Abstract
Profenofos (organophosphate) is among the major toxicant polluting freshwater bodies, exerting a significant effect on fish health. The LC50 value of Profenofos (PRO) was resolved in Grass carp (Ctenopharyngodon idella) with average body weight (55.82 ± 5.42 g) and determined the 96 h LC50 value as 7.2 µg/L for the assay. Twenty-one-day exposures to 1.8 µg/ L and 3.6 µg/ L doses were conducted to evaluate the sub-lethal effects, and various toxicological endpoints were assessed on the 1st, 7th, 15th and 21st days of exposure. Acute toxic stress was observed with fish displaying behavioral toxicity. The most hematological change was extreme microcytic hypochromic anemia. Leucocyte count increased in experimented fish. Moderate neutrophilia, monocytosis and lymphocytosis were observed. Serum total protein, albumin, and globulin concentrations were significantly diminished. Overall, increments over control were recognized in serum urea, creatinine and acid phosphatase. However, serum glucose, total lipid, cholesterol, serum ALT and AST activity showed a significant decrease in fish exposed to both concentrations of PRO. Serum IgM concentrations insignificantly changed in treated fish except for on the 21st day of exposure to 3.6 µg/ L of PRO, while serum lysozyme significantly decreased. Furthermore, total protein, lipid and glycogen concentrations in muscles and the liver exhibited a decreasing trend at all concentrations. Moreover, histopathological alterations in the liver, kidney, and muscles occurred exclusively after treatment. From the obtained results, it is assumed that profenofos induced general toxic impacts under field conditions and might disturb ecologically relevant processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Industrialization and technological advancement caused the introduction of chemicals such as agrochemicals, pesticides, halogenated polycyclic hydrocarbons and food additives (Ibeto and Okoye 2010). The environmental consequences of these substances have become a worldwide concern. Pesticides are one of the most efficient weapons that man has devised to protect agricultural products from pest attacks (El-Houseiny et al. 2022a). Although there is a lot of research in the field of pesticides, the quantity of knowledge accessible about the effects of specific pesticides on non-target organisms varies greatly.
Pesticides constitute a persistent hazard to aquatic life by affecting habitat, behavior, growth, and reproductive potential (Ibrahim et al. 2019; Taha 2022). Fish are extremely sensitive to changes in their habitats and can help to determine the risks associated with new chemical contamination in aquatic ecosystems (El-Houseiny et al. 2022a; Khalil et al. 2022). They provide a significant portion of global human nutrition, so protecting fish health is critical.
Profenofos (PRO) is an organophosphate insecticide that is utilized in agriculture to control insects. It has a short persistence and is easily decomposed; hence it was extensively applied in Egypt during those years for selective mite control on cotton, maize, and other vegetables (Sharafeldin et al. 2015). The solubility of profenofos was confirmed by HPLC, dissolved in the solvent, and tested water samples at a concentration of 1 ppm (Ghazla et al. 2019). PRO, like other insecticides, may find its way into water systems, causing harm to aquatic life. It causes substantial harm to aquatic species and even endangers human safety throughout the food chain. PRO induced marked hepatotoxic and cytotoxic impacts in Cyprinus Carpio (Joseph and Raj 2010). It induced alterations in the locomotor behavior and tissue architecture of the fish Gambusia affinis (Rao et al. 2006). Moreover, it had adverse biochemical and histopathological impacts on the liver and kidneys of white egrets, Egretta alba (Taha 2022).
Toxicity testing is critical for determining the impact and fate of toxicants in aquatic habitats. The acute toxicity trials on zebrafish (Brachydanio rerio), tilapia (Oreochromis mossambicus), and common carp (Cyprinus carpio L) have been estimated earlier (Min and Cha 2000; Rao et al. 2003; Ismail et al. 2009). It is extremely hazardous to crustaceans (Blue crab, Callinectes sapidus, has an LC50 value of 33.0 µg/L), zooplankton (Immature scud, Gammarus pseudolimnaeus, has a 96 h LC50 value of 1.30 µg/L), Bluegill (300 µg/L), Crucian carp (90.0 µg/L), and Rainbow trout (80.0 µg/L) (Tomlin 1994). 62.4 µg/L was a 96 h LC50 value for common carp (Ismail et al. 2009), and 0.057 mg/L was LC50 for zebrafish (Sultana et al. 2021). However, studies on PRO-mediated toxicities in grass carp are still scanty. Grass carp (C. idella) is a freshwater fish that has a place with the family Cyprinidae. It has a fast growth rate and high commercial values (Wang et al. 2008 and Qu et al. 2016). They effectively control aquatic vegetation plants (Cudmore et al. 2017). The impacts of contaminants on fish survival rates and health, as well as human health, have generated cause for concern due to the proliferation of contamination in waters.
Therefore, given the ecological impact of this pesticide, this study was carried out to explore the effect of PRO on the health status of grass carp (C. idella). For achieving the previous objective, the LC50 of PRO after 96 h was determined. Moreover, the sub-lethal effect of 25 and 50 % of PRO LC50 was assessed on hematological, biochemical, and immunological parameters as well as histopathological findings in certain organs in grass carp (C. idella) after 1, 7, 15 and 21 days of exposure.
Material and method
Test compounds, reagents, and chemicals
Profenofos (Ictacrune) (Nagarjuna, India) is a broad-spectrum organophosphate insecticide widely used to control Lepidoptera in cotton and soybean with strong effects against mining and sucking insects as well as mites. Each liter of Ictacrune contains 72 g of the active substance (profenofos). The total amount of PRO to be added was calculated after each aquarium’s volume was determined. All other reagents, chemicals, and stains used were purchased from (Sigma, St.Louis, MO) and were of analytical grade
Experimental fish
A total of 160 apparently healthy live grass carp (C. idella) with an average body (55.82 ± 5.42 g) were obtained from the private fish farm at Damietta province, Egypt. Fish were obtained to determine 96 h LC50 and the sublethal toxicity effect of PRO. Fish were transported to the laboratory of the National Institute of Oceanography and Fishers, Al-Qanater branch, Egypt. The fish were acclimated to the laboratory conditions for two weeks. They were fed commercial pellets daily at 3% body weight (BW) during acclimatization two times daily at 9:00 and 16:00 h.
Experimental studies
Determination of 96 h LC50 of PRO (acute toxicity test)
The test individuals were exposed to selected and serially diluted PRO concentrations as shown in Table 1. Acute toxicity bioassay was conducted with a definitive test in a semi-static system in the laboratory as per the standard methods (APHA 2005). A total of 70 grass carp (C. idella) were divided into 7 groups (10 fish/group). The first group was left as a control while 2nd, 3rd, 4th, 5th, 6th, and 7th were exposed to 2, 4, 6, 8, 10 and 12 µg/l of PRO, respectively. These concentrations were selected to determine 96 h LC50 of PRO. Fish were observed at 12 h intervals up to 96 h. The water parameters were within the recommended ranges during the experiment (pH = 7.2 ± 0.5; ammonia = 0.02 ± 0.001 mg/L, nitrite = 0.017 ± 0.001 mg/L; dissolved oxygen 6.55 mg/L; water temperature 24 °C; photoperiod 12:12 light: dark). No feed was given during the test period. Dead fish were removed immediately upon discovery. Mortalities and survival times were recorded, and then LC50 was calculated according to the equation (Behrens and Karper 1953) (Table 2).
