Abstract
The present study was designed to compare the responses in freshwater fish Oreochromis niloticus exposed to a synthetic pyrethroid, cypermethrin (CYP); an essential metal, copper (Cu); and a nonessential metal, lead (Pb). Fish were exposed to 0.05 μg/l CYP, 0.05 mg/l Cu, and 0.05 mg/l Pb for 4 and 21 days, and the alterations in serum enzyme activities, metabolite, and ion levels were determined. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities increased in response to CYP, Cu, and Pb exposures at both exposure periods. While elevations in alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) activities and in cholesterol level were observed in pesticide-exposed fish at 4 and 21 days, they increased in Cu- and Pb-exposed fish at 21 days. Although metal-exposed fish showed increases in cortisol and glucose levels at 4 days followed by a return to control levels at the end of the exposure period, their levels elevated in pesticide-exposed fish at both exposure periods. Total protein levels decreased in Pb- and pesticide-exposed fish at 21 days. Na+ and Cl− levels decreased in pesticide-exposed fish at both exposure periods and in Cu- and Pb-exposed fish at 21 days. The exposures of pesticide and metals caused an elevation in K+ level at the end of the exposure period. The present study showed that observed alterations in all serum biochemical parameters of fish-treated pesticide were higher than those in fish exposed to metals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution by toxicants has become one of the most important problems in the world (Chandran et al. 2005). The heavy metal and pesticide contamination of aquatic system has attracted the attention of researchers all over the world (Dutta and Dalal 2008) and has increased in the last decades due to extensive use of them in agricultural, chemical, and industrial processes that are becoming threats to living organisms. Fish are more frequently exposed to these pollutants because it is believed that regardless of where the pollution occurs, it will eventually end up in the aquatic environment.
Cu, an essential element for cellular metabolism, is a cofactor of redox reactions involving intracellular proteins and enzymes such as cytochrome oxidase and superoxide dismutase. Although Cu concentrations rarely exceed 3 μg/l in pristine waters, in polluted waters, they may surpass 63 μg/l and reach the toxicity threshold for some fish species (Taylor et al. 2003). Unlike Cu, Pb is a nonessential heavy metal for the biological functions of organisms even at low concentrations, and it is considered as one of the most deleterious heavy metal pollutants in aquatic systems. In polluted waters, the total Pb concentrations generally range between 0.05 and 10.0 μg/l (Galvin 1996).
Synthetic pyrethroids are insecticides that have been introduced over the past two decades for agricultural and domestic use (Sanchez-Fortun and Barahona 2005). These chemicals are potentially more toxic to fish and other aquatic organisms and are least toxic to mammals. Owing to the excessive use of synthetic pyrethroids, the environment and water resources are being polluted, thus endangering aquatic life directly and human life indirectly (Hill 1989). Due to their lipophilicity, pyrethroids have a high rate of gill absorption even when present at very low concentrations in the water. This in turn is a contributory factor to the sensitivity of the fish to aqueous pyrethroid exposures, because fish seem unable to metabolize the pyrethroids efficiently (Viran et al. 2003). Cypermethrin [(RS)-cyano-(3-phenoxyphenyl) methyl-(IRS)-cis -trans-3-(2,2-dichloroethenyl)-2,2-dimethyl-cyclopropane carboxylate] is a highly potent representative member of the type 2 synthetic pyrethroid insecticides, which have widespread application in virtually all sectors of insect control in animals, in agriculture, in the home, and in the garden as possible alternatives of some organophosphate, carbamate, or organochlorine insecticides (Jee et al. 2005). This has resulted in its discharge into the aquatic environment and consequently several laboratory studies have been performed, which evidenced that cypermethrin is extremely toxic to fish at very low concentrations with 96 h LC50 in the range of 0.4–2.2 μg/l (David et al. 2004).
Blood is a pathophysiological reflector of the whole body, and therefore, blood parameters are important in diagnosing the structural and functional status of fish exposed to toxicants (Adhikari et al. 2004). Changes in the biochemical blood profile indicate alterations in metabolism and biochemical processes of the organism, resulting from the effects of various pollutants, and they make it possible to study the mechanisms of the effects of these pollutants (Luskova et al. 2002). Previous studies have shown that metals and pesticides can cause either increase or decrease in levels of serum protein, cortisol, glucose, cholesterol, ions, and in the activities of serum enzymes depending on the toxicant type, species of fish, water quality, and length of exposure (Vaglio and Landriscina 1999; Monteiro et al. 2005; Jee et al. 2005).
