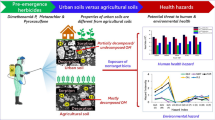
Abstract
Pesticides are the most cost-effective means of pest control; however, the serious concern is about the non-target effects due to their extensive and intensive use in both agricultural and non-agricultural settings. The degradation rate constant (k) and half-life (DT50) of four commonly used pesticides, glyphosate, 2,4-D, chlorothalonil and dimethoate were determined in five Australian urban landscape soils, with varying physicochemical characteristics, to assess their environmental and human health risks. The k values (day−1) for the selected pesticides were inversely proportional to those of organic carbon (OC), silt, clay and Fe and Al oxides, and directly proportional to pH and sand content in soils. In contrast, the calculated values of DT50 (days) of all the four pesticides in five soils positively correlated with OC, clay, silt and oxides of Fe and Al, whereas soil pH and sand content exhibited a negative correlation. The calculated values of environmental indices, GUS and LIX, for the selected pesticides indicate their potential portability into water bodies, affecting non-target organisms as well as food safety. The evaluation for human non-cancer risk of these pesticides, based on the calculated values of hazard quotient (HQ) and hazard index (HI), suggested that exposure of adults and children to soils, contaminated with 50% of initially applied concentrations, through ingestion, dermal and inhalation pathways might cause negligible to zero non-carcinogenic risks. The present data might help the stakeholders in applying recommended doses of pesticides in urban landscapes and regulatory bodies concerned in monitoring the overall environmental quality and implementing safeguard policies. Our study also clearly demonstrates the need for developing improved formulations and spraying technologies for pesticides to minimize human and environmental health risks.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, an average of three million tons of synthetic pesticides is applied on annual basis in agricultural and non-agricultural activities, resulting in unintentional toxicity to non-target biota (Fernandes et al., 2020; Shukla et al., 2006). Intensive and improper use of these agrochemicals leads to soil and water contamination causing imminent environmental and health hazards (Ahmad et al., 2019; Fernandes et al., 2020; Karimi et al., 2021). The acute human health hazards like headaches, abdominal pain, nausea, vomiting, dizziness, skin and eye irritation, etc. are due to short-term exposure to pesticides (Miah et al., 2014). Besides being possible human carcinogens, mutagens and acetylcholinesterase inhibitors, most of these pesticides cause chronic toxicities like reproductive toxicity, genotoxicity, endocrine disruption, kidney damage, metabolic alterations, liver and bladder toxicity, gastrointestinal problems, etc. (EPA, 2017; Huang et al., 2019; Ramakrishnan et al., 2018; Zhang et al., 2015). Based on the joint report of UNEP and WHO, approximately 200,000 people die throughout the world, and roughly 3 million are poisoned every year because of pesticides (Meftaul et al., 2020a; Pope et al., 1994). Though bulk amounts of pesticides are used in high-income countries, a vast majority (95%) of pesticide toxicity cases occur in developing nations due to lack of awareness, misuse, improper handling, etc. (Parven et al., 2021; Yadav et al., 2015).
Nevertheless, several pesticides are extensively used at higher doses in urban agricultural and non-agricultural settings that may result in increased contamination (Pino & Peñuela, 2011). In general, herbicides act as plant cell membrane disrupters besides being inhibitors of photosynthesis, pigment biosynthesis, lipid biosynthesis and amino acid synthesis, while fungicides inhibit protein biosynthesis, ergosterol biosynthesis and mitochondrial respiration, and insecticides mostly affect muscle and nerves, energy production and eventually growth and development of insect (Lushchak et al., 2018). The use of pesticides must ensure human and ecological safety from both the parent compounds and their hazardous metabolites (Arias-Estévez et al., 2008). Indeed, degradation is a vital process of reducing the levels of pesticide residues in soil (Hu et al., 2018; Quintero et al., 2005). The process of pesticide transformation in soil mostly occurs through microbial degradation and abiotic degradation (hydrolysis, photolysis and oxidation), wherein biodegradation is dependent on the structure and physicochemical characteristics of both soils and pesticides (Cycoń et al., 2017; Singh et al., 2006). Pesticide degradation in soils yields metabolites of varying toxicities or sometimes results in complete mineralization (Singh et al., 2006). However, the environmental fate of pesticides depends on how strongly they are bound to the soil matrix and the degradation rate (Arias-Estévez et al., 2008). Some pesticides that are insoluble in water have a tendency to get strongly sorbed to soil particles making them relatively unavailable for biodegradation and persist for longer periods thereby adversely affecting soil biology (Purnomo et al., 2011; Wang et al., 2013). The potential detrimental impacts of pesticides in soil include antagonistic effects on the population of soil microflora, alterations in activities of soil enzymes, changes in nitrogen balance of soil by inhibiting N2 fixation and ammonification, hostile effects on mycorrhizal symbiosis and nodulation in legumes, and eventually affecting soil fertility and plant growth (Das et al., 2016; Cycoń et al., 2017; El Alfy & Faraj, 2017).
In urban agricultural activities that include home gardens, flowering and ornamental plants, large trees and non-agricultural settings such as golf courses, domestic lawns, garages, driveways, footpaths and other pavements, pesticides are extensively applied for controlling undesirable noxious weeds, woody species and other invasive species (Meftaul et al., 2020b). Moreover, the application rate of pesticides in urban landscapes worldwide is tenfold higher than in agricultural farms (USFWS, 2000). For example, the annual usage of pesticides on lawns is approximately 80 million pounds of active ingredients in the USA, wherein home and garden usage accounts for 15, 10 and 8% of total insecticides/miticides, fungicides and herbicides, respectively (Bush, 2018; Grube et al., 2011). The range of organochlorine residues reported in urban soils was 0.0002–1243.68 mg kg−1 in Brazil (Fernandes et al., 2020), 0.01–62.80 µg kg−1 in Central India (Kumar et al., 2018), limit of detection (LOD)–182 ng g−1 in Novi Sad, Serbia (Škrbić et al., 2017), 1.40–60.0 ng g−1 in Nowshera, Pakistan (Zehra et al., 2015), LOD–889.0 ng g−1 in Romania (Ene et al., 2012), 4.0–1018.30 ng g−1 in Europe (Holoubek et al., 2009), 0.03–1282.58 ng g–1 (Yang et al., 2010) and 0.32–136.43 ng g–1 (Yang et al., 2012) in Beijing, China. Indeed, the above concentrations are considerably greater than reported in agricultural soils, thus representing the higher risk factor in the urban areas (Fernandes et al., 2020). Consequently, children and pets could easily be exposed to a pesticide from the urban landscape, resulting in both acute and chronic health effects (Hoover, 2005).
