Abstract
Pesticide application can have an adverse effect on pollinator honey bees, Apis mellifera L., ranging from mortality to sublethal effects. Therefore, it is necessary to understand any potential effects of pesticides. The present study reports the acute toxicity and adverse effects of sulfoxaflor insecticide on the biochemical activity and histological changes on A. mellifera. The results showed that after 48 h post-treatment, the LD25 and LD50 values were 0.078 and 0.162 µg/bee, respectively, of sulfoxaflor on A. mellifera. The detoxification enzyme activity shows an increase of glutathione-S-transferase (GST) enzyme on A. mellifera in response to sulfoxaflor at LD50 value. Conversely, no significant differences were found in mixed-function oxidation (MFO) activity. In addition, after 4 h of sulfoxaflor exposure, the brains of treated bees showed nuclear pyknosis and degeneration in some cells, which evolved to mushroom shaped tissue losses, mainly neurons replaced by vacuoles after 48 h. There was a slight effect on secretory vesicles in the hypopharyngeal gland after 4 h of exposure. After 48 h, the vacuolar cytoplasm and basophilic pyknotic nuclei were lost in the atrophied acini. After exposure to sulfoxaflor, the midgut of A. mellifera workers showed histological changes in epithelial cells. These findings of the present study showed that sulfoxaflor could have an adverse effect on A. mellifera.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 90% of flowering plant species in hot and humid environments require pollination to produce healthy fruit (Ollerton et al. 2011). Animal pollination is essential for reproduction of most flowering plants (Kremen et al. 2007). Loss of insect pollinators is a menace for global food security. Honeybees are globally considered essential pollinators in crops, fruits-bearing plants and wild species (Winfree et al. 2008). With remarkable success, bees pollinate 71 common crops from hundreds of plant species that make up 90% of the world’s food supply (Morse and Calderone 2000; Gallai et al. 2009; Artz et al. 2011). However, recently some world regions have been suffering from an increase in losses from their managed honey bee colonies. Colony Collapse Disorder (CCD) was first reported in 2006 in the USA (Neumann and Carreck 2010). The interaction between environmental stress factors, particularly exposure to pesticides and pathogens, is believed to be the main potential cause of colony collapse. It is difficult to define the main reasons for loss of colonies due to the varied social behavior of bees. They are exposed to daily human activities and other environmental factors. While numerous factors lead to losses, new reports have shown some of these factors include bee-keeping practices, pests, diseases, pesticide use, agricultural practices, and climate change (Hristov et al. 2021). Previous investigation has reported that exposure to insecticides affects colony stability (Henry et al. 2012) and homing capacity of honey bees (Tosi et al. 2017; Fulton et al. 2019), as well as memory neuronal inactivation in the mushroom body, olfactory learning, bumble bee colony growth, and queen production (Whitehorn et al. 2012). Pesticides can disrupt physiological processes unrelated to the intended modes of action (Chakrabarti et al. 2015). For example, they can cause oxidative stress and apoptosis (Gregorc et al. 2018) in bees and can exhibit smaller and irregularly shaped hypopharyngeal glands in A. mellifera (Menail et al. 2020). Neonicotinoids are the most studied pesticides in terms of side effects on pollinators with emphasis on their impacts on different bee species (Tsvetkov et al. 2017). Sulfoxaflor, the first commercial insecticide of the sulfoximines, is a systemic insecticide that translocates via treated crop plants and may contaminate pollen and nectar (Giorio et al. 2021; EFSA 2019). Sulfoxaflor acts as antagonist of the nicotinic acetylcholine receptor (nAChRs). The binding affinity of sulfoxaflor to the receptor makes it a distinctive insecticide compared to other nAChRs antagonists, which could be somewhat less toxic than other neonicotinoids such as imidaclocloprid and clothianidin (Azpiazu et al. 2021). As it is responsible for the digestion and absorption of ingested food, the insect’s midgut is a vital organ for toxicity studies. Additionally, chemical ingestion can affect other non-target insect organs as insecticides enter the midgut barrier and get distributed in the hemolymph (Catae et al. 2018) and damage mushroom bodies in the brain (de Morais et al. 2018). Sulfoxaflor is an effective insecticide against several sucking insect pests which are resistant to other insecticide classes including resistant species to the neonicotinoids. Due to its lack of cross-resistance, sulfoxaflor is a poor substrate for the metabolic enzymes endowing resistance to other classes of insecticides (Sparks et al. 2013).