Effect of Sub-lethal toxicity of PRO in C. idella
For sub-lethal toxicity tests, the fish were grouped into three batches. Each batch had 10 fish and had 3 replicates. PRO was prepared to produce the required concentration (25% (1.8 µg/ L) and 50% (3.6 µg/ L) of 96 h LC50) in which 96 h LC50 of PRO = 7.2 µg/ L. The media was renewed every alternate day. Fish were fed daily with 3% of body weight. The amount of feed was readjusted every week according to the biomass of each replicate throughout the experiment; the uneaten feed was collected by siphoning. The proximate analysis of the basal diet indicated 38.9% crude protein, 10.5% crude lipid, and 3.68% fiber, according to NRC (2011). After their respective exposure, the fish were observed daily for 21 days for any alterations in behavior. The mortalities, clinical signs, and postmortem findings were recorded.
Sampling
On the 1st, 7th, 15th and 21st days of exposure to PRO, blood samples were collected from the caudal veins of (5 fish at each time from each group, 0.5–0.0.8 ml from each fish) and were divided into two parts. The first part was collected into plain, clean, and sterile centrifuge tubes without anticoagulant to separate serum for biochemical analysis. The second part was taken in EDTA tubes for a complete blood cell count picture. Moreover, liver and muscle tissues were collected on the 1st, 7th, 15th and 21st days from exposure to PRO and stored in the freezer (at −20 °C) till used. After that, Liver and muscle samples (about 100 mg.) were homogenized, and the total proteins were precipitated by saline 0.9%, while total lipids and glycogen were precipitated by ethanol 95%. Then, centrifugation at 3000 rpm for 15 min occurred, and the supernatants were used to determine tissue biochemical parameters. Parts of the liver, Kidney, and muscles were fixed for 48 h in 10% neutral buffered formalin for histopathological examinations.
Blood cells count picture
Red blood cell counts, hemoglobin (Hb) concentration, packed cell volume (PCV), mean corpuscular volume (MCV), mean cell hemoglobin concentration (MCHC), and white blood cell counts were assessed by using an automated blood cell analyzer of Sysmex XT-2000iVKobe (Japan) (Harvey 2012). Giemsa-stained blood smears were prepared for the differential leukocytic count, including lymphocytes, neutrophils, eosinophils, and monocytes (Dacie and Lewis 1984).
Determination of some serum biochemical parameters
Liver and kidney function tests as well as immunological response
Liver and kidney injury byproducts such as alanine (ALT) and aspartate (AST) aminotransferases were determined colorimetrically using readily made kits according to the method described by Reitman and Frankel (1957). Both acid and alkaline phosphatase activities were measured using methods described by Kaplan et al. (1988) and Kind and King (1954), respectively. The serum total proteins and albumin were measured colorimetrically according to the method described by Gornall et al. (1949) and Doumas et al. (1971), respectively. Serum globulin concentration was calculated by subtracting serum albumin from total protein serum (Coles 1974). Serum creatinine was determined according to Tietz (1986). While the concentration of serum uric acid and urea were measured enzymatically according to Tietz (1990). The serum glucose, cholesterol, and total lipids were estimated according to Trinder (1969), Ellefson and Caraway (1976) and Frings and Dunn (1970), respectively. Selective immunological parameters such as IgM were measured using fish-specific ELISA kits according to the manufacturer’s instructions. Meanwhile, lysozyme activity in the serum was quantified by inhibition zone assays in agarose gel plates, as described by Mohrig and Messner (1968).
Determination of some tissue biochemical parameters
Total proteins, total lipids, and glycogen were estimated according to Gornall et al. (1949), Frings and Dunn (1970) and Seifter et al. (1950), respectively.
Histopathological studies
Samples from the liver, kidney, and muscles were collected on the 1st, 7th, 15th and 21st days from exposure to PRO for histopathological examinations. The collected samples were fixed in 10% buffered neutral formalin for 24 h, washed with running water, dehydrated in alcohol, and cleared in xylene and sections of 4–6 µ were prepared according to Bancroft et al. (1996).
Statistical analysis
All data were analyzed using the statistical package for social science (SPSS 15.0 software, 2008). The total variation was analyzed by one-way variance analysis (ANOVA). Duncan’s test was used to determine significance. Probability levels of less than 0.05 were considered significant, according to Snedecor and Cochran (1987).
Results
Toxicity test
Acute toxicity (Determination of PRO lethal concentrations)
The 96-h LC50 of PRO for C. idella was calculated. Tables 1 and 2 demonstrated the mortality of C. idella at different concentrations of PRO. The 96-h LC50 was calculated, and the results showed that the 96-h LC50 of PRO was 7.2 µg/L.
Clinical signs and postmortem
Ctenopharyngodon idella exposed to PRO swam erratically and rapidly with semi-circular swimming behavior, trying to jump out of the aquarium, increased opercular movement, rapid gulping of water as well as knocking the wall of the aquarium, then the fish showed vertical position with head downwards, loss of equilibrium and sinking to the bottom. In later stages, the exposed excited fish laid on their sides on the bottom of the aquarium, making very slight movement and remaining motionless on the aquarium bottom until death. Exposed fish to PRO showed loss of scales, excessive mucous secretion, shining skin color, fin rot, pale gills, inflamed swim bladder and congestion of internal organs.
Fish exposed to 1.8 µg/L (25% of LC50) and 3.6 µg/L (50% of LC50) of PRO showed fast movements of the operculum during the first 7 days. Later, the operculum moved normally. At the end of the exposure period, lethargy was noticed. No mortality occurred in the aquarium. Fish exposed to PRO showed abnormal swimming on its side and sank to the bottom of the aquarium. At postmortem, fish showed excessive mucous secretion, loss of scales and shined skin color, pale gills, inflamed swim bladder, gall bladder and inflamed kidney and liver.
