Abstract
Rapid climate change and invasive species introductions threaten ecological communities across the globe. Freshwaters are particularly vulnerable and impacted, especially when these stresses coincide. We document the migration of an invasive piscine predator, the Sacramento pikeminnow (Ptychocheilus grandis), within its introduced range, the South Fork Eel River, California, USA. Snorkel surveys and temperature monitoring in 2015–2019 showed that pikeminnow migrate upstream during spring and early summer, with earlier migration in warmer years. We developed a statistical temperature model to forecast the timing and extent of upstream migration by pikeminnow under varying combinations of discharge and air temperature. Modeled river temperature increased with air temperature and downstream and decreased with discharge. In years with low discharge and high air temperature, we predict pikeminnow will move upstream earlier, increasing spatial and temporal overlap in their summer range with native fishes. Managing conditions that reduce pikeminnow co-occurrence with native fishes (i.e., decreasing river temperature) could increase amount and duration of predator-free habitat for native fishes. We predict invasive pikeminnow will have larger impacts on invaded riverine communities with global warming and increasing drought severity. Knowledge of life history and phenology, for pikeminnow and other organisms, can guide effective management as conditions change and help to limit adverse impacts of introduced organisms on native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater ecosystems are extremely threatened by climate change (Rahel and Olden 2008; Jackson et al. 2001), and at the same time, are among the Earth’s most heavily invaded ecosystems (Strayer 2010). Such compounded perturbations can exert large, synergistic, and unexpected effects on communities (Paine et al. 1998). For example, altered environmental conditions can influence the phenology of native and non-native species, such as the timing of migration (Milner-Gulland et al. 2011). In animal migrations, large numbers of conspecific individuals, sometimes entire populations, periodically move en masse to track spatially shifting conditions or resources (Lucas et al. 2001; Dingle 2014). Fishes can migrate thousands of kilometers (Tyus and McAda 1984; Quinn 2018) to spawn, feed, or to avoid stressful or dangerous conditions (Northcote 1978). The timing of migrations varies, often cued by environmental conditions such as, but not limited to, water temperature (Quinn et al. 1997), the onset of seasonal rains (Campbell et al. 2006 ), elevated flows (Lucas and Batley 1996), floodplain inundation (Correa et al. 2007), or melting ice (Hughes and Reynolds 1994). As climate continues to change, temperatures will get warmer, storms more intense, and the timing of rains will shift (Pierce et al. 2018). These changes will alter the timing of fish migrations in fresh waters. For example, in a partially migratory population of European roach (Rutilus rutilus), warmer temperatures advanced the timing of spring migration and delayed fall migration between a Swedish lake and its surrounding streams (Brodersen et al. 2011). In North America, Chinook salmon (Oncorhynchus tshawytscha) smolts out-migrated earlier in years with warmer springs (Sykes et al. 2009). In the Columbia River, adult sockeye salmon (Oncorhynchus nerka) spawning migrations occurred increasingly earlier over a half-century as water temperatures warmed (Quinn et al. 1997). Timing of migration determines where and when migrants co-occur and potentially interact with other migrants and resident species. Understanding and responding to shifts in timing and impacts of native and non-native migrants will be a key to managing aquatic communities, many of which are heavily invaded, in the future.
Introductions of predatory fish are extremely common in freshwaters (Cucherousset and Olden 2011) and often adverse for native species in their introduced ranges (Zaret and Paine 1973; Ogutu-Ohwayo 1990; Brown and Moyle 1991). Sacramento pikeminnow (Ptychocheilus grandis, Fig. 1) was introduced to the Eel River, California, USA, in 1979 and, in less than a decade, became widespread in the basin (Brown and Moyle 1991). The initial introduction has been traced back to just 3–4 individuals from Clear Lake, Lake Co., California being transferred into Pillsbury Reservoir, the only large impoundment on the mainstem Eel River (Kinziger et al. 2013). Pikeminnows (genus Ptychocheilus) are North America’s largest minnows; piscivorous adults can grow to over a meter in length and are capable of moving tens to hundreds of kilometers within a year (Tyus and McAda 1984; Harvey and Nakamoto 1999; Gadomski et al. 2001). Sacramento pikeminnow in the Eel River now threaten recovering populations of cold-adapted Pacific salmonids (Oncorhynchus spp.) and other native species through predation, competition, and indirect effects (Brown and Moyle 1991; Harvey and Nakamoto 1999; Nakamoto and Harvey 2003). Additionally, the impacts of non-native predatory fish on salmonids could intensify under predicted climate change through increased duration or area of overlap (Lawrence et al. 2014) or more intense biotic interactions (Reese and Harvey 2002) or both. Unfortunately, this is likely to be true with native salmonids and non-native pikeminnow in their introduced range, and part of predicting these impacts is understanding where and when overlap between native and non-native species occurs.
