone fruit yellows diseases (Marcone et al. 1996). In Oregon, USA, clover yellow edge phytoplasma (16SrIII-B subgroup) was shown to be associated with hazelnut stunt syndrome (Jomantiene et al. 2000). Stunting and yellowing symptoms have also been observed for hazelnut trees infected with ‘Ca. P. asteris’ (16SrI group) (Cieślińska and Kowalik 2011).
Phytoplasmas are phloem-restricted, wall-less bacteria that are pathogenic to many plant species worldwide. They are classified into distinct groups, subgroups and species on the basis of molecular data obtained from 16S ribosomal (r)RNA and other conserved genes that belong to the ‘Candidatus Phytoplasma’ taxon (IRPCM 2004). Phytoplasmas are mainly spread between plants by insects of the families Cicadellidae (leafhoppers) and Psyllidae (psyllids), and the superfamily Fulgoroidea (plant hoppers). These feed on the phloem sap of infected plants, and therefore the host range of phytoplasmas is dependent upon the feeding habits of their insect vectors (Rao et al. 2018). Phytoplasmas can also be efficiently spread via vegetative propagation, such as through cuttings, grafting and micropropagation practices (Rao et al. 2018). They can also be spread via the formation of root grafts (Lešnik et al. 2008).
The aims of this study were as follows: (i) to determine whether phytoplasmas other than ‘Ca. P. fragariae’ are also associated with the decline of C. avellana in Slovenia; (ii) to determine the occurrence and geographic distribution of hazelnut trees infected with phytoplasmas in Slovenia; (iii) to check the presence of phytoplasmas in forest trees with phytoplasma-like symptoms since these trees might serve as reservoir host plants; and (iv) to characterise phytoplasma isolates in hazelnut, and to compare these with those in other plants using restricted fragment length polymorphism (RFLP) and nucleotide sequencing. These data are necessary to define the epidemiological routes and to initiate the necessary prophylactic sanitary measures.
Materials and methods
Sampling, DNA extraction and detection of phytoplasmas
After the first detection of ‘Ca. P. fragariae’ in two plantations of C. avellana in eastern Slovenia in 2017 (Mehle et al. 2018), more intensive surveys for phytoplasmas in Slovenian hazelnut trees were carried out in 2018. Shoots and roots were sampled from symptomatic and asymptomatic trees of C. avellana (Table 1). Altogether, 28 trees were sampled at locations where ‘Ca. P. fragariae’ had been found for the first time in 2017 (Slovenska Bistrica; intensive orchards #1, #2). In addition, eight trees were sampled in three other intensive orchards in eastern Slovenia, six trees in four extensive orchards in north-eastern Slovenia, one in a private garden in central Slovenia, two in two intensive orchards in south-eastern Slovenia, and one in an extensive orchard and three in an intensive orchard in western Slovenia. Furthermore, samples of shoots and roots were collected from one Carpinus betulus and two Castanea sativa trees from the surrounding forest of intensive orchard #1 (Online Resource 1). In addition, 14 forest trees were sampled in south-western Slovenia (Beka), where ‘Ca. P. ulmi’ was found in 2016 (Mehle et al. 2017): Acer campestre (×2), Carpinus betulus (×2), Crataegus laevigata (×2), Fraxinus ornus (×3), Ostrya carpinifolia (×1), Quercus cerris (×1), Quercus petraea (×2) and Sorbus aria (×1). All of these sampled forest trees showed unusually dense proliferation of sprouts from roots and/or trunks.
Total DNA was extracted from 1 g leaf mid-vein tissue and/or vascular tissue (phloem) from roots, using kits (QuickPick Plant DNA kits; Bio-Nobile, Finland) and a purification system (KingFisher mL; Thermo Scientific, USA) (Mehle et al. 2013a). The total DNA extracted from each sample was used as the template for universal phytoplasma real-time PCR assay for amplification of the phytoplasma 16S rRNA gene (Christensen et al. 2004). The phytoplasma-positive samples were then tested using specific real-time PCR for the 16SrV phytoplasma group (Hren et al. 2007).
TaqMan Universal PCR Master Mix (Applied Biosystems) was used for all of the real-time PCR assays. The final reaction volumes for the real-time PCR of 10 μL contained 2 μL 10-fold diluted DNA sample, 300 nM (universal phytoplasma assay)/ 900 nM (16SrV phytoplasma group assay) forward primer, 900 nM reverse primer and 100 nM (universal phytoplasma assay)/ 250 nM (16SrV phytoplasma group assay) probe. The real-time PCR was carried out in 384-well plates (Applied Biosystems), with the reactions run as triplicates for the detection system (ABI Prism 7900HT Fast; Applied Biosystems). The cycling condition was 2 min at 50 °C, 10 min at 95 °C, 45 cycles of 15 s at 95 °C, and 1 min at 60 °C. A sample was considered positive if it produced an exponential amplification curve that was distinguishable from the negative controls, and in such cases the quantification cycles (Cq) were determined. If no exponential amplification curve was produced, a sample was considered negative. The SDS 2.4 software (Applied Biosystems, USA) was used for fluorescence acquisition and determination of Cq. For this, the baseline was set automatically, and the fluorescence threshold was set manually at 0.065, i.e. at a level that was above the baseline and sufficiently low to be within the exponential increase region of the amplification curve. 18S rRNA prime-probe mix (Applied Biosystems) was used as the control, to evaluate the quality of the DNA in the DNA extractions (Mehle et al. 2013b).