Hematological studies
The hematological parameters were tested for the surviving fish under various doses of PRO. Fish exposed to 1.8 µg/L and 3.6 µg/L of PRO showed a significant decrease in the Hb and the hematocrit value after 1,7, 15 and 21 days of exposure, reaching a minimum value on day 1 after exposure to 3.6 µg/L of PRO, compared with control (Table 3). On 1st day of exposure to 1.8 µg/L of PRO, the red blood cell counts of fish showed a non-significant decrease compared to the control followed by a significant increase after exposure to 3.6 µg/L for 1st day. While, on 7th and 15th days of exposure to both concentrations of PRO, the red blood cells count of fish showed non-significant changes compared with control (Table 3). Later, after exposure of fish to two concentration of PRO for 21 days, a significant decrease was recorded in RBCs count. MCH and MCV showed a highly significant decrease on 1st, 7th, 15th, and 21st days, reaching to a minimum value on1st day after exposure to 3.6 µg/L of PRO. MCHC were significantly decreased, reaching a minimum value after exposure to 3.6 µg/L; however, it showed a transient non-significant decrease on 7th and 15th day (Table 3). While on the 21st day, there was a significant decrease in MCHC after exposure to 1.8 µg/L of PRO. Blood smears from different groups of fish exposed to sub-lethal concentrations of PRO revealed aberrant erythrocytic morphologies, including nuclear degeneration, micronuclear formation, binuclear development, tear drop appearance, and hypochromic erythrocytes.
Concerning white blood cell counts (WBCs), Table 3 revealed a significant increase in WBCs of C. idella on days 1, 7, 15 and 21 after exposure to 1.8 and 3.6 µg/L of PRO; also, there were significant increases in the neutrophils, monocytes, and lymphocytes counts of C. idella all over exposure periods. On the 1st, 7th, 15th, and 21st days, eosinophil counts of C. idella showed non-significant changes after exposure to 1.8 µg/L of PRO. In contrast, there was a significant decrease in eosinophils counts after exposure to 3.6 µg/L all over the periods.
Serum biochemical parameters
Liver and kidney function tests
This section deals with the study of the effects of sub-lethal exposure to 1.8 and 3.6 µg/L of PRO. The serum ALT and AST activity showed significant decreases in C. idella after exposure to both concentrations over the periods (Table 4). Significant increases were observed in the serum acid phosphatase activity of C. idella on days 1, 7, 15 and 21 after exposure to 1.8 and 3.6 µg/L of PRO. While on 1st day, the serum alkaline phosphatase activity showed a significant increase in C. idella, a sudden significant decrease in its level was recorded on day 15 after exposure to 3.6 µg/L of PRO.
As shown in Table 4, fish exposed to both concentrations of PRO showed significant decreases in the serum levels of total protein, albumin, globulin, glucose, and total lipid on 7th and 15th, and 21st days. Serum cholesterol concentrations of fish showed significant changes after exposure to 1.8 and 3.6 µg/L of PRO on the 1st, 7th, 15th, and 21st days. The serum urea and creatinine concentrations of C. idella showed significant increases after exposure to both concentrations of PRO over exposure periods (Table 4). On the 7th, 15th and 21st days, there were significant decreases in fish serum uric acid concentration after exposure to 1.8 and 3.6 µg/L of PRO.
As shown in Table 5, exposure of fish to 1.8 or 3.6 µg/L of PRO for 1, 7, 15 and 21 days induced significant decreases in the liver total protein and total lipids concentrations. However, on the 7th, 15th and 21st days, there was a significant decrease in the liver glycogen concentrations of fish exposed to both concentrations of PRO. Significant decreases were recorded in total protein, lipid concentration, and glycogen in muscles on the 7th, 15th and 21st day of exposure to both concentrations of PRO (Table 5).
Immunological Studies
As shown in Table 5, on the 1st, 7th, 15th and 21st days of exposure to both concentrations of PRO, significant decreases were recorded in the serum total lysozyme concentrations of fish. However, on the 15th and 21st day, a significant decrease was recorded in the serum IgM in both concentrations of fish.
Histopathological alterations
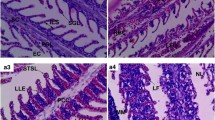
On day 1, after exposure to 1.8 and 3.6 µg/ L of PRO, the liver showed nuclear pyknosis. Moreover, moderate coagulative necrosis in the hepatocytes with a focal area of necrosis was observed (Fig. 1B). Also, edema around the hepatoportal blood vessels was noticed in fish exposed to 1.8 µg/L of PRO (Fig. 1C).
Sections of the liver of fish showing control (A) (X400), focal areas of necrosis (star) and coagulative necrosis of hepatocytes (yellow arrow) with nuclear pyknosis (red arrow) (B) (X400) (1st day-1.8 µg/L of PRO), nuclear pyknosis (yellow arrow) and edema around the hepatoportal blood vessel (red arrow) (C) (X400) (1st day-3.6 µg/ L of PRO), and vacuolar degeneration in the hepatocytes (red arrow) (D) (X400) (7th day-1.8 µg/ L of PRO). Accumulation of hemosiderin between hepatocyte (red arrow) (E, F) (X400) 7th day-3.6 µg/l and 15th day-1.8 µg/ L of PRO. Dilation and thrombosis formation in the hepatoportal blood vessel (red arrow) (G) (X400) (15th day-3.6 µg/ L of PRO). Focal areas of necrosis between hepatocytes (star), coagulative necrosis of hepatocytes (yellow arrow) with nuclear pyknosis (red arrow) (H) (X400) (21st day-1.8 µg/ L of ORO) and edema around the hepatoportal blood vessel (red arrow) (I) (X400) (21st day-3.6 µg L of PRO)
In fish exposed to both concentrations of PRO for 7 days, the liver showed vacuolar degeneration in the hepatocytes with focal areas of necrosis and nuclear pyknosis (Fig. 1D). Moreover, a slight accumulation of hemosiderin between the hepatocytes was observed in fish exposed to 3.6 µg/ L of PRO (Fig. 1E).
On day 15, after exposure to 1.8 and 3.6 µg/L of PRO, the liver showed moderate coagulative necrosis of hepatocytes. Moreover, slight accumulation of hemosiderin between the hepatocytes, focal areas of necrosis between the hepatocytes and vacuolar degeneration of hepatocytes were observed in fish exposed to 1.8 mg/ L of PRO (Fig. 1F). Also, dilation and thrombosis formation in hepatoportal blood vessels was noticed in fish exposed to 3.6 µg/L of PRO (Fig. 1G). Focal areas of necrosis between the hepatocytes and coagulative necrosis of hepatocytes were observed in the liver of C. idella exposed to both concentrations of PRO for 21 days (Fig. 1H). Moreover, edema around the hepatoportal blood vessels was noticed after exposure to 3.6 µg/ L of PRO (Fig. 1I).
On day 1, after exposure to 1.8 and 3.6 µg/ L of PRO, the kidney showed degeneration of renal tubules. Moreover, narrowing of capillary tubes of renal tubules, hemolysis in the renal blood vessel and vacuolar degeneration in the epithelial cells of renal tubules with degeneration of renal tubules were observed in fish exposed to 1.8 µg/ L of PRO (Fig. 2B). Also, depletion of hemopoietic tissue was noticed after exposure to 3.6 µg/ L of PRO (Fig. 2C). Narrowing of capillary tubes of renal tubules, vacuolar degeneration in the epithelium of renal tubules and activation in the hemopoietic tissue, accumulation of hemosiderin between renal tubules and edema in Bowman’s capsule (Fig. 2D) were observed in fish exposed to 1.8 µg/ L of PRO for 7 days. Also, depletion in the hemopoietic tissue and degenerative and necrotic changes in the renal tubules with focal areas of necrosis were noticed after exposure to 3.6 µg/ L of PRO (Fig. 2E).