Among the aquatic species, the fish are the major targets of toxicants contamination. Fish are largely being used for the assessment of the quality of aquatic environment and as such can serve as bioindicators of environmental pollution. Nile tilapia, Oreochromis niloticus, is a teleost widely distributed around the world with economic importance for fisheries and aquaculture. It is a good biological model for toxicological studies due to diverse characteristics, namely their high growth rates, efficiency in adapting to diverse diets, great resistance to diseases and to handling practices, easy reproduction in captivity and prolific rate, and, finally, good tolerance to a wide variety of environmental conditions (Fontainhas-Fernandes 1998).
Investigations into the effects of pesticides and metals on fish have a diagnostic significance in evaluating the adverse effects of these toxicants to human health. Most studies on the effects of environmental pollutants are confined to reporting biochemical and physiological changes after either pesticide treatment or metal exposure, and very little attention has been paid to compare the effects of these toxicants on biochemical parameters of fish, especially Nile tilapia. Therefore, the primary aim of this study was to compare the inducing effects of pesticide (CYP) and metals (Cu and Pb) on serum enzyme (ALT, AST, ALP, and LDH) activities, metabolites (cortisol, glucose, total protein, and cholesterol), and ions (Na+, Cl− and K+) levels of commercially valuable freshwater fish O. niloticus.
Materials and methods
Fish and experimental design
Oreochromis niloticus (56.5 ± 2.6 g of weight, 15.9 ± 1.9 cm of total length, as mean ± S.E.), obtained from Cukurova University Fish Culture Farm, were transferred to the laboratory. Fish were acclimatized to laboratory conditions in glass tanks for one month before exposure. The laboratory was illuminated for 12 h with fluorescent lamps (daylight 65/80 W). Experimental tanks contained 120 l of dechlorinated and gently aerated tap water: temperature 21.1 ± 0.4°C, pH 8.2 ± 0.8, dissolved oxygen 7.4 ± 0.3 mg/l, alkalinity 212.4 ± 3.4 mg/l CaCO3, and total hardness 329.4 ± 3.9 mg/l CaCO3. Fish were divided into four groups each containing 12 fish. Group I was held in tap water as control, and other groups were exposed to 0.05 μg/l CYP, 0.05 mg/l Cu (CuSO4·5H2O), and 0.05 mg/l Pb [Pb(NO3)2] for 4 and 21 days. The concentration of each toxicant was selected as nominal sublethal concentration and based on available literature data. Throughout the experiments, control and experimental fish were fed daily with a commercial fish food (Pinar Yem, Turkey), at approximately 3% of their body weight. Fish were maintained in static renewal conditions, where water, pesticide and metals were completely replaced every 24 h, transferring fish to freshly prepared toxicants solutions (Dutta and Arends 2003).
Serum preparation and analysis
At the end of each duration, six fish were removed from aquaria and used as replicates. Fish were immediately anesthetized with MS222 (Ethyl 3-aminobenzoate methanesulfonate salt, Sigma), and blood samples were taken from the caudal vein of each fish as described by Congleton and La Voie (2001). This blood was collected in anticoagulant-free centrifuge tubes. Serum was obtained by centrifugation of blood at 3.000 rpm for 10 min. Serum samples were then stored at −80°C until the analysis.
Biochemical parameters in the serum samples were analyzed using biochemical analyzers (Modular Roche DPP, Modular Roche E170, Hitachi Ltd, Tokyo, Japan). Reactants for all the measurements were supplied from Roche diagnostics (Mannheim, Germany) for the analyses.
Enzyme activity
ALT and AST activities were determined using UV test technique (Bergmeyer et al. 1985). The products of ALT and AST activities, pyruvate, and oxaloacetate were used to oxidize NADH to NAD+. The rate of the photometrically determined NADH decrease is directly proportional to the rate of formation of pyruvate or oxaloacetate and thus the ALT or AST activity, respectively.
ALP activity was determined by use of the colorimetric assay (Empfehlungen 1972). Measurement was based on the detection of the increase in absorbance due to the increase in the formation of p-nitrophenol in the reaction. In the presence of magnesium and zinc ions, p-nitrophenyl phosphate is hydrolyzed by phosphatases to form phosphate and p-nitrophenol. The p-nitrophenol released is proportional to the ALP activity and can be measured photometrically.