PCR and nested PCR amplification
Amplifications with PCR assays were carried out to obtain amplicons that were used for the subsequent characterisation of the phytoplasma isolates by sequencing and RFLP analysis. The 16S rRNA, intergenic 16S–23S, and a small part of the 23S rRNA gene were amplified from field-collected plant samples using the primer pair P1/P7 (Deng and Hiruki 1991; Schneider et al. 1995), followed by nested PCR with the primer pairs fU5/rU3 (Lorenz et al. 1995) and R16F2n/R16R2 (Lee et al. 1993; Gundersen and Lee 1996). For isolates characterised as 16SrV group phytoplasma, nested PCRs were carried out using the primer pairs rp(V)F1/rpR1 followed by rp(V)F2/rpR1 (Lim and Sears 1992; Lee et al. 1998) and rp(V)F1A/rp(V)R1A (Lee et al. 2004) to amplify the phytoplasma DNA segment of the ribosomal protein (rp) operon that encompassed the rplV, rpsS and rplP genes. Nested PCR were carried out using the primer pairs FD9f/FD9r (Daire et al. 1997) followed by FD9f2/FD9r (Daire et al. 1997; Angelini et al. 2001) and FD9f3b/FD9r2 (Angelini et al. 2001; Clair et al. 2003) to amplify a 16SrV group phytoplasma DNA segment of the FD9 marker. This contains the 3′ end of the rplO gene, which encodes the L15 50S rp, and a long fragment of the secY gene, which encodes a translocase protein. Nested PCR was carried out using the primer pairs FD9f5/MAPr1 followed by FD9f6/MAPr2 (Arnaud et al. 2007) to amplify a 16SrV group phytoplasma DNA segment of the secY-map genetic locus. The following primer pairs were used in the nested PCR for the amplification of DNA segments of the 16SrIX group phytoplasma isolates: (i) rpF1/rpR1(Lim and Sears 1992) followed by rpF1/rp(I)R1A (Lee et al. 2003), which amplified a phytoplasma DNA segment of the rp operon; and (ii) L15F1/MapR1 (Lee et al. 2010) followed by L15F2(IX)/MapR2(IX) (Lee et al. 2012), which amplified a phytoplasma DNA segment of the partial spc operon that includes the complete secY gene.
The PCR and nested PCR amplifications were performed in 50 μL reaction volume with 200 nM or 400 nM of each PCR primer, 2 mM MgCl2, 200 μM of each dNTP, and 0.02 or 0.03 U/μL Platinum Taq DNA Polymerase High Fidelity (Invitrogen). Two microliters of DNA extract diluted 10-fold in water was used for the first PCR, and 2 μL 100-fold diluted product from the first amplification was used as template for the nested amplification. The following conditions were used as 35-cycle or 40-cycle PCRs: denaturation at 94 °C for 15 s to 30 s (2–3 min for the first cycle), annealing at 47 °C to 58 °C for 30 s to 1 min, and primer extension at 68 °C for 1 min to 2 min. The concentrations of the reagents and the PCR conditions for each primer pair combination are listed in Online Resource 2. The PCR products were analysed using 1% agarose gel electrophoresis, with staining with ethidium bromide, and visualisation under a UV transilluminator.
Sequencing and sequence analysis
The PCR and/or nested PCR amplicons were purified (DNA Gel Extraction kits; Millipore; or MinElute PCR Purification kits; Qiagen), according to the manufacturer instructions. The forward and reverse sequencing reactions for the purified PCR and nested PCR products were performed by Macrogen Europe or by Eurofins GATC, using the Sanger method. For sequencing of the partial spc operon of the 16SrIX group phytoplasma isolate, intermediate primers (Lee et al. 2012) were used to allow the sequences to overlap. Sequences from each DNA region were assembled after each nucleotide position had been covered at least two times by sequencing. The sequences were compared with sequences from the GenBank database, using the BLAST algorithms (http://www.ncbi.nlm.nih.gov/blast). DNA sequences were aligned using the Vector NTI software. Phylogenetic trees were constructed using the MEGA 7 software (Kumar et al. 2016). Bootstrap analyses (1000 replicates) were used to estimate the stability. All of the sequences used to construct the trees were from this study or were obtained from GenBank.
Restricted fragment length polymorphism analysis
Computer-simulated RFLP analysis of sequences was performed using iPhyClassifier (Zhao et al. 2009). In addition, for the samples positive for the 16SrV phytoplasma group, the nested PCR products amplified using the primer pairs FD9f/FD9r followed by FD9f3b/FD9r2 were analysed using two enzymes: AluI and TaqI (New England Biolabs), according to the manufacturer instructions. The restriction products were then separated by electrophoresis using 2% agarose gels, and stained in ethidium bromide. The DNA bands were visualised using a UV transilluminator.
Results
Detection of phytoplasmas associated with declining of hazelnut trees
In two plantations located in Slovenska Bistrica (eastern Slovenia) that together cover 5 ha with around 1600 hazelnut trees planted 12 to 15 years ago, decline of some of the trees appeared in 2012. By October 2018, 10% of these trees had been removed, because they had died in previous years, and an additional 14% of the trees showed decaying symptoms (Online Resource 3). These decaying symptoms included dead trees that had not yet been removed (2%), trees with some dead branches (3%), and trees where yellowing and dropping of the leaves had been observed on a small part of the tree (9%). A few of the trees in these plantations (<1%) had unusual proliferation of thin twigs from the branches (i.e., witches’ broom symptom), or yellow or curled leaves only. The dead and symptomatic trees were scattered throughout the plantations with no apparent pattern. Among the cultivars, the most affected was ‘Istrska dolgoplodna leska’, which is also the most common cultivar in these plantations.
In these two plantations, 28 trees were sampled in the autumns of 2017 and 2018, as 17 trees with decaying symptoms, one with witches’ broom symptom, two with curled leaves, one with some yellowing of the leaves, and seven asymptomatic trees (Table 1). Using universal phytoplasma real-time PCR assays, we demonstrated infection with phytoplasmas in all of these hazelnut trees with decaying symptoms, and in a tree with witches’ broom symptoms, while the asymptomatic trees and trees with only yellow or curled leaves were negative for phytoplasmas. In seven out of twelve phytoplasma-positive samples where roots and shoots were tested separately, the presence of phytoplasmas was confirmed in the root samples only.