Sacramento pikeminnow (Ptychocheilus grandis, 40–50 cm individuals) holding below a bubble curtain generated by a small cascade spilling into the head of a large deep pool in the South Fork Eel River, part of their introduced range. During observation, these pikeminnow attempted to ambush out-migrating schools of juvenile Chinook salmon (Oncorhynchus tshawytscha) that entered the pool through the cascade. The pikeminnow lunged at the juvenile salmon while they tried to orient in the turbulent water (Standish-Hickey State Recreation Area, Leggett, Mendocino Co., CA, May 21, 2021)
Historically, Sacramento pikeminnow occupied middle to low elevation sections of stream in their native range and overlapped with steelhead in the middle reaches (Taft and Murphy 1950; Moyle 2002). Sacramento pikeminnow are large mobile predators, and a portion of the population appeared to migrate Sulfur Creek, Sonoma, Co. CA, part of their native range (Taft and Murphy 1950). Pikeminnow moved upstream in spring and downstream in the fall, but observations suggested some individuals might not migrate (Taft and Murphy 1950). Recently, Sacramento pikeminnow in the Sacramento River, CA, part of their native range, moved 300 km within a year (Valentine et al. 2020). Colorado pikeminnow (Ptychocheilus lucius) and northern pikeminnow (Ptychocheilus oregonensis) undertake long-distance, potamodromous, annual spawning migrations (Tyus and McAda 1984; Gadomski et al. 2001). In the Eel River, Sacramento pikeminnow were tracked up to 92 km within a year (Harvey and Nakamoto 1999). Individuals in upstream reaches tended to move downstream during the winter and some returned to the upstream locations where they were captured the following summer (Harvey and Nakamoto 1999). Due to a limited number of individual observations, and apparently partially migratory behavior, our understanding of broader trends in movement is still unclear, although evidence suggests movement is concerted among individuals and seasonally predictable (Taft and Murphy 1950; Harvey and Nakamoto 1999; Valentine et al. 2020).
Here, we describe how invasive Sacramento pikeminnow (hereafter pikeminnow) migrate in headwater reaches within their introduced range. We document a seasonal migration of pikeminnow in the South Fork Eel River of northwestern California, including temperature-dependent timing of arrival near the upstream extent of their distribution. Arrival time in this upstream portion of pikeminnows’ range determines the amount of time pikeminnow overlap with native fishes, particularly rearing juvenile salmonids; earlier arrival lengthens the time window for adverse interactions (predation, competition, and non-consumptive effects) with native fishes. We develop a statistical temperature model based on river discharge, air temperature, and distance downstream. With this model and temperature metrics calculated during pikeminnow migration, we predict the timing of pikeminnow movements and their spatial watershed distributions under different climate scenarios not observed.
Methods
Study site
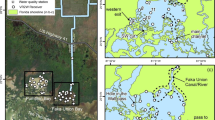
We studied pikeminnow movements in the upper reaches of the South Fork Eel River (river km 62–148, river km 0 was designated as the confluence of South Fork Eel and mainstem Eel and increased upstream), California, USA, with most of our effort centered in the Angelo Coast Range Reserve, a UC Natural Reserve System in the headwaters of the South Fork Eel River managed by the University of California, Berkeley in Mendocino Co., Northern California (river km 134.9–145.6), Fig. 2). This reach includes both the upstream extent of pikeminnow and high-quality over-summering habitat for salmonids (Brown and Moyle 1991; Georgakakos 2020). Salmonids, in particular steelhead trout (Oncorhynchus mykiss), overlap with pikeminnow in our study reach during the late spring and summer (Brown and Moyle 1991; Georgakakos 2020).
A Long profile of the study reach. The gray line shows the river channel; red and blue lines above the channel show winter (blue) and summer (red) range of pikeminnow. Dashed sections show uncertainty (winter) and variability (summer). River kilometer 0 was assigned to the confluence of the South Fork Eel and Mainstem Eel River. Crosses designate the upper (which is also the location of the USGS Branscomb Gage) and lower boundaries of where we measured and modeled temperature. B Map of the same section of upper South Fork Eel River and its tributaries shown in long profile (A). Relevant pools are shown in both long profile and map
During the 5 years of this study, summer temperature and river conditions varied dramatically. A 4-year drought in Northern California ended in December 2015 (Hahm et al. 2019; Goulden and Bales 2019). During this year, river temperatures warmed rapidly, and by July, had reached the highest recorded values of any year (Fig. 3A). In contrast, 2019 was exceptionally wet and cool. A late spring spate kept discharge high, above the 95th percentile of discharge measured from 1950 to 2019, throughout the summer (USGS 11475800, Leggett, CA). Water temperatures warmed more slowly in 2019 and remained cooler than in other years. Years 2016–2018 were intermediate in temperature (Fig. 3A). Various temperature metrics were summarized for Wilderness Pool (Table 1). Environmental conditions impact organismal performance and potentially the outcome of biotic interactions. We used interannual variability in water temperature and flow to study pikeminnow movement, and indirectly, pikeminnow’s potential to impact native fish where and when they co-occur.