The most frequently used pesticides in the urban environment include glyphosate (N-(phosphonomethyl) glycine), 2,4-D (2,4-dichlorophenoxyacetic acid), dimethoate (O,O-dimethyl-S-[2-(methylamino)-2-oxoethyl] dithiophosphate) and chlorothalonil (2,4,5,6-tetrachlorobenzene-1,3-dicarbonitrile) to control pests and diseases (Meftaul et al., 2020a). In fact, urban soil properties are considerably altered due to anthropogenic activities and are distinct from other natural or agricultural soils (Bullock & Gregory, 2009; Yu et al., 2012). The modifications that occur in the soil during urban infrastructure development are ped breakdown, micropore collapse and increase in bulk density, all of which alter the microbial activities and biomass, and soil organic matter (OM) quality (Pouyat et al., 2002; Scharenbroch et al., 2005). The application of nutrients and OM to soil greatly influences the activity and structure of fungal and bacterial populations through increased metabolism, consequently affecting pesticide degradation (Marin-Benito et al., 2012). Although pesticide application in the urban environment has been intensive throughout the globe (Eigenbrod & Gruda, 2015; Okada et al., 2020), the degradation and risks of pesticide exposure are poorly understood in urban landscape soils. Generating such detailed knowledge on the degradation of most frequently used pesticides, such as glyphosate and 2,4-D (herbicides), dimethoate (insecticide) and chlorothalonil (fungicide), in urban landscape soils is greatly warranted in assessing their potential hazards in human and environmental health. Therefore, the current novel investigation aimed to determine the half-life (DT50) of the above four extensively used pesticides in five urban landscape soils with varying physicochemical characteristics for assessing the environmental and human health risks.
Materials and methods
Chemicals
Four pesticides, viz. glyphosate, 2,4-D, chlorothalonil and dimethoate of ≥ 98% purity, were obtained from Merck. Acetonitrile and methanol (LC–MS grade), and certified standard chemicals (MS grade, ≥ 99.9% purity) like CaCl2, KCl, formic acid, acetic acid, phosphoric acid, ammonium acetate and ammonium formate were also purchased from Merck. The commercial formulations of the selected pesticides, glyphosate (100 g L‒1 isopropylamine salt) and 2,4-D amine 625 (625 g L‒1 dimethylamine and diethanolamine salts), chlortan 720 (720 g L‒1 chlorothalonil), dimethoate (400 g L‒1 dimethoate), were obtained from CRT Raymond’s Warehouse (suppliers of pesticide products in Australia). Aliquots of 1000, 160, 133.33 and 250 μL of the commercial formulations were diluted to 1.0 L with Milli-Q water to obtain 100 mg L‒1 stock solution of glyphosate and 2,4-D, chlorothalonil and dimethoate, respectively. Then, 1.0 mL of each pesticide solution (100 mg L‒1) was applied to 20 g soil to provide a final concentration of 5 mg L‒1.
Soil sampling and analysis
Five fresh urban soils, dedicated initially for growing vegetables, lawn grass, flowers, ornamental plants, etc., were obtained from the surface (0–15 cm) in the Hunter region, Australia (Fig. S1). Five bulk soil samples were collected randomly from each location and mixed thoroughly to obtain a composite sample. The soil samples were assigned with specific IDs (Table S1), air-dried, sieved through a 2-mm-diameter mesh and stored at 21 ± 1 °C. The physicochemical characteristics of each urban soil were determined using triplicate (n = 3) samples (Table S1). The hydrometer method (Gee & Or, 2002) was followed to determine the soil texture, in terms of % sand, silt and clay. Electrical conductivity (EC) and soil pH were determined using a pH meter (Laqua, Horiba Scientific) in a suspension containing 5-g soil and 25 mL Milli-Q water. The percentage of soil OC was determined in a LECO analyser equipped with a non-dispersive infrared detector (LECO Corporation, Australia). Fe and Al were extracted from 0.50 g soil samples using 5 mL aqua regia solution, digested in a Microwave Digestion System (MARS 6™, USA) and measured by ICP-OES (PerkinElmer Pvt Ltd, Singapore). Following Fourier transform infrared spectroscopy (FT-IR, Agilent Technologies, USA), the functional groups of soil organic matter were analysed (Fig. S2). The soil mineral composition was determined using peaks from X-ray diffractometer (PANalytical, the Netherlands). The physicochemical characteristics of the five selected urban landscape soils are shown in Table S1.
Pesticide degradation experiments
A set of 20-g portions of each soil, contained in centrifuge tubes covered with perforated aluminium foil, was stored in dark at 21 ± 1 °C for 30 days, and moisture content of the soil was maintained at 15% on weight basis before the start of degradation studies (Hiller et al., 2010). Soils were then spiked with 1.0 mL of 100 mg L‒1 aqueous solutions prepared from commercial formulations of the selected pesticides to provide a final pesticide concentration of ∼5 mg kg‒1 active ingredient in the soil matrix. The soils were thoroughly mixed and allowed for two h for equilibration. The soil moisture content was maintained at 70% of water-holding capacity by the addition of appropriate aliquots of Milli-Q water and incubated in dark at 21 ± 1 °C to check for microbial degradation (Hiller et al., 2010). Another set of tubes with soil-applied pesticides was incubated at ‒20 °C in a cold room to check for chemical degradation. A set of tubes that received no pesticides served as control. Duplicates of each soil sample (10 g) were taken at 0, 3, 7, 10, 15, 20, 30, 45, 60, 90, 120 and 150 days of incubation to extract and determine the residues of pesticides remained in soil samples. Pesticides were extracted by shaking the soil samples with 50 mL of methanol for three h. After centrifuging at 2750 × g for 15 min, 2 mL aliquots from supernatants were used for the analysis of glyphosate, 2,4-D and dimethoate using LC–MS, and chlorothalonil following LC-DAD system.
Analytical methods
Concentrations of glyphosate, 2,4-D and dimethoate in aliquots of the extracts were determined using an LC–MS (Agilent 1260/6150B, Agilent Technologies, USA) fitted with Zorbax Eclipse plus C18 column of 4.6 × 150 mm and 3.5 μm dia (Agilent Technologies, USA). The set parameters for single quadrupole mass spectrometer were as follows: oven temperature 60 °C (glyphosate) and 35 ℃ (2,4-D and dimethoate), capillary voltage of 4000 V, drying gas flow of 12.0 mL min‒1 at 300 ℃, 100 V fragmentor voltage, 35 psi nebuliser pressure and sheath gas flow of 3.0 mL min−1 at 150 °C, negative mode with SIM ion 124 → 168 amu (glyphosate), 219 → 221 amu (2,4-D) and positive mode with 125 → 230 amu (dimethoate). For analysis of glyphosate, mobile phases used were 1% aqueous acetic acid (A) and 1% acetic acid in methanol (B) with a flow rate of 0.4 mL min−1, following a gradient starting with 95% B at 0.0 → 1.5 min, linear ramping down to 5% B at 2.5 → 6.5 min and then increased to 95% B at 8.0 min with a post-run time of 4.0 min (Kaczyński & Łozowicka, 2015). The mobile phases included for 2,4-D were 10 mM aqueous ammonium acetate (A) and methanol (B), and the gradient used was as described earlier (Meftaul et al., 2020b). The mobile phases for dimethoate were 10 mM aqueous ammonium formate (A) and methanol (B) (Utture et al., 2012; Meftaul et al., 2020c). The data obtained were processed using Agilent OpenLAB CDS ChemStation software. The standard curves were linear over the tested concentration range of glyphosate, 2,4-D and dimethoate with R2 values of 0.9958, 0.9980 and 0.9989, respectively. The values of LOD (limit of detection) and LOQ (limit of quantitation) obtained were 0.0039 and 0.0078 mg L‒1, respectively. The mean recoveries (n = 3) of spiked glyphosate, 2,4-D and dimethoate ranged from 0.0078 to 1.0 mg L‒1, and % recoveries were in the range of 83.84–101.01, 87.98–117.94 and 96.42–107.18, respectively.