In 2018, phytoplasmas were detected also in 10 hazelnut trees among 21 tested from other locations in Slovenia (Table 1). Phytoplasmas were confirmed in three other intensive orchards in eastern Slovenia (Dramlje, Lokarje, Tabor), in an extensive orchard in north-eastern Slovenia (Petišovci), in an intensive orchard in south-eastern Slovenia (Birčna vas), in one intensive and one extensive orchard in the western part of Slovenia (Branik, Kromberk), and in a private garden in central Slovenia (Medvode). All of these phytoplasma-positive hazelnut trees had symptoms of decay. However, in some of the decaying trees, phytoplasmas were not detected. The situation in intensive orchards where the phytoplasmas were detected was as follows: (i) a 17-year-old plantation on 0.76 ha in Dramlje, with the first decaying symptoms observed in 2010, and since then, one to three dead trees confirmed each year; (ii) a 33-year-old plantation on 1.43 ha in Lokarje, with only one decayed tree; (iii) a 35-year-old plantation on 0.72 ha in Tabor, with the first decaying symptoms observed in 2015, and since then five decayed trees were confirmed each year; (iv) 10% to 15% of symptomatic trees in a 0.24-ha orchard in Birčna vas; and (v) 30% to 40% of symptomatic trees in a 1.25-ha orchard in Branik. Among these infected extensive orchards, the most severe situation was in Kromberk, where nine out of ten trees showed decaying symptoms.
RFLP and phylogenetic analyses of phytoplasma 16S rRNA gene sequences
Genetic variation of the detected phytoplasmas was studied on 18 selected samples, on the basis of the conserved 16S rRNA gene. Phylogenetic analysis and sequence analysis of the 16S rRNA gene using iPhyClassifier showed that the phytoplasma isolates identified in these decaying hazelnut trees in Slovenia belonged to three unrelated groups of phytoplasmas.
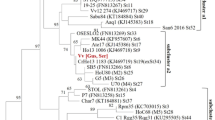
In plantations located in Slovenska Bistrica, where the presence of ‘Ca. P. fragariae’ (16SrXII-E) was confirmed for the first time in 2017 (Mehle et al. 2018), phytoplasmas from the 16SrV-C subgroup and a variant of the 16SrIX-E subgroup were also detected. Identical sequences of the 16S rRNA gene to those defined in the hazelnut trees in 2017 (MH061347, MH061346) were detected in other decaying trees from the same orchards in 2018 (D1469/18, D1473/18, D1478/18). The 16S rRNA gene sequences of samples D876/17 (MK775267) and D1474/18 were identical, and they shared 99.5% to 99.9% similarity with the reference strains of 16SrV-C subgroup (X76560, AY197642). The virtual RFLP patterns analysed using iPhyClassifier for these two samples were identical (similarity coefficient, 1.00) to the reference pattern of 16SrV-C (AY197642). The successfully sequenced 1053-bp to 1057-bp segments of the 16S rRNA gene of samples D1472/18 and D1466/18 were also identical to the 16S rRNA sequence of samples D876/17 and D1474/18. The virtual RFLP pattern of the 16S rRNA gene sequence of sample D890/17 (MK775266) was the most similar to the reference pattern of the 16Sr group IX, subgroup E (GQ925918), with a pattern similarity coefficient of 0.98. This RFLP pattern differed by one restriction site, HhaI, from subgroup 16SrIX-E. Phylogenetic analysis using partial 16S rRNA sequences of all three isolates of the phytoplasmas identified in the hazelnut trees in Slovenska Bistrica (as representative phytoplasmas of distinct phytoplasma groups or subgroups) and Acholeplasma laidlawii (as an outgroup) confirmed that the phytoplasma isolates from these hazelnut trees from Slovenska Bistrica belong to the 16SrXII-E subgroup and the 16SrV and 16SrIX group (Fig. 1).
Phylogenetic tree of the partial 16S rRNA gene sequences from the phytoplasma isolates identified in Slovenian hazelnuts (red dots) and Slovenian forest trees (light green dot), and those representative of the phytoplasma species (including representative strains of other 16SrV, 16SrIX and 16SrXII subgroups). GenBank accession numbers are shown in brackets. Sequences were aligned with CLUSTAL W. Acholeoplasma laidlawii was used as outgroup. Evolutionary history was inferred using the maximum likelihood method (1000 bootstrap replicates) with a best-fit model: Tamura-Nei model. A discrete gamma distribution was used to model the evolutionary rate differences among sites (in five categories). The rate-variation model allowed for some sites to be evolutionarily invariable. There were a total of 1280 positions in the final dataset. The proportions of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. Bootstrap values below 70 were omitted. The trees are drawn to scale, with branch lengths measured as the number of substitutions per site. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016)
Identical sequences of the 16S rRNA gene to those identified for the hazelnut trees in Slovenska Bistrica and confirmed as ‘Ca. P. fragarie’ (Mehle et al. 2018) were detected also in decaying trees from the extensive orchard in north-eastern Slovenia (D1533/18), the intensive orchard in south-eastern Slovenia (D1363/18), the intensive and extensive orchards in the western part of Slovenia (D1445/18 and D516/18), and the private garden in central Slovenia (D709/18). Based on sequence chromatograms, for many of these samples, both isolates were most probably present in the same trees [in position 305, T (MH061346), C (MH061347)]. In north-eastern Slovenia, phytoplasma from the 16SrV group was detected as well (D1532/18). This phytoplasma group has also been detected in decaying trees from other intensive orchards in eastern Slovenia (D1387/18, D1384/18). The successfully sequenced 1067-bp to 1077-bp segment of the 16S rRNA gene of samples D1532/18, D1387/18 and D1384/18 were identical to the positive samples for the 16S rRNA sequence of the 16SrV group from Slovenska Bistrica.
16SrV group phytoplasma confirmation and typing
All of the phytoplasma-positive samples were tested by 16SrV phytoplasma group-specific real-time PCR. The 16SrV phytoplasma group was confirmed in 21 hazelnut trees out of the 28 phytoplasma-positive trees (Table 1). Of 21 trees positive for the 16SrV phytoplasma group, four were co-infected by ‘Ca. P. fragariae’. In one tree (D1473/18), ‘Ca. P. fragariae’ was detected in the shoots, while the 16SrV phytoplasma group was detected in the roots. In another tree (D1469/18), both phytoplasmas were detected in the roots, and only ‘Ca. P. fragariae’ in the shoots, while in tree D1478/18, both phytoplasmas were detected in the shoots, and only the 16SrV phytoplasmas in the roots.