LDH activity was assayed using UV test technique (Wacker et al. 1956). In this analysis pyruvate is reduced to lactate. The progress of accompanying oxidation of NADH to NAD+ is monitored continuously by measuring the rate of absorbance decrease at 340 nm.
Metabolite level
Electrochemiluminometric assay was used in the determination of the cortisol levels. The test kit was prepared in accordance with the method described by Chiu et al. (2003). The serum cortisol assay is a competitive polyclonal antibody immunoassay that employs a magnetic separation step followed by electrochemiluminescence quantitation.
The enzymatic UV test was used for the determination of glucose level. The principle of the method is as described by Schmidt (1961). The enzymatic hexokinase catalyzes the reaction between glucose and adenosine triphosphate to form glucose-6phosphate and adenosine diphosphate. In the presence of NAD, the enzyme glucose-6-phosphate dehydrogenase oxidizes glucose-6-phosphate to 6-phosphogluconate. The increase in NADH concentration is directly proportional to the glucose concentration and can be measured spectrophotometrically at 340 nm.
The cholesterol level was determined by enzymatic colorimetric test (Abell et al. 1952). In this method, cholesterol esters are hydrolyzed to free cholesterol by cholesterol esterase. Free cholesterol is then oxidized by cholesterol oxidase producing hydrogen peroxide that when combined with 4-aminophenazone and phenol forms a red chromophore. Formation of this chromophore is measured at 520 nm at 37°C and is directly proportional to the cholesterol concentration of the sample.
The total protein was measured using colorimetric test. The operation of the kit was based on the method described by Weichselbaum (1946). Divalent copper reacts in alkaline solution with protein peptide bonds to form the characteristic purple-colored biuret complex. The color intensity is directly proportional to the protein concentration that can be determined photometrically.
Ion level
The electrolytes (Na+, K+, and Cl−) levels were determined with ion-selective electrodes (Tietz and Logan 1987). Synermed ISE testing reagents were used in the determination of the blood levels of these electrolytes. The principle of the test is based on the quantification of the electric potential of each ion found in the sample.
Data analysis
Data are presented as mean ± standard error. For the statistical analyses, one-way analysis of variance (ANOVA) was used, followed by the Student-Newman–Keul’s test using the SPSS version 10.0 statistical software (SPSS Inc., Chicago, IL, USA). Differences were considered significant if P < 0.05.
Results and discussion
Enzyme activity
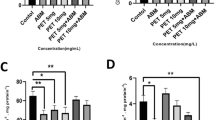
Serum ALT and AST activities of O. niloticus increased in response to Cu, Pb, and CYP exposures when compared to control during 4 and 21 days (Figs. 1 and 2). At the end of the exposure period, elevations in these enzyme activities of fish exposed to pesticide were higher when compared with metal-treated groups. While the ALP and LDH activities increased in fish exposed to pesticide at both exposure periods, an increase in their activities following metals exposure was observed at 21 days (Figs. 3 and 4).
Serum ALT activity in O. niloticus exposed to toxicants for 4 and 21 days. Data are expressed as mean ± standard error (N = 6). Different letters indicate significant differences among groups at the same time (P < 0.05). # shows significant differences between time for the same exposure group (P < 0.05)
Serum ALP activity in O. niloticus exposed to toxicants for 4 and 21 days. Data are expressed as mean ± standard error (N = 6). Different letters indicate significant differences among groups at the same time (P < 0.05). # shows significant differences between time for the same exposure group (P < 0.05)
Serum LDH activity in O. niloticus exposed to toxicants for 4 and 21 days. Data are expressed as mean ± standard error (N = 6). Different letters indicate significant differences among groups at the same time (P < 0.05). # shows significant differences between time for the same exposure group (P < 0.05)
Several of soluble enzymes of blood serum have been considered as a relevant stress indicator. Therefore, activities of serum ALT, AST, ALP, and LDH have been commonly used in the diagnosis of fish diseases as well as in the detection of tissue damage caused by environmental pollution. An increase of these enzyme activities in the extracellular fluid or serum is a sensitive indicator of even minor cellular damage (Palanivelu et al. 2005) and indicates stress-based tissue impairment. Generally, the results of ALT, AST, ALP, and LDH may indicate degeneration changes and hypofunction of liver as the toxicants effects on the hepatocytes are in the form of tissue damage in which cellular enzymes are released from the cells into the blood serum. Therefore, increases in these enzyme activities in serum of O. niloticus is mainly due to the leakage of these enzymes from the liver cytosol into the blood stream as a result of liver damage by pesticide and metals, which gives an indication of the hepatotoxic effect of toxicants. Harvey et al. (1994) concluded that blood levels of ALT, AST, and ALP may increase as due to the cellular damage in the liver and that high levels of these enzymes in serum are usually indicative of disease and necrosis in the liver of animals.