LC-DAD system (Agilent Technologies, USA) with the detector wavelength set at 233 nm was used to quantify chlorothalonil from the aqueous samples (Báez et al., 2017). The mobile phase includes 0.01 M aqueous solution of phosphoric acid (A) and acetonitrile (B) with a flow rate of 0.50 mL min‒1. The injection volume was 30 μL with a gradient involving 90% B at 0.0 → 4.0 min, 98% B at 4.0 → 9.0 min, 20% B at 9.0 → 10.0 min, then 90% B at 10.0 → 12.0 min followed by a post-run for 1.0 min (Meftaul et al., 2021b). The data obtained were processed using Agilent OpenLAB CDS ChemStation software. A linear standard curve with a correlation coefficient (R2) of 0.9989 was obtained over the concentration range used. The LOD and LOQ values for chlorothalonil were 0.0078 and 0.0156 mg L‒1, respectively. The mean recoveries (n = 3) of chlorothalonil ranged from 0.0156 to 1.0 mg L–1, whereas the recoveries were in the range of 93.43–105.84%. Thus, the methods adopted here appeared to be reliable and accurate to quantify all four pesticides in different urban landscape soils.
Data analysis
The rate of degradation of pesticides was calculated using the following equation:
where Ct and C0 are the amounts (mg kg‒1) of pesticide remaining in soil at a given time t and zero, respectively, and k is the degradation rate constant (day‒1). The DT50, which represents the time (days) needed for 50% disappearance of the initial amount of pesticide, was calculated from k using the equation (Hiller et al., 2012):
Environmental health risk assessment
The groundwater ubiquity score (GUS) and leachability index (LIX) were calculated using the Eqs. (3) and (4), respectively (Hall et al., 2015; Martins et al., 2018; Peruchi et al., 2015; Spadotto, 2002).
where DT50 is the half-life (days) of pesticide in soil, k is the degradation rate constant (day−1) and Koc is the coefficient of organic carbon (L g−1) in soil. Kd is the solid–aqueous phase distribution coefficient (Hall et al., 2015).
Human non-cancer risk assessment
The potential non-cancer health risk for adults and children was determined following the widely adopted methods of USEPA (2020). The exposure pathway of pesticide from contaminated soil via ingestion was used while considering human health risk. The non-dietary chronic daily intake (CDIi) of pesticide (mg kg−1 day−1) via the incidental ingestion of the contaminated soil in adults and children was calculated based on the following equations (Bhandari et al., 2020).
where Cs (mg kg−1) is the concentration of pesticide residue in the soil after 50% degradation, EF is the exposure frequency (days yr−1), ED is the exposure duration (yrs), IRi is the rate of contaminated soil ingestion (mg day−1), AT is the average lifetime (days), BW is the average body weight (kg) and CF is the conversion factor (kg mg−1).
where CDId is the estimated non-dietary CDI (mg kg‒1 day‒1) of pesticide-contaminated soil particles via dermal contact, DA is the exposed dermal area (cm2 day‒1), DAF is the dermal adherence factor (mg cm‒2) for soil and AF (dimensionless) is the dermal absorption factor.
where CDIih is the estimated non-dietary CDI (mg kg−1 day‒1) of pesticide-contaminated soil particles via inhalation pathway, IRih is the rate of inhalation (m3 day‒1) and PEF is the particle emission factor (m3 kg‒1).
The non-cancer risk of pesticides is expressed as hazard quotient (HQ), whereas hazard index (HI) is the sum of HQ of individual pesticides, which was calculated following the equation (Afrin et al., 2021; Nisha et al., 2021; USEPA, 2020):
where RfD is the reference dose (mg kg‒1 day‒1) of a pesticide. The maximum acceptable reference doses (RfDs) in humans for glyphosate, 2,4‒D, chlorothalonil and dimethoate considered are 1 × 10‒1, 1 × 10‒2, 1.5 × 10‒2 and 2 × 10‒4 mg kg‒1 day‒1, respectively (MMDH, 2017; OEHHA, 2017a, 2017b; USEPA, 2016; USEPA, 1987a, 1987b).
Statistical analysis
The experimental data obtained were processed using Microsoft Excel (Excel 2016). To establish the degree of correlation (P < 0.05) between multiple soil properties (predictors) and DT50 values of four pesticides in five urban soils, multivariate analysis was carried out using JMP pro 14/2021 software. Principle component analysis (PCA) was performed to determine the potential linear relationships (P < 0.05) between environmental parameters, soil Kd, GUS, LIX and DT50 values of the pesticides.
Results and discussion
Degradation of selected pesticides in urban landscape soils
The data on pesticide degradation rate constant, k (day‒1), and calculated DT50 values of four pesticides (two herbicides, one insecticide and one fungicide) in five urban landscape soils are presented in Fig. 1 and Table 1. Degradation rate constants of glyphosate, 2,4-D, chlorothalonil and dimethoate in the selected urban soils were in the range of 0.009 (ATC)–0.041 (MAR). The values of coefficient of determination (R2) for the pesticides in five soils were in the range of 0.908–0.982, 0.871–0.971, 0.901–0.960 and 0.843–0.977 for glyphosate, 2,4-D, chlorothalonil and dimethoate, respectively (Table S2). The calculated DT50 values, which can vary with the environmental conditions, soil depth and microbial activities (Oliveira et al., 2013), for glyphosate, 2,4-D, chlorothalonil and dimethoate in five urban landscape soils were in the range of 53–78, 32–75, 17–32 and 24–36, respectively (Table 1). Thus, the half-life of pesticides in soils tested followed the order: glyphosate > 2,4-D > dimethoate > chlorothalonil.
The present results of DT50 values are in conformity with the half-lives reported for glyphosate (Meftaul et al., 2020d), 2,4-D (Jote, 2019), chlorothalonil (Van Scoy & Tjeerdema, 2014) and dimethoate (Martikainen, 1996). The slower degradation rate and longer DT50 values observed for glyphosate and 2,4-D might be due to their higher sorption capacity to soil minerals or dissolved OC limiting the access of pesticides to microbial degradation. The degradation of pesticides could be largely affected by the extent of soil organic matter content (Marin-Benito et al., 2012). However, some organic amendments may decrease the degradation rate of pesticides by augmenting the sorption capacity (Fernandes et al., 2006; Rodríguez-Cruz & Lacorte, 2005), whereas some of them favour degradation through stimulating microbial activity (Kadian et al., 2008; Marin-Benito et al., 2012). In contrast, the rapid degradation rate of chlorothalonil and dimethoate associated with shorter DT50 values could be due to their lower sorption capacity to soil matrix (Dhareesank et al., 2005). Generally, pesticides are known to bind to soil particles immediately after the entry or dissolve in soil solution and then desorb making them readily available for microbial degradation (Kočárek et al., 2018). The reason for relatively weak/irreversible bonding of pesticides and their availability in soil solution for biodegradation could be due to the occurrence of undecomposed or partially decomposed OC that was observed floating in the centrifuge tubes. In some cases, pesticides sorbed on to dissolved OC could increase the bioavailability and consequent biodegradation (Marin-Benito et al., 2012).