Restricted fragment length polymorphism analysis of the FD9 marker that distinguishes different isolates of the 16SrV phytoplasma group (Angelini et al. 2001; Clair et al. 2003; Filippin et al. 2009) was performed on 15 16SrV-positive samples. Eleven of these showed RFLP patterns identical to the FD-D type isolate from grapevine, while four showed RFLP patterns identical to the FD70 type isolate from grapevine (Table 1, Fig. 2). Nucleotide sequence analysis of the FD9 amplicon was performed on sample D876/17 (MK783137), which confirmed its assignment to the FD-D type (Fig. 3a). Sample D876/17 and reference strain FD-D (AY197685) shared 99.8% sequence similarity in the 1211-bp segment of rpl15 and secY.
Phylogenetic relationships of the secY (a) and rp (b) genes among the Slovenian 16SrV phytoplasma isolates (red dots), with the other strains from the 16SrV group based on sequences obtained from GenBank. GenBank accession numbers of nucleotide sequences are given in parentheses. The trees were constructed using the maximum likelihood method (1000 bootstrap replicates) with the best-fit models: general time reversible model (a) and Tamura three-parameter model (b). Discrete gamma distributions were used to model the evolutionary rate differences among the sites (in five categories). The rate variation model allowed for some sites to be evolutionarily invariable. There were a total of 1116 (a) and 1056 (b) positions in the final datasets. The proportions of replicate trees in which the associated taxa clustered together in the bootstrap tests are shown next to the branches. Bootstrap values below 70 were omitted. The trees are drawn to scale, with branch lengths measured as the number of substitutions per site. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016)
The diversity of 13 samples was also analysed by determination of the variability of the partial rp operon. All of these samples that were characterised based on the FD9 marker as the FD-D type (D876/17, D1474/18, D1472/18, D1466/18, D1478/18, D1386/18, D1384/18, D1382/18, D1533/18) shared an identical sequence in the ~1100-bp segment of the rp operon. This sequence (MK783138) was also identical to the reference strain FD-D (AY197664) (Fig. 3b). Sequence similarity of the ~1100-bp segment of the rp operon between all four of the FD70 type samples (D1469/18, D1473/18, D1387/18, D1532/18) was 100%, and this sequence (MK783140) was clustered together with the FD70 reference strain also by phylogeny based on the rp gene (Fig. 3b).
The sequences of the map gene (674 bp) were determined for D876/17 and D1469/18. D876/17 (MK783139) had 99.7% map sequence similarity to the sequence of isolate V00-SP5 (AM384886), which was clustered by Arnaud et al. (2007) as Map-FD2, the cluster that included isolates that were FD-D type according to Angelini et al. (2001). The sequence of the map genetic loci of sample D1469/18 (MK783141) was identical to the sequence of the isolate V02–101 (AM384887), which was clustered by Arnaud et al. (2007) as Map-FD1 (a cluster that also included the FD70 isolate).
16SrIX group phytoplasma characterisation
The 16SrIX phytoplasma group isolate that was identified in one hazelnut plant in Slovenska Bistrica was further characterised by PCR-based amplification and sequence analysis of the secY and rp genes. BLAST analysis of the 1287-bp segment of secY (MK783135) showed only 96.3% sequence identity with isolate BBS3NJc5 (JN791260) and 96.0% sequence identity with isolate JunWB2Cc2 (JN791254), which are both members of the subgroup 16SrIX-E (Lee et al. 2012). The similarities of the sequenced portions of the 1137-bp segment of rp (MK783136) with representative phytoplasma strains of 16SrIX-E, as JunWB2Cc1 (JN712785) and BB3NJc1 (JN712787) (Lee et al. 2012), were 98.0% and 97.7%, respectively. The SecY and rp gene-based phylogenetic trees with strains from the different 16SrIX phytoplasma subgroups are shown in Fig. 4.
Phylogenetic relationships of secY (a) and rp (b) genes among the Slovenian 16SrIX phytoplasma isolates (red dot), with the other strains from the 16SrIX group based on sequences obtained from GenBank. GenBank accession numbers of the nucleotide sequences and 16S rRNA based classifications of these strains are given in parentheses. The tree was constructed using the maximum likelihood method (1000 bootstrap replicates) with best-fit models: general time reversible model (a), and Tamura three-parameter model (b). Discrete gamma distributions were used to model evolutionary rate differences among sites (in five categories). The rate variation model allowed for some sites to be evolutionarily invariable. There were a total of 1116 (a) and 1056 (b) positions in the final datasets. The proportions of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. Bootstrap values below 70 were omitted. The trees are drawn to scale, with branch lengths measured as the number of substitutions per site. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016)
Detection and identification of phytoplasmas in forest trees
Real-time PCR assays were carried out on the DNA extracted from leaf and root samples of Carpinus betulus and two Castanea sativa trees from the surrounding forest of the intensive orchard #1 in Slovenska Bistrica, and of 14 forest trees from south-western Slovenia (Online Resource 1). Although all of the analysed trees showed unusually dense proliferation of sprouts from roots or trunks (Online Resource 4), the presence of phytoplasmas was confirmed only for the following trees analysed from south-western Slovenia: both Acer campestre, both Carpinus betulus, one Crataegus laevigata, two Fraxinus ornus, and one Quercus petraea. In all of these cases, the presence of phytoplasma was not confirmed in DNA extracted from the leaves only.
The successfully sequenced 1333-bp segment of the phytoplasma 16S rRNA gene of both of the A. campestre, one of the C. betulus and C. laevigata, and both of the F. ornus samples were identical (one of these sequences was deposited in GenBank with the accession number MK775268). The 1132-bp sequences from the nested PCR products of 16S rRNA of the other C. betulus sample and the Q. petraea sample were also identical. BLAST analysis of the 1333-bp segment of 16S rRNA from the forest tree (MK775268) showed 99.6% sequence identity with strain ‘Straw Y’ (DQ086423). ‘Straw Y’ is a type strain of ‘Ca. P. fragariae’ (Valiunas et al. 2006) from the subgroup 16SrXII-E. In addition, the iPhyClassifier tool showed a similarity coefficient of 1.00 to the reference pattern of this phytoplasma subgroup. Phylogenetic analysis of the 16S rRNA gene also showed that this forest tree isolate clustered with the 16SrXII-E subgroup, and indicated a close phylogenetic relationship to the ‘Ca. P. fragariae’ isolate detected in the Slovenian hazelnut (MH061346) (Fig. 1). The similarity of the 16S rRNA gene of the ‘Ca. P. fragariae’ isolate detected in the Slovenian hazelnut to the isolate detected in the forest trees was 99.6%.