A Hourly temperature for the five study years sampled in the well-mixed thalweg at the tail of Wilderness Pool in the South Fork Eel River in the Angelo Coast Range Reserve, Mendocino Co. A Horizontal dashed line at 19.98 °C represents the mean temperature of the pool during the week before pikeminnow arrive across years. Color-coded arrows on x-axis correspond to date of pikeminnow arrival each year. B Linear regression (R2 = 0.798, p = 0.041) of water temperature mean (computed from May 15th to July 1st) versus date of arrival (Julian day) of Sacramento pikeminnow at Wilderness Pool in the South Fork Eel River, Angelo Coast Range Reserve, Mendocino Co., CA
Seasonal migration of Sacramento pikeminnow
Pikeminnow distribution in the South Fork Eel River headwaters was documented with snorkel surveys from 2015 to 2020 in a 10.7-km study reach centered around the Angelo Coast Range Reserve (river 134.9–145.6). Habitat units, pools, and riffles (Hawkins et al. 1993) were surveyed by two divers. Surveyors swam upstream along the edges of the unit, counting animals and communicating to avoid double counting. If the unit was too wide, or the visibility was too poor for divers to see the middle effectively, one of the divers swam back downstream surveying the middle after reaching the upstream limit of the unit. During these surveys, all fish detected were counted and body lengths were estimated categorically (Table S2). Number of units surveyed differed between years depending on physical conditions and length of river surveyed in a given year (Table S5). In all years except 2020, surveys occurred twice, once at the end of May and once at the beginning of August. In 2016–2018, all habitat units were surveyed, (maximum n = 185 units in 2016). In 2019, approximately 30% of the total units were surveyed (n = 67 and 66). In 2020, approximately 15% of units were surveyed (n = 31), and only in August. In 2015, only eight large non-contiguous pools were surveyed but revisited throughout the summer. This survey data allowed us to document pikeminnow distribution in late spring (when they were absent from the upper headwaters) and in late summer (after pikeminnow migration upstream)0.0
We also intensively monitored one pool to compare the timing of pikeminnow arrival to the upper South Fork Eel River between years. For 5 years, 2015–2019, a single diver swam an S pattern (upstream along the river left bank, downstream through the middle, and upstream along the river right bank) to monitor the survey pool, which held pikeminnow each summer (Wilderness Pool, 39.74075° N, 123.6333° W, river km 140.0, Fig. 2). This pool was surveyed daily, from mid-May until the date when pikeminnow were first seen each year. When dives were repeated to test detectability and pikeminnow were detected on the first survey, pikeminnow were always detected on repeated dives by a naive diver or by the same diver. All pikeminnow encountered were counted and their sizes were estimated to the nearest 5 cm (fork length). After pikeminnow were detected in Wilderness Pool, more infrequent surveys continued. At this site, we also measured water temperature hourly via a logger deployed at the downstream thalweg of the pool in a well-mixed location. Temperature loggers were either vacuum-sealed Maxim ibuttons (DS1990) or Onset HOBO Temperature Pendant Logger (MX2201, MX2202). We calculated various temperature metrics for different time periods before pikeminnow arrived at this location (Table 1). We also used four linear regressions with pikeminnow arrival date (Julian day) as a response variable and temperature metrics (mean, minimum, standard deviation, and degree-days) for May 15th to July 1st (Table 2) as explanatory variables. A separate model was created for each of the explanatory variables, temperature metrics, to evaluate their usefulness in predicting pikeminnow arrival. Cumulative degree-days were calculated as the hourly deviation from 15 °C (a temperature at or below which pikeminnow are less effective predators of (Vondracek 1987) and competitors with steelhead (O. mykiss, Reese and Harvey 2002), summed over the total period of interest (Eq. S1). These data allowed us to explore the influence of temperature on migration timing and identify temperature metrics that can be useful in predicting pikeminnow movement.
Climate scenario modeling and implications for pikeminnow distributions
To explore how environmental factors influenced river temperature, we used a linear mixed-effects model, with water temperature as a response variable, to evaluate a range of environmental predictors. We then used this model to locate water temperatures that coincided with pikeminnow arrival at our intensely monitored pool. In the linear mixed-effect model, independent variables included the natural logarithm of mean discharge (m3/s), mean air temperature (°C), and longitudinal river position (river km). Our response variable was mean water temperature along our study reach (river km 145.6–134.7, Fig. 2). Both response and independent variables were calculated for the period of May 15–July 1 for 2015–2019. We chose this period to capture much of the interannual variation in seasonal river warming and encompass the time of active pikeminnow migration (Fig. 3A). The model included a random effect of year.
To parameterize the linear mixed-effects model, we measured stream temperature hourly from mid-May through mid-September at 7–18 sites along a 10-km study reach from 2015 to 2019 (Fig. 2, Table S1). Deeper pools in the South Fork Eel River thermally stratify each summer, so temperature sensors were placed in the river thalweg at the outflow of pools, where water was well mixed. Stream discharge in the South Fork Eel River was estimated using a discharge rating curve (Power et al. 2008) with river stage recorded in a US Geological Survey stilling well located at the southern end of the Angelo Coast Range Reserve (Branscomb South Fork Eel gage ID 11475500, now operated by UC Berkeley Angelo Reserve researchers, data available at http://angelo.berkeley.edu/data/meteorological-data/).
We then used the parameter estimates from the mixed-effects model (Table 2) to predict river temperatures for combinations of environmental conditions which we did not observe. We generated four scenarios for the period of May 15–July 1 that simulated combinations of two mean discharges (0.5 and 8 m3/s) and two mean air temperatures (16 and 20 °C). This period was chosen to capture the period when pikeminnow are migrating and there is high interannual variation in water temperature and discharge. We constrained our predicted river temperatures to the same reach where temperature was measured. In each of our four climate scenarios, we located the longitudinal position in the study reach where predicted mean water temperature (from our linear mixed-effects model) was equal to observed mean water temperature when pikeminnow arrived at Wilderness Pool (16.3 °C, Table 1, calculated from May 15 to the date of pikeminnow arrival for 2015–2019). We used this procedure to predict where pikeminnow would be on July 1st, given combinations of discharge and air temperature represented in each of the four scenarios.