Impact of urban soil properties on pesticide degradation
Degradation or transformation of a pesticide is strongly influenced by its bioavailability and physicochemical properties of soil (Singh et al., 2006). Some pesticides are strongly bound to soil particles, become unavailable for biodegradation and persist for extended periods in soil (Purnomo et al., 2011; Wang et al., 2013). The degradation rate constant, k (day−1) of glyphosate, 2,4-D, chlorothalonil and dimethoate in five urban soils was inversely proportional to the content of soil organic carbon (OC). For instance, slower degradation followed by higher DT50 values of these pesticides were observed in soils ATC, TAR and FLE that contained higher amounts of OC (7.66, 2.02 and 1.29%, respectively) as compared to other two soils (MAR and SAL) (Table S1). Also, the presence of higher amounts of clay in soils ATC, TAR and FLE was found to be the reason for decreased rate of pesticide degradation and higher DT50 values. Thus, soil OC together and clay content seem to be the predominant factors that play a pivotal role in pesticide degradation rate in urban soils since bound residues are not readily available for further transport and degradation (Koskinen et al., 2001). These observations clearly corroborate with those reported for agricultural soils by Purnomo et al. (2011) and Wang et al. (2013). Though the selected urban soils exhibited varying levels (0.21–7.66%) of OC (Table S1), the presence of undecomposed or partially decomposed OC that was floating in the centrifuge tubes could be the fact for almost similar degradation rates of pesticides in the tested soils. Moreover, it has been well established that pesticides are strongly bound to well-decomposed organic carbon making them unavailable for biodegradation (Ren et al., 2018).
The reason for strong binding of pesticides to OC observed in the selected soils might be the occurrence of varying functional groups such as O–H, C = O, C–H and C = C (Fig. S2) that have electrostatic/covalent/H bonds. In particular, C = O and C–H groups are more reactive and are implicated in solubility, cation exchange, polarity, chemical reactivity and wettability (Meftaul et al., 2021a, 2021b). Furthermore, the negatively charged clay minerals consist of tetrahedral silicate and octahedral aluminate groups that might react with pesticides via electrostatic interaction or cation exchange. Pesticide molecules become immobilized with soil clay minerals by forming surface complexes with metal ions (Barja & dos Santos, 2005). The extent of pesticide degradation was significantly less, followed by an increase in DT50 values of pesticides in soils containing higher amounts of silt, and Fe and Al oxides. The soils TAR, ATC and FLE that contained higher amounts of silt (55, 41.20 and 23.80%, respectively) showed a slower rate of pesticide degradation and prolonged DT50 values when compared with soils MAR and SAL having 16.20 and 1.20% silt (Table S1). The rate of pesticide degradation was low in soils that contained higher amounts of Fe and Al oxides in the clay fraction (Okada et al., 2020) since negatively charged pesticides molecules have strong affinity towards transition metals to form complexes in soil solutions (Barja & dos Santos, 2005). Thus, the degradation rate of pesticides was slower in soils that contained higher amounts of OC, clay, silt and oxides of Fe and Al, while OC and clay were the significant contributors.
The urban landscape soils used in the present study had similar types of minerals, while quartz was the predominant mineral constituent among albite, zeolite, sodalite, dolomite, orthoclase, hyalophane, etc., and their role on degradation of pesticides was almost the same. In contrast, urban soils exhibited higher pH (slightly alkaline) with more sand that increased the degradation rate of pesticides. In fact, soils with higher pH decrease the partition of pesticides molecules probably due to electrostatic repulsion caused by the presence of more net negative surface charges of soil minerals (Okada et al., 2020). In most cases, the degradation rate was slower in soil ATC having pH 5.8, and faster degradation was observed in soil MAR with pH 8.0. The pesticide degradation rate was higher in soils SAL and MAR with 97.60 and 76.30% sand, whereas it was slower in soil ATC having 51.30% sand (Table S1).
The data on multivariate analysis, performed to further establish the interactions between soil characteristics (predictors) and DT50 values of pesticides, are presented in Fig. 2. Among all the predictors tested, OC (R2 = 0.276), %clay (R2 = 0.027), %silt (R2 = 0.073) and oxides of Fe (R2 = 0.101) and Al (R2 = 0.129) exhibited a significant positive correlation (P < 0.05) with DT50 values (R2 = 1.0) of all the selected pesticides. In contrast, soil pH (R2 = ‒0.08, P < 0.05) and sand content (R2 = ‒0.069) showed a negative correlation (P < 0.05) towards DT50 values of the pesticides. These observations indicate that the degradation of pesticides in soils was affected by several characteristics, including soil OC, clay and silt content, oxides of Fe and Al, and soil pH. However, the soil parameters showed a statistically significant negative correlation with the rate of pesticide degradation in most cases. Our finding clearly suggests that most of the soil properties are inversely correlated with the degradation rate and positively correlated with the observed DT50 values of pesticides.
Environmental health risk assessment
Pesticide contamination of water bodies is a growing global concern because it affects non-target organisms, drinking water quality and food safety. Assessing the leaching potential is crucial while considering the environmental risk of pesticides, which is determined by Koc based on Kd values. Using the values of DT50 and Koc, the environmental indices such as GUS and LIX were calculated, and the data are presented in Table 1. The Kd values determined for glyphosate, 2,4-D, chlorothalonil and dimethoate in the selected urban soils were in the range of 1.19–5.95 (Table 1), while the Koc values ranged from 39.57 to 1870.37 (Table S2).
The range of calculated GUS values for glyphosate, 2,4-D, chlorothalonil and dimethoate was 1.57–3.42, 1.10–4.50, 1.53–3.18 and 1.72–3.47, respectively (Table 1). Generally, soils could be considered as leachers, transitional and non-leachers if the GUS values are > 2.80, 1.80–2.80 and < 1.80, respectively (Martins et al., 2018; Meftaul et al., 2020c). Thus, soils ATC is considered as transitional, TAR and FLE soils are the potential leachers, and soils SAL and MAR are the non-leachers for glyphosate. For 2,4-D, soils ATC and FLE are the potential leachers, while soil TAR is the transitional, and soils MAR and SAL are the non-leachers. In case of chlorothalonil, soil ATC is the potential leachers, while soils TAR and FLE are the transitional, and MAR and SAL are the non-leachers. Soils ATC and MAR are the potential leachers, soils TAR and FLE are the transitional, and SAL is the non-leacher for dimethoate. Thus, our findings indicate the high leaching potential of all the four pesticides in urban soils, posing potential environmental hazards through contamination of water sources.