Chronic exposure to Sulfoxaflor at field-recommended concentrations has been shown to diminish egg-laying and impair reproductive success in bumblebees (Siviter et al. 2018, 2020). In contrast, no effect was found on learning and memory of bumblebees after acute exposure to sulfoxaflor (Siviter et al. 2019) or the escape response of locusts (Parkinson et al. 2020). Exposure of A. mellifera colonies to Sulfoxaflor in a flight enclosure caused acute toxicity but did not otherwise impact flight activity or long-term colony development (Cheng et al. 2018). Sulfoxaflor has recently been shown to increase oxidative stress and induce apoptosis in A. mellifera (Chakrabarti et al. 2020). So far, it has been shown that exposure of honey bees to sublethal doses of insecticides could affect motor activity, feeding, development, reproductive system, and enzyme (antioxidant and detoxification) mechanisms (Murawska et al. 2021). In contrast, sulfoxaflor has a low synergistic effect in bee species after treatment of bees with the LD50 value of sulfoxaflor alone or in combination with the fungicide fluxapyroxad (Azpiazu et al. 2021). Thus, in a semi-field study, no significant effects of sulfoxaflor and the fungicide azoxystrobin were found on bees (Tamburini et al. 2021). While, both forms of chlorntraniliprole (technical-grade and formulation product) had a toxic effect after 4 or 72 h post-treatments (Williams et al. 2020). On the other hand, few studies have demonstrated the detoxification enzymes to conventional insecticides in honeybees (Papadopoulos et al. 2004; Johnson et al. 2006).
Generally, the lethal and sublethal exposure to chemical or bio-insecticides may lead to changes in the biochemical and physiological characteristics of insects (Awad et al. 2022; Moustafa et al. 2022). Exposure of honey bees to insecticides may effect their motor activity, feeding, development, reproductive system and enzyme activities. Therefore, the aim of our work was to evaluate the effect of LD25 and LD50 values of sulfoxaflor on honey bee. Our present work aims to assess the toxicity and the adverse effects of the insecticide sulfoxaflor on the activity of detoxification enzymes (Glutathione-S-Transferase and Mixed-Function Oxidases). In addition, we investigated the damage caused by sulfoxaflor in histopathological alteration for each brain, midgut and the hypopharyngeal gland of Apis mellifera.
Materials and methods
Apis mellifera samples
Adult worker bees (≥21days) were collected from adequately fed, healthy, disease-free, and queen-right colonies with known history and physiological status at the apiary yard of the Faculty of Agriculture, Cairo University, Egypt. The collected bees were reared on sucrose solution in water (50% w/v) at a temperature of 25 ± 2 °C with a relative humidity of 60–70%.
Bees bioassay
Bees were assembled individually without touching with hands directly to the 15 mL falcon tube (this will make bees move normally) with ventilation holes. Non-anesthetization methods, including CO2, ether and low temperature, were used to reduce mortality. Bees were collected in the early morning directly from the opening of a tunnel, and we starved them for 2 h in the incubator before the test. Five doses were prepared in a geometric series of sulfoxaflor including; 0.039, 0.078, 0.156, 0.321, and 0.625 mg/L to determine the LD values. Ten µL of each sugary concentration solution was applied to the lid of the falcon tube. Then the tube was inverted so that it was in the bottom and turned it upside down to allow the bee to feed on. We calculated consumption by weighing the empty tube cover for each tube and then added 10 µL droplets of the pesticide in sugary solution to the cover using an adjustable micropipette (2–20 microliter). Three replicate groups of ten separated bees were conducted. For the control treatment, individual bees were fed sucrose only. After feeding the bees on the treated or untreated diet for 4 h, the live bees were transferred onto a new falcon tube attached with a 1.5 mL of Eppendorf tube that stands vertically with sucrose solution only. The mortality percentage (%) of bees was measured and recorded after 24 and 48 h post-treatment. This experiment was conducted twice.