Discussion
Phytoplasmas of three unrelated groups have been confirmed in declining hazelnut trees in Slovenia. Among these, ‘Ca. P. fragariae’ (16SrXII-E subgroup) and phytoplasmas of the 16SrV group appear to be the most devastating and widespread in Slovenian hazelnut plantations. Phytoplasmas of these two groups have been detected also in other plant species in Slovenia. On the other hand, phytoplasmas of the 16SrIX group have been detected in only one hazelnut tree, and up to now, no other plant species have been shown to be infected with phytoplasmas of this 16SrIX group in Slovenia.
The first record of ‘Ca. P. fragariae’ was from Lithuania in cultivated strawberry (Valiunas et al. 2006). It was later reported in Italy, in Cornus sanguinea and Sambucus nigra (Filippin et al. 2008; Martini et al. 2018). More recently, it has been described as causing disease in China in potatoes (Cheng et al. 2015). The first record of this phytoplasma in C. avellana was from the UK (Hodgetts et al. 2015). In Slovenia, the infection of C. avellana with ‘Ca. P. fragariae’ was confirmed for the first time on samples taken in 2017 in eastern Slovenia (Mehle et al. 2018). In the present study, we have shown that ‘Ca. P. fragariae’ has infected hazelnut trees also at other locations in Slovenia. In addition, this study presents the first finding of ‘Ca. P. fragariae’ in A. campestre, C. betulus, C. laevigata, F. ornus and Q. petraea. While all of these forest trees showed only unusually dense proliferation of sprouts from roots and/or trunks, the infected cultivated hazelnut trees in most cases showed declining symptoms. This might be due to the differences between the isolates of ‘Ca. P. fragariae’ (the similarity of the 16S rRNA gene of the ‘Ca. P. fragariae’ isolate detected in the Slovenian hazelnut trees to the isolate detected in the forest trees was 99.6%) or due to higher susceptibility of the cultivated hazelnut trees. In the UK, a high level of mortality was seen for ‘Ca. P. fragariae’ infected hazelnut trees in two plantations. The most likely scenario in the UK was that the trees planted at both sites were clonally propagated from an infected mother plant, and thus already infected at the time of planting, with the disease having progressed over a 15-year period (DEFRA 2015). In Slovenia, ‘Ca. P. fragariae’ has been detected in at least two different cultivars, as ‘Istrska dolgoplodna leska’ and ‘Istrska okrogloplodna leska’, which were planted at different locations, and thus were very unlikely to be from the same mother plant.
Both of the cultivars ‘Istrska dolgoplodna leska’ and ‘Istrska okrogloplodna leska’ from different plantations in eastern and north-eastern Slovenia were shown to be infected also with the 16SrV phytoplasma group. Moreover, this is the first report of declining symptoms on cultivated hazelnut trees associated with 16SrV phytoplasma group. The age of the 16SrV phytoplasma infected plantations were from 12 years to 35 years. Among the 16SrV phytoplasma group, isolates of 16SrV-C and 16SrV-D phytoplasma subgroups can cause serious and economically important disease of grapevines; i.e., ‘flavescence dorée’ (FD). They affect a broad range of Vitis vinifera cultivars across 10 European countries, where it is a quarantine pathogen (EPPO 2019). FD-related phytoplasma isolates can also infect Clematis vitalba, Ailanthus altissima, Alnus glutinosa and A. incana, although they remain asymptomatic (Angelini et al. 2004; Filippin et al. 2011; Mehle et al. 2011; Radonjić et al. 2013; Atanasova et al. 2014). Recently, FD-related phytoplasma isolates were also found in asymptomatic willows (Salix spp.) and in asymptomatic uncultivated hazelnut shrubs sampled in a forest close to a FD-infected vineyard in Switzerland (Casati et al. 2017). Molecular characterisation and phylogenetic analyses of the FD9 marker and rp operon sequences of isolates detected in these Slovenian hazelnut trees revealed the presence of the FD-D and FD70 type isolates, which correspond to map-type FD2 and FD1, respectively. Isolates detected in hazelnut trees in Switzerland were defined as map-type FD1, FD2 and FD3 (Casati et al. 2017).
FD-D type is the prevalent FD phytoplasma type that infects Slovenian grapevines, with 82.5% of 372 analysed grapevine samples in the period from 2005 to 2018 confirmed to be infected with FD-D type, with FD-70 assumed to be present in <7% (data not shown). FD-D type isolates have been found in Slovenia also in one of six 16SrV phytoplasma positive A. altissima trees, and in single or in mix infections with FD-70 and FD-C type isolates in A. glutinosa and A. incana trees (data not shown). These 16SrV phytoplasma positive A. altissima, A. glutinosa and A. incana samples were collected outside the winegrowing regions and outside the hazelnut plantations. As these plants were without any symptoms, they might serve as reservoir host plants. Further studies are needed to determine the status of the neighbouring plants to the phytoplasma-infected hazelnut plantations. To date, only three other trees from the neighbouring forest have been tested, and the presence of phytoplasma was not confirmed, although the trees analysed showed phytoplasma-like symptoms and although they grew in close vicinity to a hazelnut plantation infected with different phytoplasmas.