The calculated LIX values for glyphosate, 2,4-D, chlorothalonil and dimethoate were in the range of ˂0.01‒0.25, ˂0.01‒0.69, ˂0.01‒0.19 and ˂0.01‒0.36, respectively (Table 1). Generally, LIX values vary between 0.0 and 1.0, which indicate the least and profuse leaching potential, respectively (Martins et al., 2018; Meftaul et al., 2020a). Accordingly, leaching potential of the tested urban soils followed the order: TAR > FLE > ATC > SAL > MAR for glyphosate; ATC > FLE > TAR > SAL > MAR for 2,4-D; ATC > FLE > TAR > SAL > MAR for chlorothalonil; and ATC > MAR > FLE > TAR > SAL for dimethoate. These findings indicate the moderate to least leaching potential of the selected pesticides, which might pose environmental hazards by contaminating both surface and groundwater reservoirs. Likewise, the calculated values of both GUS and LIX clearly suggest that the pesticides leach into water sources from soil surface in urban landscapes and pose a potential threat to the health of aquatic organisms and other non-target biota.
To further establish the interaction effects of multiple environmental parameters (predictors) and DT50 values of pesticides, principal component analysis (PCA) was performed and the results are presented in Fig. 3. PCA is an important multivariate analysis that converts the bulk of data input variables to some common factors that are correlated (Shahid et al., 2020). Among all the predictors, Koc (R2 = 0.083), GUS (R2 = 0.365) and LIX (R2 = 0.403) exhibited a significant positive correlation (R2 = 1.0, P < 0.05) with DT50 values of pesticides. The PCA converted the whole data into four major component factors. The corresponding variance values for the predictors and DT50 values were 24.51, 17.60, 6.33 and 0.48%, respectively. The calculated eigenvalues for the above predictors were 1.22, 0.88, 0.32 and 0.03, respectively (Fig. 3). The first two principal components were attributed to approximately 51.08% of the cumulative variance with an eigenvalue of 2.55. In contrast, Kd values negative correlated (R2 = ‒0.389, P < 0.05) with DT50 values of pesticides.
Human health risk assessment
Health risk for adults and children, in terms of non-cancer ailments associated with the exposure to pesticide-contaminated soil, was evaluated using widely adopted equations and constant parameters (Table 2). The data on non-dietary chronic daily intake (CDI) of pesticide from contaminated soils through ingestion, dermal and inhalation pathways and well-established USEPA models were used to calculate the potential non-cancer risks in adults and children caused by pesticide exposure. The non-cancer risk of pesticide exposure through a pathway is denoted as the hazard quotient (HQ), which is the ratio of the estimated average non-dietary CDI value and the RfD of a contaminant (Brum et al., 2021). The data obtained for non-cancer risk associated with the exposure to pesticide-contaminated urban soils after 50% degradation are presented in Table 1 and Table S2. The calculated values of non-dietary CDI (mg kg‒1 day‒1) for adults upon exposure to pesticide-contaminated urban soils through ingestion, dermal and inhalation pathways for the four pesticides were significantly higher than those for children (Table S2). Similarly, the HQ values determined for glyphosate, 2,4-D, chlorothalonil or dimethoate exposure of adults via ingestion, dermal and inhalation pathways in five urban soils were significantly higher than those observed for children (Table 1). On the other hand, the HI values of pesticides in five urban landscape soils obtained for adults via ingestion, dermal and inhalation pathways were significantly higher than those for children (Table S3). If the value of HQ or HI is < 1, it indicates that the people exposed to pesticides are safe, whereas a value > 1 indicates a non-cancer health risk (Bhandari et al., 2020; Parven et al., 2021). The HQ and HI values for the selected four pesticides in five urban soils were several times lower than the recommended threshold values of HQ and HI. Our present data demonstrate that human exposure to pesticide residues after 50% degradation in urban soils through ingestion, dermal and inhalation pathways would cause extremely low or unnoticeable non-cancer risks for adults and children. Human non-cancer risk estimates of pesticide residues in urban soils were also lower than those reported in Central India by Kumar et al. (2018), in Novi Sad, Serbia, by Škrbić et al. (2017) and in Nepal by Bhandari et al. (2020). However, there could be a health hazard to pets and children if exposed immediately after pesticide application to the ornamental plants, lawns and parks in urban landscapes (Meftaul et al., 2021a). To our knowledge, this is the first comprehensive study that investigated the possible hazards of human and environmental health associated with four pesticides used extensively in urban landscapes.
Conclusion
The current novel study determined the values of degradation rate constant, k, and DT50 of four pesticides in five urban landscape soils to establish the associated human and environmental health risks. The k values of four pesticides significantly correlated with soil properties: a positive correlation with pH and sand content and a negative correlation with OC, clay, silt and oxides of Fe and Al. On the contrary, the calculated DT50 values of four pesticides in urban soils were positively correlated with OC, clay, silt and oxides of Fe and Al, while a negative correlation was evident with soil pH and sand content. The environmental risk assessment, in terms of GUS and LIX indices of glyphosate, 2,4-D, chlorothalonil and dimethoate indicated the portability of pesticides from the soil surface to water bodies that might affect non-target biota. Human non-cancer risk of pesticides, based on calculated values of HQ and HI indices for adults and children via ingestion, dermal and inhalation pathways, suggested that exposure to pesticide-contaminated soils, after 50% degradation, might cause zero or negligible non-carcinogenic risks. To minimize the exposure risks and safeguard the environmental and human health, improved formulations with microbially derived pesticides should be applied in urban landscapes. In addition, precision band spraying might limit the pesticide usage and its transport besides avoiding the build-up of resistant target organisms.
Data availability
The datasets used in this study are available from the corresponding author on reasonable request.
Change history
25 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10653-022-01314-9
References
Afrin, S., Alam, M. K., Ahmed, M. W., Parven, A., Jubayer, M. F., Megharaj, M., Meftaul, I. M., & Khan, M. S. I. (2021). Determination and probabilistic health risk assessment of heavy metals in widely consumed market basket fruits from Dhaka city Bangladesh. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2021.2018578
Ahmad, A., Shahid, M., Khalid, S., Zaffar, H., Naqvi, T., Pervez, A., Bilal, M., Ali, M. A., Abbas, G., & Nasim, W. (2019). Residues of endosulfan in cotton growing area of Vehari, Pakistan: An assessment of knowledge and awareness of pesticide use and health risks. Environmental Science and Pollution Research, 26, 20079–20091. https://doi.org/10.1007/s11356-018-3169-6
Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., Simal-Gándara, J., Mejuto, J. C., & García-Río, L. (2008). The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agriculture, Ecosystems & Environment, 123, 247–260. https://doi.org/10.1016/j.agee.2007.07.011
Báez, M. E., Espinoza, J., Silva, R., & Fuentes, E. (2017). Influence of selected cyclodextrins in sorption-desorption of chlorpyrifos, chlorothalonil, diazinon, and their main degradation products on different soils. Environmental Science and Pollution Research, 24, 20908–20921. https://doi.org/10.1007/s11356-017-9652-7
Barja, B., & dos Santos, A. M. (2005). Aminomethylphosphonic acid and glyphosate adsorption onto goethite: A comparative study. Environmental Science and Technology, 39, 585–592. https://doi.org/10.1021/es035055q
Bhandari, G., Atreya, K., Scheepers, P. T., & Geissen, V. (2020). Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere, 253, 126594. https://doi.org/10.1016/j.chemosphere.2020.126594
Brum, R. D. L., Penteado, J. O., Ramires, P. F., Girónes, M. C. R., Mondelongo, S. P., Armendáriz, M. D. C. R., Marina dos Santos, M. D., & Júnior, F. M. R. D. S. (2021). Recommended guidance and checklist for human health risk assessment of metal(loid)s in soil. Exposure and Health. https://doi.org/10.1007/s12403-021-00440-6
Bullock, P., & Gregory, P. J. (2009). Soils in the urban environment. Wiley.