Sections of the kidney of Ctenophyrogoden idella stained with H&E showing control (A) (X100), narrowing of renal tubules (red arrow), aggregation of inflammatory cells between renal tubules (black arrow) and hemolysis between renal tubules (star) and vacuolar degeneration in the epithelial cells of renal tubules (green arrow) and degeneration of renal tubules (yellow arrow) (B) (X400) (1st day −1.8 µg/L of PRO). Severe depletion of hemopoietic tissue (yellow arrow) and degenerative and necrotic changes in renal tubules with a focal area of necrosis (red arrow) (C) (X400) (1st day −3.6 µg/L of PRO), accumulation of hemosiderin between renal tubules (red arrow) and edema in Bowman’s capsule (yellow arrow) (D) (X400) (7th day 1.8 µg/L of PRO). Depletion of hemopoietic tissue (red arrow) and degenerative and necrotic changes with focal areas of necrosis (yellow arrow) (E) (X400) (7th day −3.6 µg/ L of PRO). Vacuolar degeneration in the epithelium of renal tubules (green arrow), depletion of hemopoietic tissue (yellow arrow) and degenerative and necrotic changes with focal areas of necrosis (red arrow) (F) (X400) in both concentrations of PRO for 15 and 21 days
In fish exposed to both concentrations of PRO for 15 and 21 days, the kidney showed vacuolar degeneration in the epithelium of renal tubules, depletion in the hemopoietic tissue and degenerative and necrotic changes in the renal tubules with focal areas of necrosis (Fig. 2F).
On day 1, the muscles of fish exposed to 1.8 and 3.6 µg/ L of PRO showed mild atrophy (Fig. 3B) to severe atrophy of muscle bundles and vacuolar degeneration in muscle bundles (Fig. 3C). Moreover, degeneration of muscle bundles was noticed in fish exposed to 3.6 µg/ L of PRO. In fish exposed to both concentrations of PRO for 7 days, the muscle showed severe vacuolar degeneration in muscle bundles. Moreover, atrophy of muscle bundles. Also, focal areas of necrosis were observed (Fig. 3D). Exposure of fish to 1.8 µg/ L of PRO for 15 days induced vacuolar degeneration in muscle bundles. Splitting of muscle bundles and severe degeneration in muscle bundles with a focal area of necrosis were observed after exposure to 3.6 µg/ L of PRO (Fig. 3E). On day 21 of exposure to both concentrations of PRO, the muscle showed severe atrophy of muscle bundles, vacuolar degeneration in muscle bundles and degeneration in muscle bundles with a focal area of necrosis (Fig. 3F).
Sections of the muscle of Ctenophyrogoden idella stained with H&E showing control (A) (X100), vacuolar degeneration in muscle bundles (yellow arrow) and mild atrophy of muscle bundles (red arrow) (B) (X400) (1st day 1.8 µg/L of PRO). Severe atrophy of muscle bundles (yellow arrow) and degeneration in muscle bundles with a focal area of necrosis (green and red arrows) (C) (X400) (1st day −3.6 µg/L of PRO), severe degeneration in muscle bundles with a focal area of necrosis (yellow and red arrows) and vacuolar degeneration in muscle bundles (black arrow) (D) (X400) 7th day of −1.8 µg/L and −3.6 µg/L of PRO and 15th day −1.8 µg/Lof PRO). Splitting of muscle bundles (yellow arrows) and severe degeneration in muscle bundles with a focal area of necrosis (red arrows) (E) (X400) (15th day −3.6 µg/L of PRO), severe atrophy of muscle bundles (black arrow), vacuolar degeneration in muscle bundles (green arrow) and degeneration in muscle bundles with a focal area of necrosis (red and yellow arrows) (A) (X400) 21st day of 1.8 µg/l and −3.6 µg/l of PRO
Discussion
The application of the LC50 has gained acceptance among toxicologists and is the highest-rated test for assessing the potential adverse effects of chemical contaminants on aquatic life (Gad and Saad 2008; Khayatzadeh and Abbasi 2010). 96 h LC50 determination has been widely recommended as a preliminary step in toxicological studies on fish (APHA 2005; Parrot et al. 2006; Moreira et al. 2008). Fish mortality because of pesticide exposure mainly depends upon its concentration, sensitivity to the toxicants and exposure time duration (Kamble et al. 2011). The 96 h LC50 of PRO on grass carp was 0.0072 mg/L. The detection of LC50 concentration of pollutants is an important step before carrying out further studies on physiological changes in animals. The data on lethal toxicity provides useful information for identifying the mode of action of a substance and also helps in comparing dose-response among different chemical substances. The acute toxicity of organophosphate (PRO) was studied on many species like Oreochromis mossambicus (Nair 2006), Cyprinus carpio (Joseph and Raj 2011) and Oreochromis niloticus (Sharafeldin et al. 2015). To our knowledge, this is the first study on grass carp.
Herein C. idella exposed to PRO swam erratically and very rapidly with a semi-circular swimming behavior and tried to jump out of the aquarium, increased opercular movement, aggressive behavior, rapid gulping of water, knocking the wall of the aquarium. Similarly, Rahman et al. (2002) observed nearly the same clinical signs. This was attributed to respiratory impairment and irritation due to toxicant in water which affects gills. Additionally, the observed difficulty in respiration mainly reflects a decreased respiratory capability because of the damage to the gills. In later stages of exposure, the exposed excited fish laid on their sides on the bottom of the aquarium, making very slight movement and remaining motionless on the aquarium bottom until death.