A good correlation between dead-branch symptoms and phytoplasma infection was observed and high susceptibility of at least two hazelnut cultivars (‘Istrska dolgoplodna leska’, ‘Istrska okrogloplodna leska’) to phytoplasma infection can now be assumed, while the susceptibility of other cultivars to phytoplasma infection needs to be examined in the future. However, since Koch’s postulates would be technically challenging to demonstrate with a phytoplasma, it cannot be said conclusively that the symptoms seen are entirely caused by phytoplasmas, although this is strongly suspected to be the case. In addition, phytoplasmas have not been detected in some decaying trees. This might be due to uneven distributions of the phytoplasmas in the trees, and consequently it is possible that the part of these trees with phytoplasmas was not included in the sample for testing. On the other hand, it is also possible, that dead-branch symptoms are due to infection with other pathogens or due to inappropriate growth conditions. Therefore, laboratory testing of symptomatic trees is needed to confirm the phytoplasma infections. Separate testing of shoot and root samples has confirmed that testing of root samples is more reliable than testing of shoot samples. The reason of this fact was not studied, but we assume that phytoplasmas are more evenly distributed in root parts as in crown. However, for reliable detection of mixed infections with different phytoplasmas, we recommend to test both roots and shoots.
Since the vector of ‘Ca. P. fragariae’ is not known and several possible vectors may be involved in transmission of FD phytoplasma (Chuche and Thiéry 2009; Dermastia et al. 2017; Filippin et al. 2009; Lessio et al. 2016; Maixner et al. 2000; Mehle et al. 2010), further studies are needed to determine the role of vectors in the phytoplasma epidemiology of hazelnuts.
References
Angelini, E., Clair, D., Borgo, M., Bertaccini, A., & Boudon-Padieu, E. (2001). “Flavescence dorée” in France and Italy – Occurrence of closely related phytoplasma isolates and their near relationships to palatine grapevine yellows and an alder yellows phytoplasma. Vitis, 40, 79–86.
Angelini, E., Squizzato, F., Luchetta, G., & Borgo, M. (2004). Detection of a phytoplasma associated with grapevine “flavescence dorée” in Clematis vitalba. European Journal of Plant Pathology, 110, 193–201.
Arnaud, G., Malembic-Maher, S., Salar, P., Bonnet, P., Maixner, M., Marcone, C., Boudon-Padieu, E., & Foissac, X. (2007). Multilocus sequence typing confirms the close genetic interrelatedness of three distinct “flavescence dorée” phytoplasma strain clusters and group 16SrV phytoplasmas infecting grapevine and alder in Europe. Applied and Environmental Microbiology, 73, 4001–4010.
Atanasova, B., Spasov, D., Jakovljević, M., Jović, J., Krstić, O., Mitrović, M., & Cvrković, T. (2014). First report of alder yellows phytoplasma associated with common alder (Alnus glutinosa) in the republic of Macedonia. Plant Disease, 98, 1268–1268.
Belisario, A., Maccaroni, M., & Coramusi, A. (2005). First report of twig canker of hazelnut caused by Fusarium lateritium in Italy. Plant Disease, 89, 106–106.
Belisario, A., Maccaroni, M., Coramusi, A., Corazza, L., Pryor, B. M., & Figuli, P. (2004). First report of Alternaria species groups involved in disease complexes of hazelnut and walnut fruit. Plant Disease, 88(4), 426–426.
Casati, P., Jermini, M., Quaglino, F., Corbani, G., Schaerer, S., Passera, A., Bianco, P. A., & Rigamonti, I. E. (2017). New insights on Flavescence dorée phytoplasma ecology in the vineyard agro-ecosystem in southern Switzerland. Annals of Applied Biology, 171(1), 37–51.
Cheng, M., Dong, J., Lee, I.-M., Bottner-Parker, K. D., Zhao, Y., Davis, R. E., Laski, P. J., Zhang, Z., & McBeath, J. H. (2015). Group 16SrXII phytoplasma strains, including subgroup 16SrXII-E (‘Candidatus Phytoplasma fragariae’) and a new subgroup, 16SrXII-I, are associated with diseased potatoes (Solanum tuberosum) in the Yunnan and Inner Mongolia regions of China. European Journal of Plant Pathology, 142, 305–318.
Christensen, N. M., Nicolaisen, M., Hansen, M., & Schulz, A. (2004). Distribution of phytoplasmas in infected plants as revealed by real-time PCR and bioimaging. Molecular Plant-Microbe Interactions, 17, 1175–1184.
Chuche, J., & Thiéry, D. (2009). Cold winter temperatures condition the egg-hatching dynamics of a grape disease vector. Die Naturwissenschaften, 96(7), 827–834.
Cieślińska, M., & Kowalik, B. (2011). Detection and molecular characterization of “Candidatus Phytoplasma asteris” in European hazel (Corylus avellana) in Poland. Journal of Phytopathology, 159(9), 585–588.
Clair, D., Larrue, J., Aubert, G., Gillet, J., Cloquemin, G., & Boudon-Padieu, E. (2003). A multiplex nested-PCR assay for sensitive and simultaneous detection and direct identification of phytoplasma in the elm yellows group and stolbur group and its use in survey of grapevine yellows in France. Vitis, 42(3), 151–157.
Daire, X., Clair, D., Reinert, W., & Boudon-Padieu, E. (1997). Detection and differentiation of grapevine yellows phytoplasmas belonging to the elm yellows group and to the “stolbur” subgroup by PCR amplification of non-ribosomal DNA. European Journal of Plant Pathology, 103, 507–514.
DEFRA (2015). Rapid pest risk analysis (PRA) for Candidatus Phytoplasma fragariae. Department for Environment Food and Rural Affairs. https://secure.fera.defra.gov.uk/phiw/riskRegister/downloadExternalPra.cfm?id=408. Accessed 20 February 2019.
Deng, S., & Hiruki, C. (1991). Amplification of 16S rRNA genes from culturable and non-culturable Mollicutes. Journal of Microbiol Methods, 14, 53–61.
Dermastia, M., Bertaccini, A., Constable, F., & Mehle, N. (2017). Grapevine yellows diseases and their phytoplasma agents. Biology and detection. Cham: Springer.
Enescu, C. M., Houston Durrant, T., de Rigo, D., & Caudullo, G. (2016). Corylus avellana in Europe: Distribution, habitat, usage and threats. In J. San-Miguel-Ayanz, D. de Rigo, G. Caudullo, T. Houston Durrant, & A. Mauri (Eds.), European atlas of forest tree species. Luxembourg: Publ. Off. EU.
EPPO (2019). EPPO Global Database (available online). https://gd.eppo.int. Accessed 26 March 2019.