Bush, E. A., (2018). Reducing pesticide use in the home lawn and garden. Virginia Cooperative Extension. VCE Publications 450/450–725.
Cycoń, M., Mrozik, A., & Piotrowska-Seget, Z. (2017). Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere, 172, 52–71. https://doi.org/10.1016/j.chemosphere.2016.12.129
Das, R., Das, S. J., & Das, A. C. (2016). Effect of synthetic pyrethroid insecticides on N2-fixation and its mineralization in tea soil. European Journal of Soil Biology, 74, 9–15. https://doi.org/10.1016/j.ejsobi.2016.02.005
Dhareesank, A., Kobayashi, K., & Usui, K. (2005). Phytotoxic activity of pethoxamid in soil under different moisture conditions. Weed Biology and Management, 5, 197–202. https://doi.org/10.1111/j.1445-6664.2005.00181.x
Eigenbrod, C., & Gruda, N. (2015). Urban vegetable for food security in cities. A review. Agronomy for Sustainable Development, 35, 483–498. https://doi.org/10.1007/s13593-014-0273-y
El Alfy, M., & Faraj, T. (2017). Spatial distribution and health risk assessment for groundwater contamination from intensive pesticide use in arid areas. Environmental Geochemistry and Health, 39, 231–253. https://doi.org/10.1007/s10653-016-9825-1
Ene, A., Bogdevich, O., & Sion, A. (2012). Levels and distribution of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in topsoils from SE Romania. Science of the Total Environment, 439, 76–86. https://doi.org/10.1016/j.scitotenv.2012.09.004
EPA. (2017). Chlorothalonil use banned outside of the workplace. Environmental Protection Authority, 27 April 2017, in New Zealand. https://www.epa.govt.nz/news-and-alerts/alerts/vtas/. (Accessed 3 July 2021).
Fernandes, C. L. F., Volcão, L. M., Ramires, P. F., De Moura, R. R., & Júnior, F. M. R. D. S. (2020). Distribution of pesticides in agricultural and urban soils of Brazil: A critical review. Environmental Science: Processes and Impacts, 22, 256–270. https://doi.org/10.1039/C9EM00433E
Fernandes, M. C., Cox, L., Hermosín, M. C., & Cornejo, J. (2006). Organic amendments affecting sorption, leaching and dissipation of fungicides in soils. Pest Management Science, 62, 1207–1215. https://doi.org/10.1002/ps.1303
Gee, G. W., & Or, D. (2002). 2.4 Particle‐size analysis. Methods of Soil Analysis: Part 4. Physical Methods, 5, 255–293.
Grube, A., Donaldson, D., Kiely, T., & Wu, L. (2011). Pesticides Industry Sales and Usage. US EPA.
Hall, K. E., Ray, C., Ki, S. J., Spokas, K. A., & Koskinen, W. C. (2015). Pesticide sorption and leaching potential on three Hawaiian soils. Journal of Environmental Management, 159, 227–234. https://doi.org/10.1016/j.jenvman.2015.04.046
Hiller, E., Čerňanský, S., & Zemanová, L. (2010). Sorption, degradation and leaching of the phenoxyacid herbicide MCPA in two agricultural soils. Polish Journal of Environmental Studies, 19, 315–321.
Hiller, E., Tatarková, V., & Šimonovičová, A. (2012). Sorption, desorption, and degradation of (4-chloro-2-methylphenoxy) acetic acid in representative soils of the Danubian Lowland, Slovakia. Chemosphere, 87, 437–444. https://doi.org/10.1016/j.chemosphere.2011.12.021
Holoubek, I., Dusek, L., Sanka, M., Hofman, J., Cupr, P., Jarkovský, J., & Klanova, J. (2009). Soil burdens of persistent organic pollutants-Their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environmental Pollution, 157, 3207–3217. https://doi.org/10.1016/j.envpol.2009.05.031
Hoover, S. (2005). National movement targets lawn care poisons. Pesticides, 25, 9–12.