Biochemical analysis
Sample preparation
Two adult honey bees were collected from each replicate cage (3 replicates) after treating the honey bees with LD25 and LD50 values of sulfoxaflor as mentioned above. Each sample was homogenized in phosphate buffer saline pH 7.4 according to according to Chakrabarti et al. (2020) using a TT-30K digital handheld homogenizer (Hercuvan Lab System, Malaysia) at 8000 rpm for two cycles of 30 s /cycle. The homogenates were centrifuged at 10,000 rpm for 5 min at 4 °C. The supernatants were kept at −20 °C until they were used.
Glutathione- S-Transferase (GST) assay
GST activity was determined according to Habig et al. (1974) and Moustafa et al. (2021a). The reaction solution was composed of 10 µL of homogenate sample as enzyme stock solution, 25 µL 30 mM CDNB, and 25 µL 50 mM GSH. The GST activity was measured at 340 nm at 25 °C for 3 min using a spectrophotometer (Jenway-7205 UV/Vis, Staffordshire, UK).
Mixed-Function Oxidases (MFO)
MFO activity was tested according to Hansen and Hodgson (1971) and Moustafa et al. (2021b). First, we incubated 100 µL of 2 mM p-nitro anisole with 90 µL of homogenate sample for 2 min at 27 °C, and we added 10 µL of 9.6 mM NADPH to initiate the reaction. Then, the activity of MFO was measured at 405 nm for 15 min using molecular devices of microplate reader (Clindiag-MR-96, ISO09001:2008, Steenberg, Belgium). Finally, we used the standard curve of p-nitrophenol to calculate the MFO activity.
Histopathological studies
Seven adult honeybees were used for each treatment; each worker bee was anesthetized by cold exposure (4 °C) and carefully dissected (Dade 1977) with a fine pointed watchmaker forceps (Dumont, No: 5) and dissection scissors (Hammacher, Solingen) under a stereomicroscope (Euromex). First, we pinned the live bees onto a glass petri dish filled with paraffin by insect pins to prevent them from moving. Then, the cuticle was removed by cutting both sides of the abdomen to expose the internal organs (midgut). Finally, the head was dissected to remove the brain and hypopharyngeal gland.
Autopsy samples were taken from the brain, hypopharyngeal gland, and main gut of bees in different groups fixed in 10% formol saline for twenty-four hours. We used tap water to wash, then serial dilutions of alcohol (methyl, ethyl, and absolute ethyl) for dehydration. We cleared specimens in xylene and embedded them in paraffin at 56 °C in a hot air oven for 24 h. We prepared paraffin bees wax tissue blocks for sectioning at 4-micron thickness by slide microtome. Then, the tissue sections were collected on glass slides, deparaffinized and stained with hematoxylin-eosin for examination through the electric light microscope (Banchroft et al. 1996).
Data analyses
The statistical analysis program LDP line was used to determine the LD values for sulfoxaflor insecticide (with 95% confidence limits). We performed a one-way ANOVA for the enzymatic activity using SAS software (SAS 2001). We separated the mean values with the Duncan’s multiple range test.
Results
Effects of sulfoxaflor on A. mellifera
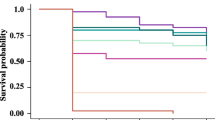
After 48 h post-treatment, the LD25 and LD50 values were 0.0785 and 0.1623 µg/bee, respectively with 95% confidence limit of 0.0511–0.1045 and 0.1242–0.2155, respectively (Table 1) of sulfoxaflor on A. mellifera. In addition, exposures to sulfoxaflor caused changes in the level of the enzyme activity of GST (Table 2). The results indicated that sulfoxaflor caused a significant increase in GST activity after 12, 24 and 48 h of post-treatment [F = 33.72, 17.51 and 110.23, P = <0.0001, 0.0008 and <0.0001] at the LD50 (2.3, 1.58 and 1.29-fold, respectively) compared to the control treatment. In contrast, sulfoxaflor at LD25 caused a significant decrease in the GST activity compared to the control (Table 2). The activity of MFO showed no significant differences after treating the A. mellifera with LD25 and LD50 of sulfoxaflor [F = 13.52, 0.23, 1.71 and 1.24, P = 0.001, 0.793, 0.234 and 0.333] compared with the control group (Table 3).