In the present investigation, C. idella showed considerable alteration in different blood parameters after exposure to sublethal concentrations of 1.8 and 3.6 µg/L of PRO. A significant decrease in Hb percentage, total erythrocyte count, and hematocrit values indicate the occurrence of anemia associated with erythropenia. The anemia may be due to the inhibition of erythropoiesis and hemosynthesis and to an increase in the rate of erythrocyte destruction in hemopoietic organs such as the kidney, as proved by histopathological studies. Reduction in Hb level may be the consequence of the toxic effects of PRO on the synthesis of this molecule. The toxicant may inhibit the synthetic pathway by affecting the activity of enzymes involved in the Hb synthesis. Similar findings were recorded in Labeo rohita exposed to lethal and sublethal concentrations of PRO (Zenebehagos et al. 2017) and in Nile tilapia, Oreochromis niloticus (Khan 2019). Erythrocytes decrease has been reported by Abdelmeguid et al. (2002) in Tilapia Zilli due to water pollution. The damage caused to the intestine by the toxicant may be a reason for impaired iron absorption that led to its deficiency, as reported by (Joshi et al. 2002). Erythrocytes are crucial for determining fish exposed to toxins’ structural and functional status. Erythrocytes can react to a few environmental stresses, and changes in fish erythrocytes (both nuclear and cellular) are the most frequent sign that pesticides are present in a body of water (Sawhney and Johal 2000). Various erythrocytic abnormalities such as binucleated, tear-drop-shaped, microcytic, and hypochromic erythrocytes, were revealed, indicating PRO toxicity. Various erythrocytic abnormalities such as binucleated, elongated shaped, tear-drop shaped, and twin were found in stained blood smears of Tilapia treated with different PRO concentrations (Khan 2019). The decline in PCV might be due to shrinking cell size after intoxication. The decrease in MCV was reasoned for the variation in red cell volume attributed to exosmosis indicated by increased electrolyte concentration inside the red cell after insecticide treatment (Reddy et al. 1991). Because MCH and MCHC are derived from Hb and RBC, any alteration in Hb and RBC levels would lead to the alteration of MCH and MCHC. It was reported that MCV indicates the status or size of RBCs (Alwan et al. 2009). We noticed a significant decrease in MCV, MCH, and MCHC concentrations in C. idella treated with PRO. A significant decline in MCH and MCV levels indicates hypochromic microcytic anemia. Similar anemia was produced due to PRO intoxication in Labeo rohita (Zenebehagos et al. 2017).
In the present investigation, WBC count concentration increased on all days of exposure periods to PRO. An increase in the WBC count may result from direct stimulation of immunological defense due to a toxic substance or may be associated with induced tissue damage. The increase of leucocytes (WBC) count of treated fish reflects a general state of toxemia exhibiting impairment of the defense mechanism and is manifested into leukocytosis to cope with such a situation. Similar results were reported in teleosts by Ramesh and Saravanan (2008) on exposure to different pesticides. Enhanced WBC count in L. rohita has been reported when exposed to profenofos (Kesharwani et al. 2017). Moreover, Shrafeldin et al. (2015) and Al-Emran et al. (2022) revealed a significant increase in WBCs counts during both acute and chronic exposure to profenofos on Nile tilapia.
Serum total proteins were useful in diagnosing fish disease (El-Houseiny et al. 2022b). Most serum proteins are impaired by nitrogen metabolism (Murray et al. 1990). It is an indicator of liver impairment (Yang and Chen 2003). Elevation in serum total protein is possibly due to several pathological conditions such as damage to the liver and kidney, relative changes in the mobilization of blood proteins, activation of metabolic systems in response to pesticides exposure, degradation of the cellular material in the liver, water loss in the serum and induction of protein synthesis in the liver. Later, significant decreases were recorded in the serum total protein, albumin and globulin concentrations after exposure to PRO. The reduction in protein, albumin and globulin concentrations of the serum in this study may be because the liver function may be impaired and no longer produce albumin or proteins. The total protein was reported as an index of liver disturbance by (Yang and Chen 2003). Reduction in the total protein level of serum due to PRO toxicity has been reported by Nagaraju and Rathnamma (2013). Firat et al. (2011) attributed the reduction in total protein to the damaging effects of pesticides on liver cells. Herein, the decrease in glucose level might be due to the increase of glucose oxidation to meet the higher energy demands during chronic exposure. Similar findings have been recorded by Al-Emran et al. (2022) on Tilapia fish after exposure to PRO. Since carbohydrates serve as the instant energy source during stress, during the acute condition, blood glucose level increases due to glycogenolysis, but reduction can be correlated to the utilization of stored glycogen to meet the energy demand or chronic exposure. In the liver, glycogen mobilized to glucose, whereas in muscle, glycogen/glucose served as readily available energy; thus, hypoglycemia was observed. The increase of cholesterol observed in the present study may be due to one or more of the following reasons: increased production by the liver and other tissues by the effect of the pesticides, release of cholesterol from damaged cell membranes, decreased hepatic excretion of cholesterol, thyroid dysfunction and finally blocked conversion of cholesterol to sex steroids as a result of gonad dysfunction and decreased activity of cytochrome P450 enzymes (Metwally 2009). The serum cholesterol level has been observed to be decreased after PRO treatment for different time intervals (Sharafeldin et al. 2015). The decline in cholesterol levels after that is due to the utilization of stored and circulatory cholesterol and other lipid fractions in pesticide-treated fish to counteract the toxic effect produced and further stabilize the toxic pesticides to prevent harm caused by them. This is mainly due to altered lipid metabolism and energy demand. The reduction in total lipid was explained as a direct effect of the utilization of body fat as an energy supply to meet the increasing physiological demands and as a result of pollutant stress which enhanced metabolic rate and reduced metabolic reserves. This may be supported by the current depression of total protein and glycogen content in muscle and liver (Sharafeldin et al. 2015).
In the present study, serum urea and creatinine significantly increased in C. idella after exposure. This result supported that PRO exerts harmful effects on the kidney tissues causing kidney dysfunction. It is well known that renal insufficiency or failure is usually associated with decreases in urea, uric acid, and creatinine excretion, thus leading to increases in serum. The elevation of urea level may be attributed to gill dysfunction (Stoskoph 1993). Chang et al. (1996) concluded that kidney damage might result in reduced renal blood flow with a reduced glomerular filtration rate, resulting in azotemia characterized by increased blood urea and creatinine. Urea in fish is synthesized by the liver and excreted primarily by the gills rather than the kidney. It is shown that the increased blood urea could occur at times of impaired kidney function, liver diseases and cardiac arrest (Abdelmoneim et al. 2008). The histopathological alterations in the studied fish’s liver, kidney, and gills supported the increased serum urea and creatinine level. In contrast, there was a decrease in the uric acid of fish after exposure to both concentrations of PRO on all days of exposure. Low uric acid levels have no significance, but their increase indicates several disturbances in the kidney (Maxine and Benjamin 1985).
The serum ALT and AST concentrations decreased in C. idella after exposure to both concentrations of PRO. On the other hand, ALP decreased after exposure to both concentrations of PRO except on 1st day after exposure. Reductions in ALT, AST and ALP values in fish exposed to various toxicants have been reported previously (Banaee et al. 2008; Gabriel et al. 2012). Impairment of the serum membrane of the liver may be the reason for the reduction in ALP activity in the liver and, subsequently, the sera of the tested fish. The lower values of AST, ALT and ALP enzyme activities were suggested by inactive transamination and oxidative deamination (Gabriel et al. 2012) and the inhibition of intermediary metabolic processes (Begum 2005). Adeyemi et al. (2008) also believed that any alteration at the subcellular level might affect the activity of ALT and AST enzymes. The activity of enzymes produced in the liver is consistent with the serum protein concentrations in this experiment.