We used R statistical software (R Development Core Team, 2018, version 3.14) and the Python programming language (https://www.python.org/) for all analyses and data management.
Results
Seasonal migration of Sacramento pikeminnow
Headwater snorkel surveys revealed Sacramento pikeminnow migrated upstream in the South Fork Eel River during all 5 years of the study period (2016–2020, Fig. 2). In late May in 2016, 2017, and 2019, pikeminnow were only detected in the most downstream large pool in our headwater surveys (Hunters Pool, river km 134.9) (mean density across years averaged over the 10.7 km survey reach = 0.33 individuals /km). In contrast, by early August, pikeminnow were detected further upstream, producing higher reach-scale densities (mean across years = 38.96 individuals /km), with substantial variation among years (Table 3). Our intensive sampling of Wilderness Pool revealed that pikeminnow arrival times differed between years by up to 49 days but generally occurred in late spring/early summer (Fig. 3A). Pikeminnow arrived earliest to this site in the warmest year, 2015, on June 6th, and latest in the coolest year, 2019 on August 4th. Arrival date (measured as Julian date) varied significantly with mean water temperature at this pool (Table 2, Fig. 3B). While arrival date varied across years, the temperature conditions the week before pikeminnow arrived in the pool were similar across years (Table 1). Mean water temperature for the week preceding arrival was 20 °C (± 0.75 °C SD), mean maximum weekly average temperature (MWAT) across years was 21.7 °C (± 0.6 °C SD). Mean accumulated degree-days above 15 °C from May 15th to the date of arrival across years was 36.0 (± 6.0 SD) and mean temperature over this time interval was 16.3 °C (± 0.78 °C SD). We used the mean temperature (16.3 °C) as the threshold value, representing the conditions in which pikeminnow arrive, in our scenario-based modeling. The extent of upstream movement from varied from river km 140.4 km in 2015 to 144.2 km in 2018.
Climate scenario modeling and predicting pikeminnow distribution
In our linear mixed-effects model, mean air temperature, river position, and the natural logarithm of mean discharge over the May 15–July 1 window significantly predicted mean river temperature during the same period. As expected, river temperatures increased with air temperatures, decreased with greater discharge, and increased downstream. Together, these three environmental predictors explained 96% of the observed variation in mean river temperature and were all significant or marginally significant (Table 2). When we used the parameter estimates to create four predictive scenarios, river temperatures were hottest when discharge was low (0.5 m3/s) and mean air temp was high (20 °C, Fig. 4A); coolest when discharge was high (8 m3/s) and air temperatures were cool (16 °C, Fig. 4D); and intermediate in low discharge—low temperature (Fig. 4B) and high discharge—high temperature (Fig. 4C) scenarios.
Gridded maps with river channel colored as predicted mean temperature from May 15 to July 1 in four climate scenarios. Q = mean discharge (m3/S) and Tair = mean air temperature (°C). Scenarios are combinations of two mean discharge conditions (0.5 and 8.0 m3/s, x-axis) and two mean air temperatures (16 and 20 °C, y-axis). Mean river temperatures are calculated from the parameter estimates of a linear mixed-effects model. Temperatures are predicted for the reach in which temperature was measured. The pikeminnow icon is placed at mean water temperature of 16.3 °C, which was the mean temperature from May 15 to the date of pikeminnow arrival at Wilderness pool from 2015 to 2019 and is an estimate of pikeminnows upstream distribution on July 1 in each scenario. In scenario A, the whole reach is above 16.3 °C, and in scenario D, the 16.3 °C temperature threshold is downstream our study reach
In our four scenarios, we identified the river position of the 16.3 °C May 15–July 1 mean temperature, our estimate of upstream extent of pikeminnow on July 1. This predicted threshold was farthest upstream when discharge was low (0.5 m3/s) and mean air temp was high (20 °C, Fig. 4A). In contrast, when discharge was high (8 m3/s) and air temperatures were cool (16 °C), river temperatures did not exceed 16.3 °C in the entire 10.8 km study reach. In this case, we predict that no pikeminnow would arrive in our study reach by July 1 (Fig. 4D). In both our low discharge—low temperature (Fig. 4B) and high discharge—high temperature (Fig. 4C), pikeminnow were predicted to reach intermediate positions (9.9 and 6.8 km upstream from Hunter’s Pool, respectively) by July 1.
Discussion
We show here that pikeminnow migrate in part of their introduced range. The seasonal migration begins in late spring when pikeminnow move from downstream wintering habitat upstream to the headwaters of the South Fork Eel River (Fig. 2). At our daily monitoring location in the seasonally occupied upstream portion of pikeminnow range (Wilderness Pool), pikeminnow arrived earlier in years with warmer spring water temperature from 2015 to 2019 (Fig. 3). Thermal conditions when pikeminnow arrived at this intensely monitored pool were similar across years (mean weekly temperature, maximum weekly average temperature, cumulative degree days, Table 1, Fig. 3A, B). This consistency suggests that monitoring temperature within a year can help predict the timing of pikeminnow migration. River temperatures in our study reach (and in general) are strongly influenced by discharge, air temperature and river position (Table 1, Fig. 4), which correlates with solar radiation input and drainage area (Bode et al. 2014). These relationships suggest that temperature models can help predict the phenology and spatial dynamics of pikeminnow migration and probably other life-history events of importance to conservation and management.