Histopathological changes of brain, hypopharyngeal glands and midgut of A. mellifera
Brain
We observed no histopathological alteration in the control of A. mellifera brain (Fig. 1A). The brain of A. mellifera L. manifests normal structure, Kenyon cells of the mushroom bodies were apparent, well-developed spherical nuclei and clear nucleoli. After 4 h the brain of treated bees with insecticide (sulfoxaflor) showed nuclear pyknosis and degeneration in some cells (Fig. 1B). The histopathology brain of bees treated with sulfoxaflor shows that most neuronal cells have nuclear pyknosis and degeneration for 24 h (Fig. 1C). After 48 h, the mushroom-shaped tissue showed loss of most neurons and was replaced by vacuoles in magnification.
Light micrographs of the brain of Apis mellifera L. (H&E X160) A the control bees showing no histopathological alteration B Brain of treated bees administrated insecticide (sulfoxaflor) for 4 h. Showing nuclear pyknosis (pk) and degeneration in some few cells. C Brain of treated bees with administrated insecticide (sulfoxaflor) For 24 h. showing most of the neuronal cells have nuclear pyknosis and degeneration. (D) (x80) after 48 h. The mushroom shaped tissue showed loss of most neurons and replaced by vacuoles in magnification showing vacuolization replacing the damage neurons. Nb: mushroom body (Mb), calyx (Ca), kenyon cells (kc), pyknosis (pk), damage neurons(dn)
Hypopharyngeal gland
The hypopharyngeal gland of A. mellifera L. contains secretory cells, the cytoplasm of which contains a variable number of secretory vesicles that appear almost as unstained as the control (Fig. 2A). There was a slight effect in secretory vesicles of treated bees with sulfoxaflor after 4 h (Fig. 2B). After 24 h, the treated bees showed atrophy with nuclear pyknosis in most cells, showing a loss of cytoplasmic fat vacuoles (Fig. 2C). After 48 h, the vacuolar cytoplasm and basophilic pyknotic nuclei were lost in the atrophied acini.
Light micrographs of the hypopharyngeal gland of Apis mellifera L. (H&E X160). A the control bees showing cytoplasm of the secretory cell seen contain a variable number of secretory vesicles that are almost unstained. B hypopharyngeal gland of treated bees with administrated insecticide (sulfoxaflor) for 4 h, showing slightly effect in secretory vesicles (vs). C hypopharyngeal gland of treated bees with administrated insecticide (sulfoxaflor) for 24 h, showing atrophy with nuclear pyknosis (pk) in most of the cells showing loss of cytoplasmic fat vacuoles. (D) (X80) after 48 h. There were loss of the vacuolar cytoplasm and basophilic pyknotic nuclei in the atrophied acini
Midgut
The midgut epithelial cells of A. mellifera workers (control) show normal nuclei, and the cytoplasm was densely homogeneous (Fig. 3A). After 4 h exposure to sulfoxaflor, the midgut epithelial cells of A. mellifera workers showed epithelium villi, many cytoplasmic vacuoles, and degenerated vacuolar lumen. The midgut region of bees treated with sulfoxaflor for 24 h showed the necrobiotic change in the lining epithelial cells. After 48 h, the mucosal lining epithelium showed necrosis with loss of the histological structure and was replaced by pigmented material. Other areas of the mucosa had degenerative vacuolar changes.