Hu, Y., Qi, S., Yuan, L., Liu, H., & Xing, X. (2018). Assessment of organochlorine pesticide contamination in waterbirds from an agricultural region, Central China. Environmental Geochemistry and Health, 40, 175–187. https://doi.org/10.1007/s10653-016-9891-4
Huang, F., Li, Z., Zhang, C., Habumugisha, T., Liu, F., & Luo, X. (2019). Pesticides in the typical agricultural groundwater in Songnen plain, northeast China: Occurrence, spatial distribution and health risks. Environmental Geochemistry and Health, 41, 2681–2695. https://doi.org/10.1007/s10653-019-00331-5
Jote, C. A. (2019). A review of 2, 4-D environmental fate, persistence and toxicity effects on living organisms. Organic and Medicinal Chemistry International Journal, 9, 22–32. https://doi.org/10.19080/OMCIJ.2019.09.555755
Kaczyński, P., & Łozowicka, B. (2015). Liquid chromatographic determination of glyphosate and aminomethylphosphonic acid residues in rapeseed with MS/MS detection or derivatization/fluorescence detection. Open Chemistry, 13, 1011–1019. https://doi.org/10.1515/chem-2015-0107
Kadian, N., Gupta, A., Satya, S., Mehta, R. K., & Malik, A. (2008). Biodegradation of herbicide (atrazine) in contaminated soil using various bioprocessed materials. Bioresource Technology, 99, 4642–4647. https://doi.org/10.1016/j.biortech.2007.06.064
Karimi, H., Mahdavi, S., Lajayer, A. B., Moghiseh, E., Rajput, V. D., Minkina, T., & Astatkie, T. (2021). Insights on the bioremediation technologies for pesticide-contaminated soils. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-01081-z
Kočárek, M., Kodešová, R., Sharipov, U., & Jursík, M. (2018). Effect of adjuvant on pendimethalin and dimethenamid-P behaviour in soil. Journal of Hazardous Materials, 354, 266–274. https://doi.org/10.1016/j.jhazmat.2018.04.073
Koskinen, W. C., Cox, L., & Yen, P. (2001). Changes in sorption/bioavailability of imidacloprid metabolites in soil with incubation time. Biology and Fertility of Soils, 33, 546–550. https://doi.org/10.1007/s003740100366
Kumar, B., Mishra, M., Verma, V., Rai, P., & Kumar, S. (2018). Organochlorines in urban soils from Central India: Probabilistic health hazard and risk implications to human population. Environmental Geochemistry and Health, 40, 2465–2480. https://doi.org/10.1007/s10653-018-0112-1
Liu, Y. (2008). New insights into pseudo-second-order kinetic equation for adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 320, 275–278. https://doi.org/10.1016/j.colsurfa.2008.01.032
Lushchak, V. I., Matviishyn, T. M., Husak, V. V., Storey, J. M., & Storey, K. B. (2018). Pesticide toxicity: A mechanistic approach. EXCLI Journal, 17, 1101. https://doi.org/10.17179/excli2018-1710
Marin-Benito, J. M., Andrades, M. S., Sanchez-Martin, M. J., & Rodríguez-Cruz, M. S. (2012). Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. Journal of Agricultural and Food Chemistry, 60, 6936–6945. https://doi.org/10.1021/jf301322h
Martikainen, E. (1996). Toxicity of dimethoate to some soil animal species in different soil types. Ecotoxicology and Environmental Safety, 33, 128–136. https://doi.org/10.1006/eesa.1996.0016
Martins, E. C., Bohone, J. B., & Abate, G. (2018). Sorption and desorption of atrazine on soils: The effect of different soil fractions. Geoderma, 322, 131–139. https://doi.org/10.1016/j.geoderma.2018.02.028
Meftaul, I. M., Venkateswarlu, K., Annamalai, P., Parven, A., & Megharaj, M. (2021a). Glyphosate use in urban landscape soils: Fate, distribution, and potential human and environmental health risks. Journal of Environmental Management, 292, 112786. https://doi.org/10.1016/j.jenvman.2021.112786
Meftaul, I. M., Venkateswarlu, K., Annamalai, P., Parven, A., Sobhani, Z., & Megharaj, M. (2021b). Behavior and fate of fungicide chlorothalonil in urban landscape soils and associated environmental concern. Journal of Environmental Science and Health, Part B. https://doi.org/10.1080/03601234.2021.2014255
Meftaul, I. M., Venkateswarlu, K., Dharmarajan, R., Annamalai, P., Asaduzzaman, M., Parven, A., & Megharaj, M. (2020d). Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environmental Pollution. https://doi.org/10.1016/j.envpol.2020.114372
Meftaul, I. M., Venkateswarlu, K., Dharmarajan, R., Annamalai, P., & Megharaj, M. (2020a). Pesticides in the urban environment: A potential threat that knocks at the door. Science of the Total Environment, 711, 134612. https://doi.org/10.1016/j.scitotenv.2019.134612
Meftaul, I. M., Venkateswarlu, K., Dharmarajan, R., Annamalai, P., & Megharaj, M. (2020b). Movement and fate of 2, 4-D in urban soils: A potential environmental health concern. ACS Omega, 5, 13287–13295. https://doi.org/10.1021/acsomega.0c01330
Meftaul, M. I., Venkateswarlu, K., Rajarathnam, D., Annamalai, P., & Megharaj, M. (2020c). Sorption-desorption of dimethoate in urban soils and potential environmental impacts. Environmental Science: Processes and Impacts, 22, 2256–2265. https://doi.org/10.1039/D0EM00337A
Miah, S. J., Hoque, A., Paul, A., & Rahman, A. (2014). Unsafe use of pesticide and its impact on health of farmers: A case study in Burichong Upazila, Bangladesh. Cancer, 21, 22–30.
MMDH. (2017). Toxicological summary for: Glyphosate. Health Risk Assessment Unit, Environmental Health Division, 2–10. https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/glyphosatesumm.pdf. Retrieved January 10th, 2021.
Nisha, U. S., Khan, M. S. I., Prodhan, M. D. H., Meftaul, I. M., Begum, N., Parven, A., Shahriar, S., Juraimi, A. S., & Hakim, M. A. (2021). Quantification of pesticide residues in fresh vegetables available in local markets for human consumption and the associated health risks. Agronomy, 11, 1804. https://doi.org/10.3390/agronomy11091804
OEHHA. (2017b). Initial statement of reasons: Glyphosate. Proposed amendment to: Section 25705(b) Specific regulatory levels posing no significant risk. Glyphosate. https://oehha.ca.gov/media/downloads/crnr/glyphosate032917isor.pdf. Retrieved December 8th, 2021
OEHHA. (2017a). Glyphosate to be listed under proposition 65 as known to the state to cause cancer. https://oehha.ca.gov/media/downloads/public-information/press-releaseproposition-65/glyphosatepress032817.pdf. Retrieved December 23rd, 2020
Okada, E., Allinson, M., Barral, M. P., Clarke, B., & Allinson, G. (2020). Glyphosate and aminomethylphosphonic acid (AMPA) are commonly found in urban streams and wetlands of Melbourne, Australia. Water Research, 168, 115139. https://doi.org/10.1016/j.watres.2019.115139
Oliveira, R. S., Koskinen, W. C., Graff, C. D., Anderson, J. L., Mulla, D. J., & Nater, E. A. (2013). Acetochlor persistence in surface and subsurface soil samples. Water, Air, and Soil Pollution, 224, 1747. https://doi.org/10.1007/s11270-013-1747-2
Parven, A., Khan, M. S. I., Prodhan, M. D. H., Venkateswarlu, K., Mallavarapu, M., & Meftaul, I. M. (2021). Human health risk assessment through quantitative screening of insecticide residues in two green beans to ensure food safety. Journal of Food Composition and Analysis, 103, 104121. https://doi.org/10.1016/j.jfca.2021.104121
Peruchi, L. M., Fostier, A. H., & Rath, S. (2015). Sorption of norfloxacin in soils: Analytical method, kinetics and Freundlich isotherms. Chemosphere, 119, 310–317. https://doi.org/10.1016/j.chemosphere.2014.06.008
Pino, N., & Peñuela, G. (2011). Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. International Biodeterioration and Biodegradation, 65, 827–831. https://doi.org/10.1016/j.ibiod.2011.06.001
Pope, J. V., Skurky-Thomas, M., & Rosen, C. (1994). Toxicity, organochlorine pesticides. Medscape Education.