Water temperature is a good predictor of site-specific arrival times and spatial movements of pikeminnow within a year. Temperature could trigger pikeminnow migration directly, by affecting their physiology and performance, and indirectly, by influencing other organisms. Warming strongly influences spawning time (Webb and McLay 1996) and developmental rates in cyprinids (Herzig and Winkler 1986). Additionally, as summer progresses and river waters warm, headwaters become more physiologically favorable for pikeminnow (Vondracek 1987; Reese and Harvey 2002). However, temperature alone is not sufficient to predict pikeminnow migration and arrival. Using temperature only, we predicted that pikeminnow would migrate farthest upstream in a low discharge (0.5 m3/s), high mean air temperature (20 °C) year (Fig. 4A). The year 2015 (mean air temperature 18.9 °C, mean discharge 0.59 m 3/s) most closely approximated these conditions, and the whole study reach was above the 16.3 °C threshold, which we identified as thermally suitable for pikeminnow by July 1. However, in summer 2015, pikeminnow migration terminated at the most downstream location of any year of survey from 2015 to 2020. We hypothesize that at these extremely low flows, passage was impeded, which could have limited upstream movement despite suitable thermal conditions. In addition to forecasting temperature regimes under different discharge-air temperature scenarios, future spatially explicit models predicting pikeminnow migration should locate potential migration barriers limiting passage for these large predators and estimate low-flow thresholds at which they may become impassable. Water-penetrating LiDAR facilitates longitudinal bathymetric studies of rivers and is increasingly available (Mandlburger et al. 2020), including for the South Fork Eel River (Power 2013).
The adverse effects of pikeminnow on salmonids and other native fishes will likely worsen as climate warms. Unfortunately, as we found here, pikeminnows’ impacts under warmer conditions will be more prolonged, passage permitting, and more spatially extensive; earlier arrivals in upstream reaches will expand and lengthen pikeminnow co-occurrence with native species. In warmer conditions, these interactions will not only be prolonged but likely also more intense. Pikeminnow out-competed size-matched dominant steelhead at 20–23 °C but not at 15–18 °C (Reese and Harvey 2002). In addition, physiological studies of pikeminnow (lower gut passage and faster digestion rates at 20 °C versus 15, 10, or 5 °C (Vondracek 1987)) suggest their impacts as predators will intensify in warmer water. In addition to these competitive and predatory effects, pikeminnow also alter the behavior of potential prey. Adult coastal roach (Hesperoleucus venustus), juvenile Sacramento sucker (Catostomus occidentalis), and juvenile steelhead (O. mykiss), all potential pikeminnow prey, use pool habitats when pikeminnow are absent, but in the presence of pikeminnow, shift to riffles and shallower habitats that pikeminnow do not frequent during the day (Brown and Moyle 1991; Brown and Brasher 1995; Harvey and Nakamoto 1999). This could be particularly detrimental for over-summering salmonids that would, in the absence of pikeminnow, use deep stratified pools as thermal refuges during the warmest, most physiologically stressful periods of summer (Nielsen et al. 1994). Finally, Pikeminnow also impede salmonids from tracking growth opportunities through the interconnected mosaic of habitats created by asynchronous temperature regimes and productivity peaks in rivers. Warm water reaches provide seasonal growth opportunities for cool-water fishes (Armstrong et al. 2021, Rossi et al. 2021) that can move amongst these habitats (Armstrong et al. 2010). Earlier upstream pikeminnow arrivals could make traveling between habitat patches riskier and exclude access to some.
To reduce pikeminnow impacts on native salmonids, water withdrawal prescriptions should vary depending on environmental conditions during a given summer. In years with cool springs and high discharge, river temperatures should remain cool throughout the reach, despite some water withdrawals (Fig. 4D). In years with hot spring air temperatures and low discharge, any water withdrawals could increase temperatures to adverse thresholds. In this case, water withdrawals should be very limited, not only to reduce river temperature for salmonids but also to delay pikeminnow migration and make conditions less physiologically favorable for them (Fig. 4A). For intermediate scenarios (warm air temperatures and high discharge (Fig. 4B) or low air temperature and low discharge (Fig. 4C), we suggest limiting water withdrawals during the summer months for environmental flows. Withdrawing water during winter months and storing it for summer use could also reduce the potential for warming during the spring and summer, which accelerates the timing of pikeminnow migration, and subsequent onset of overlap with native fishes upstream. While our temperature model can predict discharge-temperature relationships for our study reach, additional temperature data from downstream locations would be needed to model a greater spatial extent, where parameter estimates would likely change. Additional parameters such as watershed lithology (Dralle et al. 2023), accounting for autocorrelation of explanatory variables at larger scales, and the influence of tributary confluences on mainstem temperatures would likely be needed to maintain model accuracy. Within our relatively limited spatial range, we show the feasibility of our approach for understanding how environmental drivers can predict temperature in a given year, and therefore, the timing of pikeminnow migration and arrival.