Light micrographs of the midgut of Apis mellifera L. (H&E X160) A the control bees showing single layered epithelium with columnar cells containing spherical nucleus (N) and apical surface with peritrophic matrix layers (pm). B Midgut region of treated bees with administrated insecticide (sulfoxaflor) for 4 h. Showing vacuolar degeneration lumen (L) epithelium of villi (ev). C Midgut region of treated bees with Administrated Insecticide (sulfoxaflor) for 24 h. showing necrobiotic change was detected in the lining epithelial cells. (D) (x80) after 48 h. The mucosal lining epithelium showed necrosis with lose of the histological structure and replaced by pigmented material. Other’s areas of the mucosa had vacuolar degenerative changes
Discussion
This study provides new insight into sulfoxaflor’s lethal and sublethal effects on honeybees. To understand this insecticide’s negative effects, both mortality and biochemical parameters on A. mellifera were measured. Results confirmed that sulfoxaflor is very toxic (LD50 = 0.078 µg/mL) to honeybees, which have fewer detoxification genes than insect pests, thus making honeybees more susceptible to pesticide exposure (Sadd et al. 2015). Other studies have classified sulfoxaflor toxicity on honeybees with oral and contact LD50 values of 0.05 and 0.13 µg/mL a.i/bee, respectively (Cheng et al. 2018), while its LC50 value was 1.72 mg/L after 96 h post-treatment (Li et al. 2021). In parallel, in the Pesticide Properties Data Base, the contact LD50 of sulfoxaflor was 0.379 μg/bee and for acute LD50 was 0.146 μg/bee for honeybees (Li et al. 2021).
The biochemical and physiological effects of insecticides can render individual honeybees unable to perform their mission smoothly, thus affecting the colony’s performance (Chakrabarti et al. 2020). GST and MFO are important metabolic enzymes and are essential elements in developing insecticide detoxication on insects (Bird et al. 2022). In the current study, GST exhibited higher activity in A. mellifera to sulfoxaflor. In addition, the activity of mixed-function oxidase did not significantly increase in bees. These results agree with Hu et al. (2014) and Zhang et al. (2016) who found an overexpression of GST related to resistance to diamide insecticides. There is also a positive correlation between the toxicity of Lambda-cyhalothrin and the activity of GST enzyme (Zhu et al. 2017), while no effect on esterase and GST activity was observed after spraying A. mellifera with sulfoxaflor (Zhu et al. 2017).
Thus, the exposure of honey bee workers to sulfoxaflor for 48 h caused a negative effect on the mushroom bodies. This damage may affect walking behavior (Martin et al. 1998; Helfrich-Förster et al. 2002), reduce memory ability and learning (Peng and Yang 2016) and influence the sensory organs in the head (Hansson and Anton 2000; Paulk et al. 2009; de Morais et al. 2018). As shown above, there was a slight effect in secretory vesicles after 4 h from exposure to sulfoxaflor, while after 24 h the effect has increased to atrophy with nuclear pyknosis in most of the cells showing loss of cytoplasmic fat vacuoles and after 48 h increased in basophilic pyknotic nuclei in the atrophied acini. This corresponds with the findings of several authors who confirmed the same histological effects after exposure to insecticides. When honey bees are exposed to insecticides, the cytoplasm degenerates and the nuclei of the secretory vesicles of the hypopharyngeal gland undergo pyknosis, which ultimately results in vacuoles and heterogeneous secretory vesicle content (Halberstadt 1980; Silva de Moraes and Bowen 2000).
Minor targets in insects may be affected. Although the insecticide’s major target is (nAChR) in insect nervous systems (Tomizawa and Casida 2003; Tan et al. 2007) and the organs concerned the metabolism. The ultrastructure changes were revealed through ultrastructural study. Structures harmed by being exposed to sulfoxaflor were given to bees. This finding corresponds with Oliveira et al. (2014), who showed that morphological ultrastructure in the midguts of adults and larvae of A. mellifera are exposed to insecticides. A study on the midgut of Africanized honeybee with a dose of 0.428 ng/mL of thiamethoxam per day reduced the number of regenerative cells in the epithelium and incited cytoplasmic vacuolization. The midgut is the part of the digestive tract responsible for most food processing and absorption, and it is known as the functional stomach (Cruz-Landim 2009). Because the pesticide was taken orally, the midgut was one of the first organs to be exposed to the lethal dose, and it suffered immediate consequences at the start of the exposure. In addition, our results correspond with Abd Alla and El-Wassef (2019) who reported that the neonicotinoids insecticides were lethal for worker honeybees and revealed changes that happened in the midgut. The nuclei were abnormal, small-size and deep-blue color (nucleus pyknosis), most of the lining mucosal epithelium showed vacuolar degeneration in the cytoplasm after being treated with a recommended dose of imidacloprid.