It was observed that the exposure to the heavy metals resulted in increases in ALT, AST, and ALP activities of plasma/serum of fish Sparus aurata (Vaglio and Landriscina 1999) and Cyprinus carpio (Karan et al. 1998). The present results are in agreement with the findings of Jee et al. (2005) who found that an increase in activities of serum ALT, AST, and LDH in Korean rockfish (Sebastes schlegeli) exposed to cypermethrin. Exposure to cypermethrin produced a significant increase in the activities of serum ALP and LDH in fish Rhamdia quelen (Borges et al. 2007) and Labeo rohita (Das and Mukherjee 2003), respectively. Long-term exposure (28 days) of Nile tilapia to subacute dose (1.46 μg/l) of another pyrethroid, deltamethrin also caused an increase in serum ALP (El-Sayed and Saad 2008). The researchers concluded that necrosis of liver and subsequent leakage of this enzyme into blood stream might be responsible for increase of this enzyme in blood.
Metabolite level
Although Cu- and Pb-exposed fish showed increases in cortisol and glucose levels at 4 days followed by a return to control levels at the end of the exposure period, their levels elevated in pesticide-exposed fish at both exposure periods (Table 1).
Serum cortisol levels are widely used as a primary response to stressors such as metals and pesticides. The hypothalamo-pituitary-interrenal (HPI) axis of fish is activated to produce cortisol and other corticosteroid hormones for the maintenance of disturbed homeostasis (Gagnon et al. 2006). Dethloff et al. (1999) reported that cortisol is released to the blood via stimulation of the HPI axis by heavy metal exposure. Cortisol is not stored in the interrenal tissue, but is synthesized on demand (Sumpter 1997) and so, in this study, the elevation of circulating cortisol may be a function of de novo stimulation of the HPI axis in response to pesticide and metal stress. A raised cortisol level in the serum has generally been used as a sign of stress response in fish (Wendelaar Bonga 1997).
The increase in blood glucose concentrations is known as a general secondary response to stress of fish to acute toxic effects and is considered as a reliable indicator of environmental stress (Sepici-Dinçel et al. 2009). Increase in serum glucose levels in fish under stress was reported by Cicik and Engin (2005). Hyperglycemic response illustrated in the present study is an indication of a disruption in carbohydrate metabolism, possibly due to enhanced glucose 6-phosphatase activity in liver, elevated breakdown of liver glycogen, or the synthesis of glucose from extrahepatic tissue proteins and amino acids. Heavy metals increase the glucose content in blood because of intensive glycogenolysis and the synthesis of glucose from extrahepatic tissue proteins and amino acids (Almeida et al. 2001). Raja et al. (1992) suggested that the increase in blood glucose by pesticide treatment may indicate disrupted carbohydrate metabolism due to enhanced breakdown of liver glycogen, possibly mediated by increase in adrenocorticotrophic and glucagon hormones and/or reduced insulin activity. Cypermethrin-induced hyperglycemia has been recorded in L. rohita (Das and Mukherjee 2003) and S. schlegeli (Jee et al. 2005).
Stress is an energy demanding process and the animal mobilizes energy substrates to cope with stress metabolically (Vijayan et al. 1997). Glucose is one of the most sensitive indices of the stress state of an organism: its high concentrations in blood indicate that the fish is in stress and it is intensively using energy reserves i.e., glycogen in liver and muscles (Vosyliene 1999). The stress hormone cortisol has been shown to increase glucose production in fish, by both gluconeogenesis and glycogenolysis, and likely play an important role in the stress-associated increase in plasma glucose concentration (Iwama et al. 1999). Plasma glucose and cortisol levels, used as stress indicators, increased during the waterborne copper exposure period, are significantly correlated with each other (Monteiro et al. 2005). In our work, cortisol and glucose levels might elevate to cope with the increased energy demand during pesticide- and metal-induced stress, both of which are important pathways for the recovery from stress. Increases in the cortisol and glucose levels were reported in Prochidolus lineatus (Martinez et al. 2004) and O. niloticus (Monteiro et al. 2005) in response to Pb and Cu, respectively. Borges et al. (2007) suggested that cypermethrin-induced hyperglycemia in R. quelen is likely to be a sign of stress and associated with the increase in cortisol levels.