Fish migrate to access spawning sites, forage, or seek refuge from undesirable or dangerous conditions (Northcoat 1978). The upstream and downstream components of migrations can be motivated by different factors. For example, pikeperch (Sander lucioperca) in River Gudenaa, Denmark, migrate upstream in the spring to spawning locations, and downstream to sites where prey concentrate in the fall (Koed et al. 2005). Similarly, European roach (Rutilus rutilus) migrate from lakes to spawn in tributary streams. Then as flows increase, roach return to slower water refuge areas after spawning (Vøllestad and L'Abée-Lund 1987). The upstream component of pikeminnow migration is likely to spawn and/or forage. In addition to accessing potential spawning sites, foraging becomes more profitable in the headwaters as spring transitions to summer. Pikeminnow’s most numerous prey, coastal roach (SM Fig. S3), become denser upstream as inundated habitats contract during summer river recession and following roach reproduction. Additionally, as suggested by Harvey and Nakamoto (1999), pikeminnow moving into the upstream end of their range in spring are likely to encounter juvenile salmonids that have never encountered piscivorous pikeminnow before. These naive animals are likely easier to capture than out-migrating salmonids that have navigated reaches where pikeminnow are more abundant. With the onset of fall rains, pikeminnow may move downstream to seek refuge from high flows during winter (Harvey and Nakamoto 1999). The extent of pikeminnows’ downstream migration and overwintering locations are currently unknown. There are few winter observations of pikeminnow in the upper and middle reaches of the South Fork Eel River, despite searches during periods of good water clarity (Pers. obs. P. Georgakakos and Pers. comm., Chris Loomis, California Department of Fish and Wildlife Biologist). In 2021, as flows allowed, we surveyed five pools downstream of our main study reach during early spring to determine the extent of downstream travel and timing of upstream migration and found no fish larger than 30 cm upstream of Piercy, Mendocino Co., CA until May (Table S4). This suggests that most pikeminnow larger than 40 cm overwinter downstream of Piercy (Fig. 2A). However, migration distance might vary between years, all individuals do not appear to migrate, and there appears to be size structured movement dynamics. Further work tagging individuals would help address these outstanding questions and clarify what is motivating movement.
Like Sacramento pikeminnow in the Eel River, other invasive species’ phenologies are tied to seasonal environmental regimes in their non-native environments. Understanding how temperature, moisture, flow velocities, or other seasonal factors control behavior, movement patterns, and the timing of life history events in other invasive species will help us anticipate and manage their impacts on native species and ecosystems under global change. For example, cane toads (Rhinella marina) are more active and move greater distances in wet seasons, when they will likely have greater impacts but possibly would be easier to trap (Brown et al. 2011). Invasive fire ants (Linepithema humile) are less active in winter months when they retreat from satellite nests and concentrate in fewer larger nest sites, where they may become more vulnerable to control (Heller and Gordon 2006). Understanding how non-native organisms react to environmental controls over the timing of major life history events will help us manage these organisms and reduce their impacts on native organisms. In our system, and perhaps in others, these controls over spatial distributions, movements, and timing of life history events operate over annual time scales. Therefore, they can inform predictions and management choices that can adapt to the inter-annual variation in climate parameters predicted and already manifest in our wetter, hotter, or drier years ahead.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Armstrong JB, Schindler DE, Omori KL, Ruff CP, Quinn TP (2010) Thermal heterogeneity mediates the effects of pulsed subsidies across a landscape. Ecology 91:1445–1454. https://doi.org/10.1139/cjfas-2015-0420
Armstrong JB, Fullerton AH, Jordan CE, Ebersole JL, Bellmore JR, Arismendi I, Penaluna BE, Reeves GH (2021) The importance of warm habitat to the growth regime of cold-water fishes. Nat Clim Chang 11:354–361. https://doi.org/10.1038/s41558-021-00994-y
Bode CA, Limm MP, Power ME, Finlay JC (2014) Subcanopy Solar Radiation model: Predicting solar radiation across a heavily vegetated landscape using LiDAR and GIS solar radiation models. Remote Sens Environ 154:387–397. https://doi.org/10.1016/j.rse.2014.01.028
Brodersen JA, Nicolle A, Nilsson PA, Skov C, Brönmark C, Hansson LA (2011) Interplay between temperature, fish partial migration and trophic dynamics. Oikos 120:1838–1846. https://doi.org/10.1111/j.1600-0706.2011.19433.x
Brown GP, Kelehear C, Shine R (2011) Effects of seasonal aridity on the ecology and behaviour of invasive cane toads in the Australian wet-dry tropics. Funct Ecol 25:1339–1347. https://doi.org/10.1111/j.1365-2435.2011.01888.x
Brown LR, Brasher AM (1995) Effect of predation by Sacramento squawfish (Ptychocheilus grandis) on habitat choice of California roach (Lavinia symmetricus) and rainbow trout (Oncorhynchus mykiss) in artificial streams. Can J Fish Aquat Sci 52:1639–1646. https://doi.org/10.1139/f95-758
Brown LR, Moyle PB (1991) Changes in habitat and microhabitat partitioning within an assemblage of stream fishes in response to predation by Sacramento squawfish (Ptychocheilus grandis). Can J Fish Aquat Sci 48:849–856. https://doi.org/10.1139/f91-101