Conclusions
This study elucidated the adverse effects of sulfoxaflor insecticide on A. mellifera. Sulfoxaflor caused significant effects on mortality after 48 h post-treatment. Conversely, treated bees show significant differences in GST enzyme compared with the control group. The above findings of the present study show that sulfoxaflor has an adverse effect on tissue structure in the different organs (brain, hypopharyngeal gland and midgut) of the worker honeybee A. mellifera L, which eventually leads to death.
References
Abd Alla A, El-Wassef RAM (2019) Lethal and histological effects of Neonicotinoids pesticides on Apis mellifera (Hymenoptera, Apiadae). J Plant Prot Pathol Mansoura Univ 10:543–548
Artz DR, Hsu CL, Nault BA (2011) Influence of honey bee, Apis mellifera, hives and field size on foraging activity of native bee species in pumpkin fields. Environ Entomol 40:1144–1158
Awad M, Ibrahim ES, Osman EI, Elmenofy WH, Mahmoud AM, Atia MAM, Moustafa MAM (2022) Nano-insecticides against the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae): Toxicity, development, enzyme activity, and DNA mutagenicity. PLoS ONE 17:e0254285
Azpiazu C, Bosch J, Bortolotti L, Medrzycki P, Teper D, Molowny-Horas R, Sgolastra F (2021) Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci Rep 11:6821
Banchroft JD, Stevens A, Turner DR (1996) Theory and practice of histological techniques, 4th ed. Churchill Livingstone, New York, London, San Francisco, Tokyo
Bird L, Miles M, Quade A, Spafford H (2022) Insecticide resistance in Australian Spodoptera frugiperda (J.E. Smith) and development of testing procedures for resistance surveillance. PLoS ONE 17(2):e0263677
Catae AF, Roat TC, Pratavieira M, da Silva Menegasso ARD, Palma MS, Malaspina O (2018) Exposure to a sublethal concentration of Imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology 27:109–121
Chakrabarti P, Carlson EA, Lucas HM, Melathopoulos AP, Sagili RR (2020) Field rates of SivantoTM (flupyradifurone) and Transform (sulfoxaflor) increase oxidative stress and induce apoptosis in honey bees (Apis mellifera L.). PLOS ONE 15:e0233033
Chakrabarti P, Rana S, Sarkar S, Smith B, Basu P (2015) Pesticide induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidologie 46:107–129
Cheng Y, Bu Y, Tan L, Wu W, Li J, Zhou J (2018) A semi-field study to evaluate effects of sulfoxaflor on honey bee (Apis mellifera). Boll. Insectol 71:225–233
Cruz-Landim C (2009) Abelhas: morfologia e função de sistemas. S~ao Paulo: Editora UNESP. 408 p
Dade HA (1977) Anatomy and dissection of honey bee. Book published by the London Internat. Bee Research association. 158p
de Morais CR, Travençolo BAN, Carvalho SM, Beletti ME, Vieira Santos VS, Campos CF, de Campos Júnior EO, Pereira BB, Carvalho Naves MP, de Rezende AAA, Spanó MA, Vieira CU, Bonetti AM (2018) Ecotoxicological effects of the insecticide fipronil in Brazilian native stingless bees Melipona scutellaris (Apidae: Meliponini). Chemosphere 206:632–642
European Food Safety Authority (EFSA), Abdourahime H, Arena M, Auteri D, Barmaz S, Ctverackova L et al. (2019) Peer review of the pesticide risk assessment for the active substance sulfoxaflor in light of confirmatory data submitted. EFSA J17:e05633
Fulton CA, Huff Hartz KE, Fell RD, Brewster CC, Reeve JD, Lydy MJ (2019) An assessment of pesticide exposures and land use of honey bees in Virginia. Chemosphere 222:489–493
Gallai N, Salles JM, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821
Giorio C, Safer A, Sánchez-Bayo F, Tapparo A, Lentola A, Girolami V, van Lexmond MB, Bonmatin JM (2021) An update of the worldwide integrated assessment (WIA) on systemic insecticides. Part 1: new molecules, metabolism, fate, and transport. Environ Sci Pollut Res 28:1–33