While there were no significant changes in total protein levels in all toxicants-exposed fish at 4 days, it decreased in Pb- and CYP-exposed fish at the end of the exposure periods (Table 1). Total serum protein, the majority of serum proteins which are synthesized in the liver, is used as an indicator of liver impairment (Yang and Chen 2003). Rivarola and Balegno (1991) reported that the reduction in plasma protein in animals treated with pesticides could be attributed to changes in protein and free amino acid metabolism and their synthesis in the liver. In addition, the decrease in blood protein may be due to loss of protein by either reduced protein synthesis or increased proteolytic activity or degradation (Shakoori et al. 1990). The decrease in total proteins could be attributed in part to the damaging effects of pesticide and metal on liver cells as confirmed by the increase in the activities of serum AST and ALT observed in this study. A decline in serum total protein level was reported in fish R. quelen (Borges et al. 2007) and O. niloticus (Öner et al. 2008) in response to cypermethrin and Cu exposure, respectively.
While an elevation in cholesterol level was observed in pesticide-exposed fish at both exposure periods, its level in fish under metals exposure increased at 21 days (Table 1). The present results are in agreement with the findings of Öner et al. (2008) who found that cholesterol concentrations in the serum of metal-exposed O. niloticus generally increased when compared to that of the control value. They concluded that the concentrations of cholesterol, an essential structural component of membranes and the precursor of all steroid hormones, may increase due to liver and kidney failure causing the release of cholesterol into the blood. In the present study, exposure of fish to pesticide and metal caused increase in serum cholesterol concentration indicating the hypercholesteremia, which may be due to the stress induced by toxicants. The hypercholesteremia following cypermethrin exposure was observed in R. quelen (Borges et al. 2007). Yousef et al. (2003) reported that changes in blood cholesterol levels are related to changes caused by pesticides in the permeability of hepatic cells and that accumulation of pesticides in the liver disrupt lipid metabolism and increase serum cholesterol levels.
Ion level
The present study showed that toxicants in sublethal concentration caused the alterations in serum ion levels of O. niloticus (Table 2). Na+ and Cl− levels decreased in Cu- and Pb-exposed fish at 21 days and in pesticide-exposed fish at both exposure periods. The exposures of Cu, Pb, and CYP did not cause any significant changes in K+ level of fish at 4 days, while they caused an elevation in its level at the end of the exposure period. Shifts in the hydromineral balance may be a consequence of the action of pollutants on organs involved in osmoregulation, on endocrine system, on metabolism, or on active transport processes (Martinez and Colus 2002). Possibly, observed changes in the levels of serum ions of O. niloticus could be attributed to pathological changes in tissues such as gills and kidney involved in the exchange of ions between the fish and the surrounding water and to the reduction of Na+/K+-ATPase activity, which plays a central role in whole body ion regulation, due to pesticide and heavy metals toxicity. Previous studies (Reddy and Philip 1994; De Boeck et al. 2001; Monteiro et al. 2005) reported that the osmoregulatory disturbances induced by pesticides and metals were associated with an increased epithelial permeability and inhibition of active ion uptake, subsequently to the decline of Na+/K+- ATPase activity and a decrease in the number of active chloride cells.
In general, plasma/serum Na+ and Cl− tend to be similarly affected by waterborne toxicants (McDonald et al. 1989) as shown in our work. Levels of Na+ and Cl− decreased in fish P. lineatus (Mazon et al. 2002) and Cyprinion mhalensis (Al-Attar 2006) exposed to heavy metals. Cd effect of O. mykiss caused an increase in level of K+ (Chowdhury et al. 2004). They concluded that increased K+ is probably attributable to stress and/or acidosis and reflects an efflux of K+ from the intracellular compartment of white muscle. K+ is normally excreted by the kidneys, so disorders that decrease the function of the kidneys can result in hyperkalemia. The present results are in agreement with the results of Bernard and Grazyna (1999) who found that Na+ and Cl− levels decreased in serum of C. carpio, although K+ level elevated following deltamethrin exposure as a pyrethroid insecticide.