Campbell IC, Poole C, Giesen W, Valbo-Jorgensen J (2006) Species diversity and ecology of Tonle Sap Great Lake, Cambodia. Aquat Sci 68:355–373. https://doi.org/10.1007/s00027-006-0855-0
Correa SB, Winemiller KO, Lopez-Fernandez H, Galetti M (2007) Evolutionary perspectives on seed consumption and dispersal by fishes. Bioscience 57:748–756. https://doi.org/10.1641/B570907
Cucherousset J, Olden JD (2011) Ecological impacts of nonnative freshwater fishes. Fisheries 36:215–230. https://doi.org/10.1080/03632415.2011.574578
Dingle H (2014) Migration: The biology of life on the move, 2nd edn. University of Oxford Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199640386.001.0001
Dralle DN, Ross G, Georgakakos P, Hahm WJ, Rempe DM, Blanchard M, Power ME, Dietrcich WE, Carlson SM (2023) The salmonid and the subsurface: hillslope storage capacity determines the quality and distribution of fish habitat. Ecosphere 14:e4436. https://doi.org/10.1002/ecs2.4436
Gadomski DM, Barfoot CA, Bayer JM, Poe TP (2001) Early life history of the northern pikeminnow in the lower Columbia River basin. Trans Am Fish Soc 130:250–262. https://doi.org/10.1577/1548-8659(2001)130%3c0250:ELHOTN%3e2.0.CO;2
Georgakakos PB (2020) Impacts of native and introduced species on native vertebrates in a salmon-bearing river under contrasting thermal and hydrologic regimes. PhD Dissertation, University of California, Berkeley. https://escholarship.org/uc/item/0wf2q0pk
Goulden ML, Bales RC (2019) California forest die-off linked to multi-year deep soil drying in 2012–2015 drought. Nat Geosci 12:632–637. https://doi.org/10.1038/s41561-019-0388-5
Hahm WJ, Dralle DN, Rempe DM, Bryk AB, Thompson SE, Dawson TE, Dietrich WE (2019) Low subsurface water storage capacity relative to annual rainfall decouples mediterranean plant productivity and water use from rainfall variability. Geophys Res Lett 46:6544–6553. https://doi.org/10.1029/2019GL083294
Harvey BC, Nakamoto RJ (1999) Diel and seasonal movements by adult Sacramento pikeminnow (Ptychocheilus grandis) in the Eel River, northwestern California. Ecol Freshw Fish 8:209–215. https://doi.org/10.1111/j.1600-0633.1999.tb00072.x
Hawkins CP, Kershner JL, Bisson PA, Bryant MD, Decker LM, Gregory SV, McCullough DA, Overton CK, Reeves GH, Steedman RJ, Young MK (1993) A hierarchical approach to classifying stream habitat features. Fisheries 18:3–12
Heller NE, Gordon DM (2006) Seasonal spatial dynamics and causes of nest movement in colonies of the invasive Argentine ant (Linepithema humile). Ecol Ent 31:499–510. https://doi.org/10.1111/j.1365-2311.2006.00806.x
Herzig A, Winkler H (1986) The influence of temperature on the embryonic development of three cyprinid fishes, Abramis brama Chalcalburnus chalcoides mento and Vimba vimba. J Fish Biol 28:171–181. https://doi.org/10.1111/j.1095-8649.1986.tb05155.x
Hughes NF, Reynolds JB (1994) Why do arctic grayling (Thymallus arcticus) get bigger as you go upstream. Can J Fish Aquat Sci 51:2154–2163. https://doi.org/10.1139/f94-21
Jackson RB, Carpenter SR, Dahm CN, McKnight DM, Naiman RJ, Postel SL, Running SW (2001) Water in a changing world. Ecol App 11:1027–1045. https://doi.org/10.2307/3061010
Kinziger AP, Nakamoto RJ, Harvey BC (2013) Local-scale invasion pathways and small founder numbers in introduced Sacramento pikeminnow (Ptychocheilus grandis). Conserv Genet 15:1–9. https://doi.org/10.1007/s10592-013-0516-5
Koed A, Mejlhede P, Balleby K, Aarestrup K (2005) Annual movement and migration of adult pikeperch in a lowland river. J Fish Biol 57:1266–1279. https://doi.org/10.1111/j.1095-8649.2000.tb00486.x
Lawrence DJ, Stewart-Koster B, Olden JD, Ruesch AS, Torgersen CE, Lawler JJ, Butcher DP, Crown JK (2014) The interactive effects of climate change, riparian management, and a nonnative predator on stream-rearing salmon. Ecol Appl 24:895–912. https://doi.org/10.1890/13-0753.1
Lucas MC, Batley E (1996) Seasonal movements and behaviour of adult barbel Barbus barbus, a riverine cyprinid fish: implications for river management. J App Ecol 33:1345
Lucas MC, Baras E, Thom TJ, Duncan A, Slavík O (2001) Migration of freshwater fishes. Blackwell, Oxford. https://doi.org/10.1002/9780470999653
Mandlburger G, Pfennigbauer M, Schwarz R, Flöry S, Nussbaumer L (2020) Concept and performance evaluation of a novel UAV-borne topo-bathymetric LiDAR sensor. Remote Sens 12:986. https://doi.org/10.3390/rs12060986
Milner-Gulland EJ, Fryxell JM, Sinclair AR (eds) (2011) Animal migration: a synthesis. OUP Oxford. https://doi.org/10.1002/jwmg.839
Moyle PB (2002) Inland fishes of California; revised and expanded. University of California Press
Nakamoto RJ, Harvey BC (2003) Spatial, seasonal, and size-dependent variation in the diet of Sacramento pikeminnow in the Eel River, northwestern California. Calif Fish Game 89:30–45
Nielsen JL, Lisle TE, Ozaki V (1994) Thermally stratified pools and their use by steelhead in northern California streams. Trans Am Fish Soc 123:613–626. https://doi.org/10.1577/1548-8659(1994)123%3c0613:tspatu%3e2.3.co;2
Northcote TG (1978) Migratory strategies and production in freshwater fishes. In: Gerking SD (ed) Ecology of freshwater fish production. Blackwell, Oxford, pp 326–359
Ogutu-Ohwayo R (1990) The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environ Biol Fish 27:81–96. https://doi.org/10.1007/BF00001938
Paine RT, Tegner MJ, Johnson EA (1998) Compounded perturbations yield ecological surprises. Ecosystems 1:535–545. https://doi.org/10.1007/s100219900049
Pierce DW, Kalansky JF, Cayan DR (2018) Climate, drought, and sea level rise scenarios for California's fourth climate change assessment. California’s Fourth Climate Change Assessment, California Energy Commission. Publication Number: CNRA-CEC-2018–006.