Filippin, L., Angelini, E., & Borgo, M. (2008). First identification of a phytoplasma infecting Cornus sanguinea and Sambucus nigra. Plant Pathology, 57, 1175–1175.
Filippin, L., De Pra, V., Zottini, M., Borgo, M., & Angelini, E. (2011). Nucleotide sequencing of imp gene in phytoplasmas associated to “flavescence dorée” from Ailanthus altissima. Bulletin of Insectology, 64(Suppl), S49–S50.
Filippin, L., Jović, J., Cvrković, T., Forte, V., Clair, D., Toševski, I., Boudon-Padieu, E., Borgo, M., & Angelini, E. (2009). Molecular characteristics of phytoplasmas associated with “flavescence dorée” in clematis and grapevine and preliminary results on the role of Dictyophara europaea as a vector. Plant Pathology, 58, 826–837.
Gundersen, D. E., & Lee, I. M. (1996). Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathologia Mediterranea, 35, 144–151.
Hodgetts, J., Flint, L., Davey, C., Forde, S., Jackson, L., Harju, V., Skelton, A., & Fox, A. (2015). Identification of 'Candidatus Phytoplasma fragariae' (16Sr XII-E) infecting Corylus avellana (hazel) in the United Kingdom. New Disease Reports, 32, 3.
Hren, M., Boben, J., Rotter, A., Kralj, P., Gruden, K., & Ravnikar, M. (2007). Real-time PCR detection systems for “flavescence dorée” and “bois noir” phytoplasma in grapevine: A comparison with the conventional PCR detection system and their application in diagnostics. Plant Pathology, 56, 785–796.
IRPCM. (2004). ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. International Journal of Systematic and Evolutionary Microbiology, 54, 1243–1255.
Jomantiene, R., Postman, J. D., Montano, H. G., Maas, J. L., Davis, R. E., & Johnson, K. B. (2000). First report of clover yellow edge phytoplasma in Corylus (hazelnut). Plant Disease, 84(1), 102–102.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874.
Lamichhane, J. R., Fabi, A., & Varvaro, L. (2012). Severe outbreak of bacterial blight caused by Xanthomonas arboricola pv. corylina on hazelnut cv. Tonda di Giffoni in central Italy. Plant Disease, 96(10), 1577–1577.
Lee, I.-M., Bottner-Parker, K. D., Zhao, Y., Davis, R. E., & Harrison, N. A. (2010). Phylogenetic analysis and delineation of phytoplasmas based on secY gene sequences. International Journal of Systematic and Evolutionary Microbiology, 60, 2887–2897.
Lee, I.-M., Bottner-Parker, K. D., Zhao, Y., Bertaccini, A., & Davis, R. E. (2012). Differentiation and classification of phytoplasmas in the pigeon pea witches’ broom group (16SrIX): An update based on multiple gene sequence analysis. International Journal of Systematic and Evolutionary Microbiology, 62, 2279–2285.
Lee, I.-M., Gundersen-Rindal, D. E., Davis, R. E., & Bartoszyk, I. M. (1998). Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. International Journal of Systematic Bacteriology, 48, 1153–1169.
Lee, I.-M., Hammond, R. W., Davis, R. E., & Gundersen, D. E. (1993). Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasma-like organisms. Phytopathology, 83, 834–842.
Lee, I.-M., Martini, M., Bottner, K. D., Dane, R. A., Black, M. C., & Troxclair, N. (2003). Ecological implications from a molecular analysis of phytoplasmas involved in an aster yellows epidemic in various crops in Texas. Phytopathology, 93, 1368–1377.
Lee, I.-M., Martini, M., Marcone, C., & Zhu, S. F. (2004). Classification of phytoplasma strains in the elm yellows group (16SrV) and proposal of ‘Candidatus Phytoplasma ulmi’ for the phytoplasma associated with elm yellows. International Journal of Systematic and Evolutionary Microbiology, 54, 337–347.
Lešnik, M., Brzin, J., Mehle, N., & Ravnikar, M. (2008). Transmission of 'Candidatus phytoplasma mali' by natural formation of root bridges in M9 apple rootstock. Agricultura (Slovenia), 5(2), 43–46.
Lessio, F., Picciau, L., Gonella, E., Mandrioli, M., Tota, F., & Alma, A. (2016). The mosaic leafhopper Orientus ishidae: Host plants, spatial distribution, infectivity, and transmission of 16SrV phytoplasmas to vines. Bulletin of Insectology, 69(2), 277–289.
Lim, P. O., & Sears, B. B. (1992). Evolutionary relationships of a plant pathogenic mycoplasmalike organism and Acholeplasma laidlawii deduced from two ribosomal protein gene sequences. Journal of Bacteriology, 174, 2606–2611.
Lorenz, K. H., Schneider, B., Ahrens, U., & Seemüller, E. (1995). Detection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and non-ribosomal DNA. Phytopathology, 85, 771–776.
Maixner, M., Reinert, W., & Darimont, H. (2000). Transmission of grapevine yellows by Oncopsis alni (Schrank) (Auchenorrhyncha: Macropsinae). Vitis, 39, 83–84.
Marcone, C., Ragozzino, A., & Semüller, E. S. (1996). Association of phytoplasmas with the decline of European hazel in southern Italy. Plant Pathology, 45, 857–863.
Martini, M., Mesaglio, A., Savian, F., Loschi, A., Loi, N., & Ermacora, P. (2018). Identification of ‘Candidatus Phytoplasma fragariae’ (16SrXII-E) infecting Cornus sanguinea in Friuli Venezia Giulia (North-Eastern Italy). In Dermastia, M. (ed.), proceedings of the 5th European bois noir workshop, City Hotel, Ljubljana, Slovenia, 18-19 September 2018. https://www2.cd-cc.si/Skripte/boisn/CongressManuscripts.html. Accessed 23 February 2019.
Mehle, N., Dermastia, M., Brus, R., & Jurc, D. (2017). First report of ‘Candidatus Phytoplasma ulmi’ in Ulmus minor and Ulmus glabra in Slovenia. Plant Disease, 101(10), 1819–1819.