Gregorc A, Alburaki M, Rinderer N, Sampson B, Knight PR (2018) Effects of coumaphos and Imidacloprid on honey bee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Sci Rep. 9(8):15003
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halberstadt K (1980) Elektrophoretische Untersuchungen zur sekretionstatigkeit der hypopharynxdrüse der Honigbiene (Apis mellifera L.). Insectes Soc 27:61–77
Hansen LG, Hodgson E (1971) Biochemical characteristics of insect microsomes and O-demethylation. Biochem Pharm 20:1569–1578
Hansson BS, Anton S (2000) Function and morphology of the antennal lobe: New developments. Annu Rev Entomol 45:203–231
Helfrich-Förster C, Wulf J, de Belle JS (2002) Mushroom body influence on locomotor activity and circadian rhythms in Drosophila melanogaster. J Neurogenet 16:73–109
Henry M, Béguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Hristov P, Shumkova R, Palova N, Neov B (2021) Honey bee colony losses: Why are honey bees disappearing? Review. Sociobiology 68:e-5851
Hu Z, Feng X, Lin Q, Chen H, Li Z, Yin F, Liang P, Gao X (2014) Biochemical mechanism of chlorantraniliprole resistance in the diamondback moth, Plutella xylostella Linnaeus. J Integr Agric 13:2452–2459
Johnson RM, Wen Z, Schuler MA, Berenbaum MR(2006) Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J Econ Entomol 99:1046–1050
Kremen C, Williams NM, Aizen MA, Gemmill-Herren BG, LeBuhn G, Minckley R et al. (2007) Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
Li J, Zhao L, Qi S, Zhao W, Xue X, Wu L, Huang S (2021) Sublethal effects of Isoclast™ Active (50% sulfoxaflor water dispersible granules) on larval and adult worker honey bees (Apis mellifera L.). Ecotoxicol Environ Saf 220:112379
Martin JR, Ernst R, Heisenberg M (1998) Mushroombodies suppress locomotor activity in Drosophila melanogaster. Learn Mem 5:179–191
Menail AH, Boutefnouchet-Bouchema WF, Haddad N, Taning CNT, Smagghe G, Loucif-Ayad W(2020) Effects of thiamethoxam and spinosad on the survival and hypopharyngeal glands of the African honey bee (Apis mellifera intermissa) Entomol General 40:207–215
Morse RA, Calderone NW (2000) The value of honey bee as pollinator of U.S. crops. Bee Cult 128:1–15
Moustafa MAM, Awad M, Amer A, Hassan NN, Ibrahim ES, Ali HM, Akrami M, Salem MZM (2021a) Insecticidal activity of lemongrass essential oil as an eco-friendly agent against the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Insects 12:737
Moustafa MAM, Fouad EA, Abdel-Mobdy Y, Hamow KÁ, Mikó Z, Molnár BP, Fónagy A (2021b) Toxicity and sublethal effects of chlorantraniliprole and indoxacarb on Spodoptera littoralis (Lepidoptera: Noctuidae). Appl Entomol Zool 56:115–124
Moustafa MAM, Elmenofy WH, Osman EA, El-Said NA, Awad M (2022) Biological impact, oxidative stress and adipokinetic hormone activities of Agrotis ipsilon in response to bioinsecticides. Plant Prot Sci. 58:326–337
Murawska A, Migdał P, Roman A (2021) Effects of plant protection products on biochemical markers in honey bees. Agriculture 11:648
Neumann P, Carreck NL (2010) Honey bee colony losses. J Apicult Res 49:1–6
Oliveira RA, Roat TC, Carvalho SM, Malaspina O (2014) Side-effects of thiamethoxam on the brain and midgut of the Africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ Toxicol 10:1122–1133
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Papadopoulos AI, Polemitou I, Laifi P, Yiangou A, Tananaki C, Glutathione S (2004) transferase in the insect Apis mellifera macedonica. Comp Biochem Physiol C Toxicol Pharmacol 139:93–97
Parkinson RH, Zhang S, Gray JR (2020) Neonicotinoid and sulfoximine pesticides differentially impair insect escape behavior and motion detection. Proc Natl Acad Sci USA 117:5510–5515
Paulk AC, Dacks AM, Phillips-Portillo J, Fellous JM, Gronenberg W (2009) Visual processing in the central bee brain. J. Neurosci 29:9987–9999