The obtained results showed an appreciable decline in different biochemical constituents of the fish tissues (glycogen, total lipid and total protein levels in liver and muscle) under pesticide stress. In the present study, the results clearly indicated a decrease in glycogen content to resist the effects of pesticides. The decrease in the glycogen concentration of the tissues of C. idella can be due to its enhanced utilization as an immediate source to meet energy demands under PRO stress. The glycogen content was observed in a decreasing manner with increasing concentrations. Because of the stress, the fish make suitable adjustments for which the stored energy is utilized. This may be the reason for the decreased amount of glycogen content consumed to provide immediate energy to the body’s fighting elements and protect all body systems from the harmful effect of pesticides. It could also be due to the prevalence of hypoxic or anoxic conditions, which normally enhances glycogen utilization (Dezwaan and Zandee 1973). Depleted glycogen levels following chromium stress were reported in Cyprinus carpio communis (Ambrose et al. 1994) under hypoxic conditions and in O. niloticus exposed to PRO (Shrafeldin et al. 2015). The protein content in the muscle and liver of C. idella is decreased with increasing concentrations of PRO. The decrease in protein content indicates that the tissue protein undergoes proteolysis. The decrease in tissue lipids and proteins might be partly due to their utilization in cell repair and tissue organization with the formation of lipoproteins, which are important cellular constituents of cell membranes, and cell organelles present in the cytoplasm (Harper 1983). A decrease in the lipid concentration observed in the present study can also be attributed to its utilization in cell repair and tissue organization. Moreover, the decrease in the total lipid of the liver with the increase in the concentration of PRO may be attributed to the utilization of energy storage to meet more energy demands for the detoxification process and also to balance the hindrance of normal metabolism (Bawa et al. 2017). Similar effects of PRO on total protein and lipids in Tilapia were demonstrated by Shrafeldin et al. (2015).
In our study, there was a significant decrease in lysozyme activity in serum of fish exposed to PRO. This result is in accordance with the study of Rahman et al. (2020), who observed a significant decrease in lysozyme activity in the plasma of Cyprinus carpio exposed to PRO. There were no significant changes in serum IgM of fish exposed to PRO all over exposure periods except in fish exposed to 3.6 µg/L of PRO on the 21st day. Similarly, Girón-Pérez et al. (2008) found that the IgM levels in plasma from Nile tilapia exposed to 0.39 and 0.78 mg/L diazinon were not affected. But at 1.96 mg/L, diazinon increased IgM concentrations. This finding agrees with a previous study in which intermediate doses were tested (Garg et al. 2004).
Tissue histology is regarded as a marker of exposure to pollutants and is an effective tool to evaluate the pollution level, particularly for sublethal impacts (El-Houseiny et al. 2022c). In the present study, histopathological data revealed that profenofos exhibited tissue alterations in fish liver, kidney, and muscle. Several changes were produced, such as coagulative necrosis of hepatocytes with nuclear pyknosis, vacuolar degeneration, and accumulation of hemosiderin between hepatocytes. Changes in the liver could be because the liver is the main site of detoxification, and it is expected that the toxicant would reach there abundantly for detoxification and disposal (Mushigeri and David 2005). The inability of fish to regenerate new liver cells may also have led to necrosis of hepatic cells of sinusoids. The renal tissue showed narrowing of renal tubules, aggregation of inflammatory cells and hemolysis between renal tubules, degenerative and necrotic changes, severe depletion of hemopoietic tissue, and accumulation of hemosiderin between renal tubules. Lesions recorded in the kidney indicate nephrotoxicity caused by the tested compound and its metabolites since kidneys are the way to eliminate most of the organophosphorus compound. Vacuolar degeneration, severe atrophy and degeneration in muscle bundles with a focal area of necrosis were observed in muscles. Our results parallel findings on the histological changes of different fish species exposed to pesticides (Mohamed et al. 2019). Various liver and kidney alterations were revealed in PRO-exposed Cyprinus carpio (Rahman et al. 2020) and in PRO-exposed Egretta alba (Taha 2022).
Conclusion
Along these lines, PRO in the aquatic medium is a major factor responsible for drastic changes in the fish blood and tissues. Abnormal behavior, hypochromic microcytic anemia, leukocytosis, and negative biochemical and histopathological effects on the liver, muscles, and kidney were common features of fish health status due to PRO toxicity. So, this issue must be considered during toxicological analysis and control of the main aquatic pollutant.
Data availability
Data of the present article are available under request.
References
Abdelmeguid N, Kheirallah AM, Abou-Shabana KA, Abdel-Moneim A (2002) Histochemical and biochemical changes in liver of Tilapia zilli G. as a consequence of water pollution. Online J. Biol. Sci. 2:224–229
Abdelmoneim A, AbouShabana N, Khadre S, Abdel K (2008) Physiological and histopathological effects in catfish (Clarias lazera) exposed to dyestuff and chemical water. Int J Zoological Res 4(4):189–202
Adeyemi O, Oloyede OB, Oladiji AT (2008) Biochemical evaluation of oxidative damage induced by leachate contaminated groundwater on selected tissues of rats. Int J Toxicol 4:1–7
Al-Emran M, Hasan NA, Khan MP et al. (2022) Alterations in hematological parameters and the structure of peripheral erythrocytes in Nile tilapia (Oreochromis niloticus) exposed to profenofos. Environ Sci Pollut Res 29:29049–29061
Alwan SF, Hadi AA, Shokr AE (2009) Alterations in haematological parameter of freshwater fish. Tilapia Zilli exposed to Aluminium. J Sci Appl 3(1):12–19
Ambrose T, Cyril Arun Kumar L, Vincent S, Roselyn L (1994) Biochemical responses of Cyprinus carpio communis to toxicity of tannery effluent. J Ecobiol 6(3):213–216
APHA (American Public Health Association) (2005) Standard methods for examination of water including bottom sediments and sludges. Standard Methods, (19th ed.), 874
Banaee M, Mirvaghefi AR, Ahmadi K, Banaee S (2008) Determination of LC50 and investigation of acute toxicity effects of diazinon on hematology and serology indices of common carp (Cyprinus carpio). J Marine Sci Technol Res 3(2):1–10
Bancroft JD, Stevens A, Turner DR (1996) Theory and practice of histological technique, 4th edition. Churchill, Livingston, New York, London, San Francisco, Tokyo, p 125
Bawa V, Kondal JK, Hundal SS, Kaur H (2017) Biochemical and histological effects of glyphosate on the liver of Cyprinus carpio (Linn.). Am J Life Sci 5(3-1):71–80
Begum G (2005) In vivo biochemical changes in liver and gill of Clarias battrachus during cypermethrin exposure and following cessation of exposure. Pesticide Biochem Physiol 82:185
Behrens AS, Karper L (1953) Determination of LC50. Arch. Exp. Path. Pharm. 28:177–183
Chang L, Magos L, Suzuki T (1996) Toxicology of metals. Lewis Publishers, New York
Coles EH (1974) Veterinary clinical pathology. W.B. Sounders company, Philadelphia, London, Toronto, p 211–213
Cudmore B, Jones LA, Mandrak NE, Dettmers JM, Chapman DC, Kolar CS, Conover G (2017) Ecological risk assessment of Grass carp (Ctenopharyngodon idella) for the great lakes basin. Can Sci Advis Sec Res Doc 2016/118:vi–115