Pouyat, R., Groffman, P., Yesilonis, I., & Hernandez, L. (2002). Soil carbon pools and fluxes in urban ecosystems. Environmental Pollution, 116, S107–S118. https://doi.org/10.1016/S0269-7491(01)00263-9
Purnomo, A. S., Mori, T., Takagi, K., & Kondo, R. (2011). Bioremediation of DDT contaminated soil using brown-rot fungi. International Biodeterioration and Biodegradation, 65, 691–695. https://doi.org/10.1016/j.ibiod.2011.04.004
Quintero, J. C., Moreira, M. T., Feijoo, G., & Lema, J. M. (2005). Effect of surfactants on the soil desorption of hexachlorocyclohexane (HCH) isomers and their anaerobic biodegradation. Journal of Chemical Technology and Biotechnology, 80, 1005–1015. https://doi.org/10.1002/jctb.1277
Ramakrishnan, B., Venkateswarlu, K., Sethunathan, N., & Megharaj, M. (2018). Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance? Science of the Total Environment, 654, 177–189. https://doi.org/10.1016/j.scitotenv.2018.11.041
Ren, X., Zeng, G., Tang, L., Wang, J., Wan, J., Liu, Y., Yu, J., Yi, H., Ye, S., & Deng, R. (2018). Sorption, transport and biodegradation—An insight into bioavailability of persistent organic pollutants in soil. Science of the Total Environment, 610, 1154–1163. https://doi.org/10.1016/j.scitotenv.2017.08.089
Rodríguez-Cruz, S., & Lacorte, S. (2005). Degradation of alachlor in natural and sludge-amended soils, studied by gas and liquid chromatography coupled to mass spectrometry (GC−MS and HPLC−MS). Journal of Agricultural and Food Chemistry, 53, 9571–9577. https://doi.org/10.1021/jf051920+
Scharenbroch, B. C., Lloyd, J. E., & Johnson-Maynard, J. L. (2005). Distinguishing urban soils with physical, chemical, and biological properties. Pedobiologia, 49, 283–296. https://doi.org/10.1016/j.pedobi.2004.12.002
Shahid, M., Khalid, S., Murtaza, B., Anwar, H., Shah, A. H., Sardar, A., Shabbir, Z., & Niazi, N. K. (2020). A critical analysis of wastewater use in agriculture and associated health risks in Pakistan. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00702-3
Shukla, G., Kumar, A., Bhanti, M., Joseph, P., & Taneja, A. (2006). Organochlorine pesticide contamination of ground water in the city of Hyderabad. Environment International, 32, 244–247. https://doi.org/10.1016/j.envint.2005.08.027
Singh, B. K., Walker, A., & Wright, D. J. (2006). Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: Influence of different environmental conditions. Soil Biology and Biochemistry, 38, 2682–2693. https://doi.org/10.1016/j.soilbio.2006.04.019
Škrbić, B. D., Marinković, V., Antić, I., & Gegić, A. P. (2017). Seasonal variation and health risk assessment of organochlorine compounds in urban soils of Novi Sad, Serbia. Chemosphere, 181, 101–110. https://doi.org/10.1016/j.chemosphere.2017.04.062
Spadotto, C. A. (2002). Screening method for assessing pesticide leaching potential. Pesticidas: Revista de Ecotoxicologia e Meio Ambiente, 12, 69–78.
USEPA. (1987b). 2,4-Dichlorophenoxyacetic acid (2,4-D), IRIS Assessments. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=150. Retrieved August 14th, 2021.
USEPA. (1987a). Chlorothalonil, IRIS Assessments. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=143. Retrieved August 14th, 2021.
USEPA. (2016). Dimethoate, IRIS Assessments. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=44. Retrieved August 14th, 2021.
USEPA. (2020). Regional Screening Table - User’s Guide, Mid-Atlantic Risk Assessment. Retrieved on: https://www.epa.gov/risk/regional-screening-levels-rslsgeneric-tables. Retrieved August 14th, 2021.
USFWS. (2000). Homeowner’s guide to protecting frogs-lawn and garden care. Division of Environmental Contaminants. https://www.fws.gov/dpps/visualmedia/printingandpublishing/publications/2003_HomeownersGuidetoProtectingFrogs.pdf. Retrieved August 20th, 2021.
Utture, S. C., Banerjee, K., Kolekar, S. S., Dasgupta, S., Oulkar, D. P., & Patil, S. H. (2012). Food safety evaluation of buprofezin, dimethoate and imidacloprid residues in pomegranate. Food Chemistry, 131, 787–795. https://doi.org/10.1016/j.foodchem.2011.09.044
Van Scoy, A. R., & Tjeerdema, R. S. (2014). Environmental fate and toxicology of chlorothalonil. Reviews of Environmental Contamination and Toxicology, 232, 89–105. https://doi.org/10.1007/978-3-319-06746-9_4
Wang, Q., Xie, S., & Hu, R. (2013). Bioaugmentation with Arthrobacter sp. strain DAT1 for remediation of heavily atrazine-contaminated soil. International Biodeterioration and Biodegradation, 77, 63–67. https://doi.org/10.1016/j.ibiod.2012.11.003
Yadav, I. C., Devi, N. L., Syed, J. H., Cheng, Z., Li, J., Zhang, G., & Jones, K. C. (2015). Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Science of the Total Environment, 511, 123–137. https://doi.org/10.1016/j.scitotenv.2014.12.041
Yang, L., Xia, X., & Hu, L. (2012). Distribution and health risk assessment of HCHs in urban soils of Beijing, China. Environmental Monitoring and Assessment, 184, 2377–2387. https://doi.org/10.1007/s10661-011-2124-5
Yang, L., Xia, X., Liu, S., & Bu, Q. (2010). Distribution and sources of DDTs in urban soils with six types of land use in Beijing, China. Journal of Hazardous Materials, 174, 100–107. https://doi.org/10.1016/j.jhazmat.2009.09.022
Yu, S., Zhu, Y.-G., & Li, X.-D. (2012). Trace metal contamination in urban soils of China. Science of the Total Environment, 421, 17–30. https://doi.org/10.1016/j.scitotenv.2011.04.020
Zehra, A., Eqani, S. A. M. A. S., Katsoyiannis, A., Schuster, J. K., Moeckel, C., Jones, K. C., & Malik, R. N. (2015). Environmental monitoring of organo-halogenated contaminants (OHCs) in surface soils from Pakistan. Science of the Total Environment, 506–507, 344–352. https://doi.org/10.1016/j.scitotenv.2014.10.055
Zhang, M., Zeiss, M. R., & Geng, S. (2015). Agricultural pesticide use and food safety: California’s model. Journal of Integrative Agriculture, 14, 2340–2357. https://doi.org/10.1016/S2095-3119(15)61126-1
Acknowledgements
MMI acknowledges the Australian Government for Research Training Program (RTP) scholarship and Sher-e-Bangla Agricultural University, Bangladesh, for granting study leave for PhD programme.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding was provided by University of Newcastle (Grant No. RTP scholarship).
Author information
Authors and Affiliations
Contributions
IMM was responsible for investigation, methodology, data curation, validation and writing the original draft. KV comprehensively edited and revised the manuscript. PA was involved in methodology; AP carried out data curation and validation. MM took part in conceptualization, supervision, sriting, reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Table 1 and Electronic Supplementary Material were incorrectly published. Both have been updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meftaul, I.M., Venkateswarlu, K., Annamalai, P. et al. Degradation of four pesticides in five urban landscape soils: human and environmental health risk assessment. Environ Geochem Health 45, 1599–1614 (2023). https://doi.org/10.1007/s10653-022-01278-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01278-w