Since freshwater fish are hyperosmotic to the surrounding water, disruption of the gill epithelium that occurs after long-term metal exposures will increase epithelium permeability, water influx, and salt efflux (Lauren and McDonald 1985; Richards and Playle 1999), and thus lead to a final significant decrease in plasma osmolality and ion concentrations (Monteiro et al. 2005). This may explain the decline in serum Na+ and Cl− with increasing duration of exposure.
Conclusion
Biochemical profiles of blood can provide important information about the internal environment of the organism, as the unfavorable changes of the ambient environment are the first ones to earliest affect the blood. The present study showed that the changes in the serum enzymes activities, metabolites, and ions levels in pesticide- and metal-exposed fish were regarded as the biochemical manifestation of the toxic actions of toxicants. Observed increases/decreases in all serum biochemical parameters of fish-treated pesticide were higher than those in fish exposed to metals. We concluded that the alterations in serum parameters may be a result of the target tissue (i.e., liver, gill, and kidney) damage and dysfunction induced by the toxicants and that these parameters can be thus used as rapid and sensitive indicators of monitoring toward the impact of toxicants on aquatic organisms and ultimately whole of the ecosystem.
References
Abell LL, Levy BB, Brodie BB, Kendall FE (1952) A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem 195:357–366
Adhikari S, Sarkar B, Chatterjee A, Mahapatra CT, Ayyappan S (2004) Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxol Environ Saf 58:220–226. doi:10.1016/j.ecoenv.2003.12.003
Al-Attar AM (2006) The physiological responses of the fish, Cyprinion mhalensis to mercury intoxication. J Egypt Ger Soc Zool 51A:123–137
Almeida JA, Novelli ELB, Dal-Pai Silva M, Alves-Junior R (2001) Environmental cadmium exposure and metabolic responses of the Nile tilapia Oreochromis niloticus. Environ Pollut 114:169–175
Bergmeyer HU, Horder M, Rej R (1985) International federation of clinical chemistry (IFCC) scientific committee. J Clin Chem Clin Biochem 24:481–495
Bernard K, Grazyna L (1999) Effect of a sublethal concentration of deltametrin on biochemical parameters of the blood serum of carp (Cyprinus carpio L.). Acta Ichth Piscat 29:109–117
Borges A, Scotti LV, Siqueira DR, Zanini R, Amaral F, Jurinitz DF, Wassermann GF (2007) Changes in hematological and serum biochemical values in jundiá Rhamdia quelen due to sub-lethal toxicity of cypermethrin. Chemosphere 69:920–926. doi:10.1016/j.chemosphere.2007.05.068
Chandran R, Sivakumar AA, Mohandass S, Aruchami M (2005) Effect of cadmium and zinc on antioxidant enzyme activity in the gastropod, Achatina fulica. Comp Biochem Physiol 140C:422–426. doi:10.1016/j.cca.2005.04.007
Chiu SK, Collier CP, Clark AF, Wynn-Edwards KE (2003) Salivary cortisol on ROCHE Elecsys immunoassay system: pilot biological variation studies. Clin Biochem 36:211–214. doi:10.1016/S0009-9120(02)00471-X
Chowdhury MJ, Pane EF, Wood CM (2004) Physiological effects of dietary cadmium acclimation and waterborne cadmium challenge in rainbow trout: respiratory, ionoregulatory, and stress parameters. Comp Biochem Physiology 139C:163–173. doi:10.1016/j.cca.2004.10.006
Cicik B, Engin K (2005) The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turk J Vet Anim Sci 29:113–117
Congleton JL, La Voie WJ (2001) Comparison of blood chemistry values for samples collected from juvenile Chinook salmon by three methods. J Aquat Anim Health 13:168–172. doi:10.1577/1548-8667(2001)013<0168:COBCVF>2.0.CO;2
Das BK, Mukherjee SC (2003) Toxicity of cypermethrin in Labeo rohita fingerlings: biochemical, enzymatic and haematological consequences. Comp Biochem Physiol 134C:109–121