Dacie, J., Lewis, S. (1984). Estimation of plasma haemoglobin. In: Practical Haematology, 6th Ed. Churchill Livingstone, London, pp. 139–140
Dezwaan A, Zandee DI (1973) Body distribution and seasonal changes in glycogen content of the common sea mussel, Mytilus edulis. Comp Biochem Physiol 43:53–55
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96
El-Houseiny W, Abd El-Hakim YM, Metwally MMM, Abdel Ghfar SS, Khalil AA (2022a) The single or combined Silybum marianum and co-enzyme Q10 role in alleviating fluoride-induced impaired growth, immune suppression, oxidative stress, histological alterations, and reduced resistance to Aeromonas sobria in African catfish (Clarias gariepinus). Aquaculture 548:737693
El-Houseiny W, Khalil AA, Abd-Elhakim YM, Arisha AH, Moselhy AA, Dahshan H, Ahmed MM (2022c) Alleviative effects of dietary Silybum marianum and co-enzyme Q10 on waterborne nickel-induced impaired growth, immunosuppression, tissue damage, immune-related genes dysregulation, and reduced resistance to Pseudomonas aeruginosa in Oreochromis niloticus. Aquaculture Reports 26:101308
El-Houseiny W, Algharib SA, Mohamed EAA, Metwally MMM, Mahmoud YK, Alghamdi YS, Soliman MM, Abd-Elhakim YM, El-Murr AE (2022b) Dietary parsley seed mitigates methomyl-induced impaired growth performance, hemato-immune suppression, oxidative stress, hepato-renal damage, and Pseudomonas aeruginosa Susceptibility in Oreochromis niloticus. Antioxidants 11:1185. https://doi.org/10.3390/antiox11061185
Ellefson RD, Caraway WT (1976) Fundamentals of clinical chemistry. In: Tietz NW, editor. Saunders WB, Philadelphia, USA
Firat O, Cogun H, Yuzereroglu T, Gok G, Frat O, Kargin F, Kotemen Y (2011) A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 37:657–666
Frings CS, Dunn RT (1970) Colorimetric method for determination of total plasma lipids based on the sulphophospho-vanilin reaction. Am J Clin Path 53:89–91
Gabriel UU, Akinrotimi OA, Ariweriokuma VS (2012) Changes in metabolic enzymes activities in selected organs and tissue of Clarias gariepinus exposed to cypermethrin. J Environ Eng Technol 1:13–19
Gad NS, Saad AS (2008) Effect of environmental pollution by Phenol on some physiological parameters of Oreochromis niloticus. Global Veterinaria 2(6):312–319
Garg UK, Pal AK, Jha GJ, Jadhao SB (2004) Haemato-biochemical and immunopathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks. Int Immunopharmacol 15:1709–22
Ghazla G, Sultana S, Al-Ghanim KA, Mahboob S (2019) The effect of profenofos on the nutritive composition of major carp for estimating maximum allowable toxicant concentration of the pesticide. Polish J Environ Stud 28(3):1127–1133. https://doi.org/10.15244/pjoes/85671
Girón-Pérez MI, Zaitseva G, Casas-Solis J, Santerre A (2008) Effects of diazinon and diazoxon on the lymphoproliferation rate of splenocytes from Nile tilapia (Oreochromis niloticus): the immunosuppresive effect could involve an increase in acetylcholine levels. Fish Shellfish Immunol 25(5):517–521
Gornall AC, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–66
Harper AH (1983) Review of Biochemistry 20th ed. Lange medical publications co, California, 1012
Harvey JW (2012) Veterinary hematology: a diagnostic guide and color atlas. St. Elsevier, Louis, Missouri
Ibeto CN, Okoye COB (2010) High levels of Heavy metals in Blood of Urban population in Nigeria. Res. J. Environ. Sci 4(4):371–382
Ibrahim RE, El-Houseiny W, Behairy A, Mansour MF, Abd-Elhakim YM (2019) Ameliorative effects of Moringa oleifera seeds and leaves on chlorpyrifos induced growth retardation, immune suppression, oxidative stress, and DNA damage in Oreochromis niloticus. Aquaculture 50:225–234
Ismail M, Ali R, Ali T, Waheed U, Khan QM (2009) Evaluation of the acute toxicity of profenofos and its effects on the behavioral pattern of fingerling common carp (Cyprinus carpio L., 1758). Bull Environ Toxicol 82:569–573
Joseph B, Raj SJ (2011) Impact of pesticide toxicity on selected biomarkers in fishes. Int J Zool Res 7:212–222
Joshi P, Harish D, Bose M (2002) Effect of Lindane and Malathion exposure to certain blood parameters in a freshwater teleost fish Clarias batrachus. Poll Res 21(2):55–57
Kamble SM, Bhagwan HK, Chinte DN (2011) Acute toxicity of Sevin concentration on mortality and behaviour of freshwater fish Barilius barila. Int Ref Res J 2(23):15–17
Kaplan A, Ozabo LL, Ophem KE (1988) Clinical chemistry: interpretation and techniques, 3rd ed. Lea and Febiger, Philadelphia
Kesharwani SS, Dube KK, Khan R (2017) Effect of Profenofos on Rohu Fish (Labio rohita): A Fish Widely Cultivated In Rural Areas of India. Int J Curr Microbiol App Sci. 6(5):1889–1893
Khalil AA, Abd-Elhakim YM, Said EN, Moselhy AAA, Abu-Elsaoud AM, El-Houseiny W (2022) Milk thistle and co-enzyme Q10 fortified diets lessen the nickel chloride-induced neurotoxic and neurobehavioral impairments in Oreochromis niloticus via regulating the oxidative stress response, acetylcholinesterase activity, and brain nickel content. Aquaculture 553:738102
Khan MP (2019) Effects of profenofos, an organophosphate pesticide, on the hematological parameters of nile tilapia (Oreochromis niloticus). Master Thesis. Department of fisheries management, Bangladesh Agricultural University
Khayatzadeh J, Abbasi E (2010) The effects of heavy metals on aquatic animals. The 1st International applied geological congress. Department of Geology, Islamic Azad University-Mashad Branch, Iran, p 688–694
Kind PRN, King EJ (1954) Estimation of plasma phosphatase by determination of hydrolised phenol with amino-antipyrine. J Clin Pathol 7(4):322–326
Maxine M, Benjamin BS (1985) Outline of veterinary clinical pathology, 3rd ed. Colorado State University Printed in India at Rekha printers Pvt. Ltd., New Delhi, India
Metwally MA (2009) Effect of garlic (Allium sativum) on some heavy metal (copper and zinc) induced alteration in serum lipid profile of Oreochromis niloticus. World J Fish Marine Sci 1(1):1–6
Min KJ, Cha CG (2000) Determination of the bioconcentration of phosphamidon and profenofos in Zebra fish (Brachydanio rerio). Bull Environ Contam Toxicol 65:611–617
Mohamed WA, El-Houseiny W, Ibrahim RE, Abd-Elhakim YM (2019) Palliative effects of zinc sulfate against the immunosuppressive, hepato-and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus). Aquaculture 504:227–238
Mohrig W, Messner G (1968) Immuneraktionen bilnsektionen lysosamals grundlegender antibakterieller factor in humoralen abwehr mechanismus der insekten. Biol Zent Bl 87:439–447
Moreira SM, Donato C, Lopes I, Ribeiro R (2008) Avoidance tests with small fish: determination of the median avoidance concentration and of the lowest observed-effect gradient. Environ Toxicol Chem 27:1576–1582
Murray R, Mayes P, Granner D, Radwel V (1990) Harper’s biochemistry?, 22 Edition. Appleton and Lange, London, Toronto
Mushigeri SB, David M (2005) Fenvalerate induced changes in the Ach and associated AchE activity in different tissues of fish Cirrhinus mrigala (Hamilton) under lethal and sub-lethal exposure period. Environ Toxicol Pharmacol 20(1):65–72
Nagaraju B, Venkata RV (2013) Effect of profenofos on organophosphate of protein levels in some tissues of fresh water fish Labeo Rohita (Hamilton). Inter J Pharm Pharmace Sci 5(1):0975–1491
Nair R (2006) Studies on acute toxicity and mortality response of Curacron (Profenofos) and Karate (Lambda cyhalothrin) of Oreochromis mossambicus. Article in Ecology. Environ Conserv 12(3):427–430
NRC (2011). Nutrient Requirements of Fish and Shrimp. National Academies Press, Washington, D.C.