Peng YC, Yang EC (2016) Sublethal dosage of Imidacloprid reduces the microglomerular density of honey bee mushroom bodies. Sci Rep 6:19298
SAS (2001) User guide: Statistics (Release 8.02). SAS Institute, Cary, North Carolina
Sadd BM, Barribeau SM, Bloch G, de Graaf DC, Dearden P, Elsik CG et al. (2015) The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol 16:76
Silva de Moraes RL, Bowen ID (2000) Modes of cell death in the hypopharyngeal gland of the honey bee (Apis mellifera L). Cell Biol Int 24:737–743
Siviter H, Brown MJF, Leadbeater E (2018) Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 561:109–112
Siviter H, Folly AJ, Brown MJF, Leadbeater E (2020) Individual and combined impacts of sulfoxaflor and Nosema bombi on bumblebee (Bombus terrestris) larval growth. Proc Biol Sci 287:20200935
Siviter H, Scott A, Pasquier G, Pull CD, Brown MJF, Leadbeater E (2019) No evidence for negative impacts of acute sulfoxaflor exposure on bee olfactory conditioning or working memory. PeerJ 7:e7208
Sparks TC, Watson GB, Loso MR, Geng C, Babcock JM, Thomas JD (2013) Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic Biochem Physiol 107:1–7
Tamburini G, Pereira-Peixoto M-H, Borth J, Lotz S, Wintermantel D, Allan MJ, Dean R, Schwarz JM, Knauer A, Albrecht M, Klein AM (2021) Fungicide and insecticide exposure adversely impacts bumblebees and pollination services under semi-field conditions. Environ Inter 157:106813
Tan J, Galligan JJ, Hollingworth RM (2007) Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology 28:829–842
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48:339–364
Tosi S, Burgio G, Nieh JC (2017) A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci Rep 7:1201
Tsvetkov N, Samson-Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V, Zayed A (2017) Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356:1395–1397
Whitehorn PR, O’Connor S, Wackers FL, Goulson D (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336:351–352
Williams JR, Swale DR, Anderson TD (2020) Comparative effects of technical-grade and formulated chlorantraniliprole to the survivorship and locomotor activity of the honey bee, Apis mellifera (L.). Pest Manag Sci 76:2582–2588
Winfree R, Williams NM, Gaines H, Ascher JS, Kremen C (2008) Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J Appl Ecol 45:793–802
Zhang S, Zhang X, Shen J, Mao K, You H, Li J (2016) Susceptibility of field populations of the diamondback moth, plutella xylostella, to a selection of insecticides in Central China. Pestic Biochem Physiol 132:38–46
Zhu YC, Yao J, Adamczyk J, Luttrell R (2017) Synergistic toxicity and physiological impact of Imidacloprid alone and binary mixtures with seven representative pesticides on honey bee (Apis mellifera). PLOS ONE 12:e0176837
Acknowledgements
We would like to express our special thanks to Egyptian Knowledge Bank (EKB) for improving the manuscript considerably, including English editing and grammar. Special thanks goes to Stephen Giles (English Language Support Manager, Harper Adams University, UK) for improving the revised manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
E-DSI, AEAA, MSE-M, RAS, NNH, and MAMM, helped in the layout, data collection, systematic layout, data, and carrying the experimental. All authors contributed to writing the draft and the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
All authors consent their participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, ED.S., Abd Alla, A.E., El-Masarawy, M.S. et al. Sulfoxaflor influences the biochemical and histological changes on honeybees (Apis mellifera L.). Ecotoxicology 32, 674–681 (2023). https://doi.org/10.1007/s10646-023-02677-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02677-0