David M, Mushigeri SB, Shivakumar R, Philip GH (2004) Response of Cyprinus carpio (Linn) to sublethal concentration of cypermethrin: alterations in protein metabolic profiles. Chemosphere 56:347–352. doi:10.1016/j.chemosphere.2004.02.024
De Boeck G, Vlaeminck A, Balm PH, Lock RA, De Wachter B, Blust R (2001) Morphological and metabolic changes in common carp, Cyprinus carpio, during short-term copper exposure: interactions between Cu2+ and plasma cortisol elevation. Environ Toxicol Chem 20:374–380
Dethloff GM, Schlenk D, Khan S, Bailey HC (1999) The effects of copper on blood and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol 36:415–423
Dutta HM, Arends DA (2003) Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environ Res 91:157–162. doi:10.1016/S0013-9351(02)00062-2
Dutta HM, Dalal R (2008) The effect of endosulfan on the ovary of bluegill sunfish: a histopathological study (Lepomis macrochirus sp). Int J Environ Res 2:215–224
El-Sayed YS, Saad TT (2008) Sub-acute intoxication of a deltamethrin-based preparation (Butox® 5% EC) in monosex Nile tilapia, Oreochromis niloticus L. Basic Clin Pharmacol Toxicol 102:293–299. doi:10.1111/j.1742-7843.2007.00157.x
Empfehlungen D (1972) Deutschen gesselschaft für klinische chemie. Z Klin Chem Klin Biochem 10:182–192
Fontainhas-Fernandes AA (1998) Tilapia production. In: Reis-Henriques MA (ed) Aquaculture handbook. Academic Press, California, pp 135–150
Gagnon A, Jumarie C, Hontela A (2006) Effects of Cu on plasma cortisol and cortisol secretion by adrenocortical cells of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 78:59–65. doi:10.1016/j.aquatox.2006.02.004
Galvin RM (1996) Occurrence of metals in water. an overview. Water SA 22:7–18
Harvey RB, Kubena LF, Elissalde M (1994) Influence of vitamin E on aflatoxicosis in growing swine. Am J Vet Res 55:572–577
Hill JR (1989) Aquatic organisms and pyrethroids. Pestic Sci 27:429–465
Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA (1999) Heat shock proteins and physiological in fish. Am Zool 39:901–909
Jee J-H, Masroor F, Kang J-C (2005) Responses of cypermethrin-induced stress in haematological parameters of Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquac Res 36:898–905. doi:10.1111/j.1365-2109.2005.01299.x
Karan V, Vitorovic S, Tutundzic V, Poleksic V (1998) Functional enzymes activity and gill histology of carp after copper sulfate exposure and recovery. Ecotoxol Environ Saf 40:49–55
Lauren DJ, McDonald DG (1985) Effects of copper on branchial ionoregulation in the rainbow trout, Salmo gairdneri Richardson: modulation by water hardness and pH. J Comp Physiol 155B:635–644
Luskova V, Svoboda M, Kolarova J (2002) The effects of diazinon on blood plasma biochemistry in carp (Cyprinus carpio L.). Acta Vet Brno 71:117–123
Martinez CBR, Colus IMS (2002) Biomarcadores em peixes neotropicais para o monitoramento da poluicao aquatica na bacia do rio Tibagi. In: Medri ME, Bianchini E, Shibatta AO, Pimenta JA (eds) a bacia do rio Tibagi. Londrina, Parana, pp 551–577
Martinez CBR, Nagae MY, Zaia CTBV, Zaia DAM (2004) Acute morphological and physiological effects of lead in the Neotropical fish, Prochidolus lineatus. Braz J Biol 64:797–807
Mazon AF, Monteiro EAS, Pinheiro GHD, Fernandes MN (2002) Hematogical and physiological changes induced by short-term exposure to copper in the freshwater fish, Prochilodus scrofa. Braz J Biol 62:621–631
McDonald DG, Reader JP, Dalziel TRK (1989) The combined effects of ph and trace metals on fish ion regulation. In: Morris R, Taylor EW, Brown JA (eds) Acid toxicity and aquatic animals. Soc Exp Biol Semin Scr 31:221–242
Monteiro SM, Mancera JM, Fernandes AF, Sousa M (2005) Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comp Biochem Physiol 141C:375–383. doi:10.1016/j.cbpc.2005.08.002
Öner M, Atli G, Canli M (2008) Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ Toxicol Chem 27:360–366. doi:10.1897/07-281R.1