Parrot JL, Mcmaster ME, Hewitt LM (2006) A decades of research on the environmental impacts of pulp and paper mill effluents in Canada: Development and application of fish bioassays. J Toxicol Environ Health 9:297–317
Qu J-H, Sun D-W, Cheng J-H, Pu H (2016) Mapping moisture contents in grass carp (Ctenopharyngodon idella) slices under different freeze drying periods by Vis-NIR hyperspectral imaging. LWT - Food Sci Technol 75:529–536
Rahman ANA, Mohamed AAR, Mohammed HH, Elseddawy NM, Salem GA, El-Ghareeb WR (2020) The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquaculture 517:734777
Rahman MZ, Hossain Z, Mellah MFR, Ahmed GU (2002) Effect of Diazinon 60EC on Anabus testudineus, Channa punctatus, and Barbados gomonotus. NAGA. The ICLARM Quarterly 25:8–11
Ramesh M, Saravanan M (2008) Haematological and biochemical responses in a freshwater fish, Cyprinus carpio exposed to Chlorpyrifos. Int J Integr Biol 3(1):80–83
Rao JV, Shilpanjali D, Kavitha P, Madhavendra SS (2003) Toxic effects of profenofos on tissue acetylcholinesterase and gill morphology in a euryhaline fish, Oreochromis mossambicus. Arch Toxicol 77:227–232
Rao JV, Begum G, Jakka NM, Srikanth K, Rao RN (2006) Sublethal Effects of Profenofos on Locomotor Behavior and Gill Architecture of the Mosquito Fish, Gambusia affinis. Drug Chem Toxicol 29(03):255–267
Reddy PM, Philip GH, Bashamohideen M (1991) Fenvalerate induced biochemical changes in the selected tissues of freshwater fish, Cyprinus carpio. BiochemInt 23:1087–1096
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvate transaminase. Am J Clin Pathol. 28:56
Sawhney A, Johal M (2000) Erythrocyte Alterations Induced by Malathion in Channa punctatus (Bloch). Bull Environ Contam Toxicol 64:398–405
Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem Biophys 50:191–200
Sharafeldin KM, Abdel-Gawad HA, Ramzy EM, Sweilum MA, Mossad MN (2015) Bioaccumulation of profenofos and its impact on hematological parameters of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758). Int J Aquatic Sci 6(2):48–59
Snedecor GW, Cochran WG (1987) Statistical methods, 6th Ed. Iowa State University Press, Ames, Iowa, USA
Stoskoph, M. (1993) Fish Medicine. St. Louis: W.B. Saunders Company. 116:128–129
Sultana Z, Khan MM, Mostakim GM et al. (2021) Studying the effects of profenofos, an endocrine disruptor, on organogenesis of zebrafish. Environ Sci Pollut Res 28:20659–20667. https://doi.org/10.1007/s11356-020-11944-0
Taha A (2022) Assessment of non-target toxicity of profenofos insecticide on the aquatic bird; the white egret egretta alba. Egyptian J Aquatic Biol Fish 26(2):263–276
Tietz NW (1990) Clinical guide to laboratory tests, 2nd ed. W.B. Saunders Company, Philadelphia, USA, p 554–556
Tietz NW (1986) Textbook of clinical chemistry. W.B. Saunders (Ed.), Philadelphia, pp 1271–1281
Tomlin CDS (1994) The pesticide manual, incorporating the agrochemicals handbook, 10th edn. The Royal Society of Chemistry and British Crop Protection Council, Cambridge
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6(1):24–27
Wang C, Chen Q, Lu G, Xu J, Yang Q, Li S (2008) Complete mitochondrial genome of the grass carp (Ctenopharyngodon Teleostei): Insight into its phylogenic position within Cyprinidae. Sifa L / Gene 424:96–101
Yang JL, Chen HC (2003) Effects of gallium on common carp (Cyprinus carpio): acute test, serum biochemistry, and erythrocyte morphology. Chemosphere 53:877–882
Zenebehagos Z, Chaitanya K, Krishnan GKG, Teka Z, Mulugeta M (2017) Toxic effect of profenofos on blood parameters in the freshwater fish, Labeo rohita (Hamilton). Innovat Int J Med Pharmaceut Sci. 2(2):14–18
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, ZME-b, FASM, MAWE, WE Methodology, ZE, FASM, MAWE, WE formal analysis, ZE, WE; investigation, ZE, FASM, MAWE, WE; resources, ZME-b, FASM, MAWE; WE; writing—original draft preparation, MAWE, WE; writing-review and editing, WE.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All experimental procedures with live fish were approved by the animal welfare and ethical review committee of Faculty of Veterinary Medicine, Zagazig University, Egypt.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-bouhy, Z.M., Mohamed, F.A.S., Elashhab, M.W.A. et al. Toxicity bioassay and sub-lethal effects of profenofos-based insecticide on behavior, biochemical, hematological, and histopathological responses in Grass carp (Ctenopharyngodon idella). Ecotoxicology 32, 196–210 (2023). https://doi.org/10.1007/s10646-023-02628-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02628-9