Power ME (2013) South Fork Eel River, CA Watershed morphology. Distributed by Open Topography. https://doi.org/10.5069/G9639MPN. Accessed: 2023–07–26
Power ME, Parker MS, Dietrich WE (2008) Seasonal reassembly of a river food web: floods, droughts, and impacts of fish. Ecol Monogr 78:263–282. https://doi.org/10.1890/06-0902.1
Quinn TP, Hodgson S, Peven C (1997) Temperature, flow, and the migration of adult sockeye salmon (Oncorhynchus nerka) in the Columbia River. Can J Fish Aquat Sci 54:1349–1360. https://doi.org/10.1139/f97-038
Quinn TP (2018) The behavior and ecology of pacific salmon and trout, 2nd edn. University of Washington Press, Seattle
Rahel FJ, Olden JD (2008) Assessing the effects of climate change on aquatic invasive species. Conserv Biol 22:521–533. https://doi.org/10.1111/j.1523-1739.2008.00950.x
Reese CD, Harvey BC (2002) Temperature-dependent interactions between juvenile steelhead and Sacramento pikeminnow in laboratory streams. Trans Am Fish Soc 131:599–606. https://doi.org/10.1577/1548-8659(2002)131%3c0599:TDIBJS%3e2.0.CO;2
Rossi GJ, Power ME, Pneh S, Neuswanger JR, Caldwell TJ (2021) Foraging modes and movements of Oncorhynchus mykiss as flow and invertebrate drift recede in a California stream. Can J Fish Aquat Sci 78:1045–1056. https://doi.org/10.1139/cjfas-2020-0398
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174. https://doi.org/10.1111/j.1365-2427.2009.02380.x
Sykes GE, Johnson CJ, Shrimpton JM (2009) Temperature and flow effects on migration timing of Chinook salmon smolts. Trans Am Fish Soc 138:1252–1265. https://doi.org/10.1577/T08-180.1
Taft AC, Murphy GI (1950) The life history of the Sacramento squawfish (Ptychocheilus grandis). Calif Fish and Game 36:147–164
Tyus HM, McAda CW (1984) Migration, movements and habitat preferences of Colorado squawfish, Ptychocheilus lucius, in the Green, White and Yampa rivers, Colorado and Utah. J Anim Ecol 29:289–299. https://doi.org/10.2307/3671360
Valentine DA, Young MJ, Feyrer F (2020) Sacramento pikeminnow migration record. J Fish Anim Manag 11:588–592. https://doi.org/10.3996/JFWM-20-038
Vondracek B (1987) Digestion rates and gastric evacuation times in relation to temperature of the Sacramento squawfish, Ptychocheilus grandis. Fish Bull 85:159–163
Vøllestad LA, L’Abée-Lund JH (1987) Reproductive biology of stream-spawning roach, Rutilus rutilus. Environ Biol Fish 18:219–227. https://doi.org/10.1007/BF00000361
Webb JH, McLay HA (1996) Variation in the time of spawning of Atlantic salmon (Salmo salar) and its relationship to temperature in the Aberdeenshire Dee, Scotland. Can J Fish Aquat Sci 53:2739–2744. https://doi.org/10.1139/f96-240
Zaret TM, Paine RT (1973) Species introduction in a tropical lake: a newly introduced piscivore can produce population changes in a wide range of trophic levels. Science 182:449–455. https://doi.org/10.1126/science.182.4111.449
Acknowledgements
We thank scientists of the Eel River Critical Zone Observatory for discussion and feedback during all stages of this project. This manuscript received thoughtful and helpful reviews from Julie Gonzalez, Wayne Sousa, Stephanie Carlson, and two anonymous reviewers. We also thank Peter Steel for logistical support at the Angelo Reserve.
Funding
This project was funded through the National Science Foundation CZP EAR-1331940, agreement no. Q1996052 from the California Department of Fish and Wildlife, the Carol Baird Graduate Student Award for Field Research, the UC Natural Reserve System’s Mildred E. Mathias Award, and through the Institute for the Study of Ecological Effects of Climate Impacts of the University of California Natural Reserve System.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Fieldwork and data collection were conducted by Philip Georgakakos. Data manipulation and analyses were performed by David Dralle and Philip Georgakakos with feedback from Mary Power. The first draft of the manuscript was written by Philip Georgakakos, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because this study was entirely observational, and no animals were handled.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Georgakakos, P.B., Dralle, D.N. & Power, M.E. Spring temperature predicts upstream migration timing of invasive Sacramento pikeminnow within its introduced range. Environ Biol Fish 106, 2069–2082 (2023). https://doi.org/10.1007/s10641-023-01486-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01486-y