Palanivelu V, Vijayavel K, Ezhilarasibalasubramanian S, Balasubramanian MP (2005) Influence of insecticidal derivative (Cartap Hydrochloride) from the marine polychaete on certain enzyme systems of the freshwater fish Oreochromis mossambicus. J Environ Biol 26:191–196
Raja M, Al-Fatah A, Ali M, Afzal M, Hassan RA, Menon M, Dhami MS (1992) Modification of liver and serum enzymes by paraquat treatment in rabbits. Drug Metab Drug Inter 10:279–291
Reddy PM, Philip GH (1994) In vivo inhibition of AChE and ATPase activities in the tissues of freshwater fish, Cyprinus carpio exposed to technical grade cypermethrin. Bull Environ Contam Toxicol 52:619–626
Richards JG, Playle RC (1999) Protective effects of calcium against the physiological effects of exposure to a combination of cadmium and copper in rainbow trout (Oncorhynchus mykiss). Can J Zool 77:1035–1047
Rivarola VA, Balegno HF (1991) Effect of 2, 4-dichlorophenxyacetic acid on polyamine synthesis in Chinese hamster ovary cells. Toxicol Lett 56:151–157
Sanchez-Fortun S, Barahona MV (2005) Comparative study on the environmental risk induced by several pyrethroids in estuarine and freshwater invertebrate organisms. Chemosphere 59:553–559. doi:10.1016/j.chemosphere.2004.12.023
Schmidt FH (1961) Enzymatic determination of glucose and fructose simultaneously. Klin Wochenschr 39:1244–1250
Sepici-Dinçel A, Benli AÇK, Selvi M, Sarıkaya R, Şahin D, Özkul IA, Erkoç F (2009) Sublethal cyfluthrin toxicity to carp (Cyprinus carpio L.) fingerlings: biochemical, hematological, histopathological alterations. Ecotoxicol Environ Saf 72:1433–1439. doi:10.1016/j.ecoenv.2009.01.008
Shakoori AR, Aziz F, Alam J, Ali SS (1990) Toxic effects of talastar, a new synthetic pyrethroid, on blood and liver of rabbit. Pak J Zool 23:289–300
Sumpter JP (1997) Fish stress and health in aquaculture. In: Iwama GK, Rickering AD, Sumpter JP, Schreck CB (Eds) The endocrinol, stress. Cambridge Univ Press, Cambridge, pp 95–118
Taylor LN, Wood CM, McDonald DG (2003) An evaluation of sodium loss and gill metal binding properties in rainbow trout and yellow perch to explain species differences in copper tolerance. Env Toxicol Chem 22:2159–2166. doi:10.1897/02-256
Tietz NW, Logan NM (1987) Fundamentals of clinical chemistry. WB Saunders, Philadelphia
Vaglio A, Landriscina C (1999) Changes in liver enzyme activity in the teleost Sparus aurata in response to cadmium intoxication. Ecotoxicol Environ Saf 43B:111–116
Vijayan MM, Cristina Pereira E, Grau G, Iwama GK (1997) Metabolic responses associated with confinement stress in tilapia: the role of cortisol. Comp Biochem Physiol 116C:89–95
Viran R, Erkoç FÜ, Polat H, Koçak O (2003) Investigation of acute toxicity of deltamethrin on guppies Poecilia reticulata. Ecotoxicol Environ Saf 55:82–85. doi:10.1016/S0147-6513(02)00096-9
Vosyliene MZ (1999) The effects of heavy metals on haematological indices of fish. Act Zool Lit Hydro 9:76–82
Wacker WEC, Ulmer DD, Vallee BL (1956) Metalloenzymes and myocardial infarction. New Eng J Med 255:449
Weichselbaum TE (1946) An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol 16:40–48
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 7:591–625
Yang JL, Chen HC (2003) Effects of gallium on common carp (Cyprinus carpio): acute test, serum biochemistry, and erythrocyte morphology. Chemosphere 53:877–882. doi:10.1016/S0045-6535(03)00657-X
Yousef MI, El-Demerdash FM, Kamel KI, Al-Salhen KS (2003) Changes in some hematological and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicology 189:23–234. doi:10.1016/S0300-483X(03)00145-8
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fırat, Ö., Cogun, H.Y., Yüzereroğlu, T.A. et al. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus . Fish Physiol Biochem 37, 657–666 (2011). https://doi.org/10.1007/s10695-011-9466-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9466-3