Mehle, N., Nikolić, P., Rupar, M., Boben, J., Ravnikar, M., & Dermastia, M. (2013a). Automated DNA extraction for large numbers of plant samples. In M. Dickinson & J. Hodgetts (Eds.), Phytoplasma: Methods and protocols, methods in molecular biology (Vol. 938, pp. 139–145). New York: Springer Science and Business Media LLC.
Mehle, N., Prezelj, N., Hren, M., Boben, J., Gruden, K., Ravnikar, M., & Dermastia, M. (2013b). A real-time PCR detection system for the bois noir and flavescence dorée phytoplasmas and quantification of the target DNA. In M. Dickinson & J. Hodgetts (Eds.), Phytoplasma: Methods and protocols, methods in molecular biology (Vol. 938, pp. 253–268). New York: Springer Science and Business Media LLC.
Mehle, N., Ravnikar, M., Dermastia, M., Solar, A., Matko, B., & Mešl, M. (2018). First report of ‘Candidatus Phytoplasma fragariae’ infection of Corylus avellana (hazelnut) in Slovenia. Plant Disease, 102, 2636–2636.
Mehle, N., Ravnikar, M., Seljak, G., Knapic, V., & Dermastia, M. (2011). The most widespread phytoplasmas, vectors and measures for disease control in Slovenia. Phytopathogenic Mollicutes, 1, 65–76.
Mehle, N., Seljak, G., Rupar, M., Ravnikar, M., & Dermastia, M. (2010). The first detection of a phytoplasma from the 16SrV (elm yellows) group in the mosaic leafhopper Orientus ishidae. New Disease Reports, 22, 11.
Minutolo, M., Nanni, B., Scala, F., & Alioto, D. (2016). Sphaceloma coryli: a reemerging pathogen causing heavy losses on hazelnut in southern Italy. Plant Disease, 100(3), 548–554.
Molnar, T. J., Capik, J., Zhao, S., & Zhang, N. (2010). First report of Eastern filbert blight on Corylus avellana ‘Gasaway’ and ‘VR20-11’ caused by Anisogramma anomala in New Jersey. Plant Disease, 94(10), 1265–1265.
Radonjić, S., Hrnčić, S., Krstić, O., Cvrković, T., Mitrović, M., Jović, J., & Toševski, I. (2013). First report of alder yellows phytoplasma infecting common and grey alder (Alnus glutinosa and A. incana) in Montenegro. Plant Disease, 97, 686–686.
Rao, G. P., Bertaccini, A., Fiore, N., & Liefting, L. W. (2018). Phytoplasmas: Plant pathogenic bacteria – I. characterisation and epidemiology of phytoplasma – Associated diseases. Singapore: Springer.
Santori, A., Vitale, S., Luongo, L., & Belisario, A. (2010). First report of Fusarium lateritium as the agent of nut gray necrosis on hazelnutin Italy. Plant Disease, 94, 484.
Schneider, B., Seemüller, E., Smart, C. D., & Kirkpatrick, B. C. (1995). Phylogenetic classification of plant pathogenic mycoplasma like organisms or phytoplasmas. In S. Razin & J. G. Tully (Eds.), Molecular and diagnostic procedures in mycoplasmology (Vol. 1, pp. 369–380). San Diego, CA (US): Academic Press.
Scortichini, M. (2002). Bacterial canker and decline of European hazelnut. Plant Disease, 86, 704–709.
Scortichini, M., Rossi, M. P., Loreti, S., Bosco, A., Fiori, M., Jackson, R. W., Stead, D. E., Aspin, A., Marchesi, U., Zini, M., & Janse, J. D. (2005). Pseudomonas syringae pv. coryli, the causal agent of bacterial twig dieback of Corylus avellana. Phytopathology, 95, 1316–1324.
Solar, A. (2019). Lupinarji: oreh, leska, kostanj, mandelj (247 p). Kmečki glas: Ljubljana.
Solar, A., & Stampar, F. (2011). Characterisation of selected hazelnut cultivars: Phenology, growing and yielding capacity, market quality and nutraceutical value. Journal of the Science of Food and Agriculture, 91(7), 1205–1212.
Valiunas, D., Staniulis, J., & Davis, R. E. (2006). ‘Candidatus Phytoplasma fragariae’, a novel phytoplasma taxon discovered in yellows diseased strawberry, Fragaria x ananassa. International Journal of Systematic and Evolutionary Microbiology, 56, 277–281.
Zhao, Y., Wei, W., Lee, I.-M., Shao, J., Suo, X., & Davis, R. E. (2009). Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). International Journal of Systematic and Evolutionary Microbiology, 59(10), 2582–2593.
Acknowledgements
The sampling and analysis of the hazelnut trees was financed by the Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection. The samples of forest trees were analysed in the framework of the Euphresco research topic ‘Study of the diversity of phytoplasmas detected in European forests (2016-F-211)’. The data on the diversity of FD isolates of grapevines were collected in the framework of the Euphresco research topic ‘Modelling the epidemiology of Flavescence dorée in relation to its alternate host plants and vectors (2016-F-196)’. The authors thank Tomaž Adamič, Leonida Lešnik, Ana Mihevc and Domen Bajec for helping with sample collection, and the coordinator at the Phytosanitary Administration RS, MSc Erika Orešek. Many thanks to Prof Dr. Maja Ravnikar for fruitful discussion, and to Larisa Gregur for excellent technical help in the laboratory, and other colleagues who were involved in sample preparation. The data on the hazelnut production in Slovenia were provided by Dr. Anita Solar. Language revision was carried out by Dr. Christopher Berrie.
Funding
This study was supported by the Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection and by the Slovenian Research Agency (grant number P4-0165).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors bear all the ethical responsibilities for this manuscript.
Conflict of interest
They declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Human and animal rights
They declare that the research was conducted in the absence of any commercial or financial relationships that it does not include any animal and/or human trials.
Informed consent
All authors consent to this submission.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mehle, N., Jakoš, N., Mešl, M. et al. Phytoplasmas associated with declining of hazelnut (Corylus avellana) in Slovenia. Eur J Plant Pathol 155, 1117–1132 (2019). https://doi.org/10.1007/s10658-019-01839-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01839-3