Abstract
We tracked locations of three fish species in two bays with differing hydrology in SW Florida in 2018–2020 to test the hypotheses about fish residency, movements, and environmental variables. Due to extensive watershed modification, one bay receives less freshwater and the other receives more relative to natural conditions. Home range duration differed for gray snapper (54 ± 6 days), red drum (132 ± 39), and goliath grouper (226 ± 63). Distances between relocation movements were similar for gray snapper and red drum (~ 1.2 km), but farther for goliath grouper (2.3 ± 0.3 km). Relocations were primarily seaward for gray snapper (83%) but varied for the other species. Home range duration related to age for goliath grouper (< 100 days for 1–1.5-year-olds, 300–425 days for 4–4.5-year-olds). Generalized additive models marginally related probability of gray snapper relocating to salinity and temperature whereas relocations of the other species occurred during all environmental conditions. Movement simulations lacking environmental cues produced similar emigration patterns as observed in tagged fish. Overall, results suggest that movements here are not strongly linked to environmental conditions, will be resilient to watershed restoration that should moderate salinity, and have implications for understanding the impacts of localized depletion due to recreational fishing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding when and why fish move is an essential aspect of defining their ecosystem roles and environmental requirements. This is especially important for juvenile and estuarine life stages due to their vulnerability to coastal stressors such as pollution, watershed impacts, and habitat loss (Beck et al., 2001; Gillanders & Kingsford, 2002; Adams et al., 2006). Beyond simply knowing that juvenile and young–adult fish use estuaries for their home range, more detailed information is needed regarding movement patterns within these nursery habitats. For example, how long are home ranges occupied? How often, in what direction (e.g., inshore, offshore), and over what distances do they shift home ranges within their juvenile and young–adult habitats? When maturing fish emigrate from estuaries, do they prefer particular routes or corridors? This information is critical for informing spatial (e.g., protected areas, migration corridors) and temporal (e.g., seasonal fishing restrictions, freshwater releases) management strategies for fisheries (Brownscombe et al., 2022).
Equally as important as quantifying home range parameters, is identifying relationships between the timing of movements and environmental cues such as temperature, salinity, or other factors (Adams & Tremain, 2000; Livernois et al., 2021). For example, estuarine species like juvenile gray snapper, Lutjanus griseus (Linnaeus, 1758), have been observed to move to intermediate salinities in choice experiments (Serrano et al., 2010). Juvenile goliath grouper, Epinephelus itajara (Lichtenstein, 1822), have been trapped more commonly at intermediate temperatures (24–31°C) and salinities (10–30 ppt) (Shideler et al., 2015). Juvenile red drum, Sciaenops ocellatus (Linnaeus, 1766), in some estuaries are more likely to move when temperatures drop below 14°C and salinity is decreasing (Dance & Rooker, 2015). Of note, however, temperature, salinity, and other environmental variables are subject to unprecedented change in many areas due to coastal and watershed modification (Gillanders & Kingsford, 2002; Adams et al., 2009; Michot et al., 2017), or more broadly due to sea level rise and climate change (Erwin, 2009; Krauss et al., 2011; Lammers et al., 2013).
In other cases, links between juvenile and young–adult fish movements and the environment have proven more challenging to identify, and movements have been attributed to a variety of other processes such as a shift in dietary requirements (Livernois et al., 2021) or attaining a certain minimum size associated with maturity or spawning (Welsh et al., 2013; Huijbers et al., 2015; Daly et al., 2021). In fact, some researchers have suggested that observed movement patterns may be due merely to random processes or at least are caused by such a diversity of cues that they appear random. Some studies have compared observed movements to those created by random-movement simulations to determine if the observed patterns could have arisen by random processes alone (Crook, 2004; Sims et al., 2006; Börger et al., 2008; Cramer et al., 2021). Collectively, these studies suggest that it is important to not only examine the timing of fish movements in the context of potential environmental cues, but also compare those patterns to random or simulated movements to determine if observed patterns could have arisen in the absence of the environmental drivers.
The Ten Thousand Islands (TTI) area of southwest Florida, USA, offers a natural experimental setting to investigate home range shifts and potential environmental cues for fish movement. The region includes multiple bays with differing hydrologic characteristics. Specifically, due to watershed manipulations in the 1960s, each bay receives significantly more or less freshwater relative to natural conditions (Booth & Knight, 2021). As a result, otherwise similar bays but that experience very different levels of freshwater input can be used to test hypotheses about environmental influences on fish movements (Adams et al., 2009; Kendall et al., 2022; Williams et al., 2023). Understanding the relationships between fish movements and water flow in this region is timely, given that a major watershed restoration currently underway (i.e., the Comprehensive Everglades Restoration Plan) is expected to result in more natural flow regimes and parity of salinity between these bays (US Army Corp of Engineers, 2004).
Three fish species were the focus of this study due to their prevalence in mangrove estuaries as juveniles, importance to recreational fishing, roles in the estuarine ecosystem, or conservation status. Gray snapper, red drum, and goliath grouper are all known to rely on estuarine habitats through the juvenile and young–adult life stages prior to moving offshore to occupy adult and spawning habitats (Luo et al., 2009; Koenig et al., 2011). In estuaries, all three species feed on small fish and crustaceans either along the mangrove fringe or in adjacent soft-bottom (Thayer et al., 1987; Sadovy & Eklund, 1999; Frias-Torres, 2006). Gray snapper and red drum have long been popular targets for recreational anglers (Kinch & O’Harra, 1976), whereas goliath grouper harvest had been prohibited since 1990 until limited take was opened in 2023 in Florida (Bertoncini et al., 2018; McCawley, 2022). A limited-take goliath grouper harvest was authorized by Florida Fish and Wildlife Conservation Commissioners beginning in 2023 that will focus on large juveniles (McCawley, 2022). Understanding the movements and residency patterns of goliath grouper will be important for determining the impact of this harvest.
General habitat preferences have been studied for these three species as well as aspects of fine scale movements such as diel habitat shifts (Luo et al., 2009) and incidence of migration to offshore habitat (Koenig et al., 2011). In this study, we focus on an intermediate and unstudied scale of movement: shifts among home range areas within estuaries during the juvenile and young–adult life stage. Although all three species utilize mangrove fringe, channels, or flats as juvenile habitat, their site fidelity, shifts in home range location, and routes and timing of their emigration from juvenile and young–adult habitats in relation to environmental conditions such as salinity and temperature are not well described. To address this knowledge gap, we implanted acoustic transmitters into fish to track their movements within two estuarine bays in southwest Florida with differing hydrologic characteristics. Our objectives were to evaluate the following: (1) home ranges including the duration, direction, and distance of home range shifts, and if they differed between the study bays, (2) relationships between dates of fish movement and environmental variables or other potential cues for movement such as fish age, (3) if emigration time-of-day and routes are random or occur through specific corridors, (4) if observed emigration rates could be due to random movements of fish, and (5) if changes in flow from watershed restoration are expected to influence future occupancy patterns.
Methods
Study area
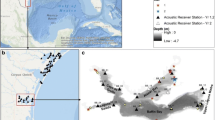
The study area consisted of two estuarine bays in southwest Florida: Pumpkin Bay and Faka Union Bay (Fig. 1a). These two bays are similar in area (~ 250–300 ha), average depth (~ 1 m overall with ~ 7 m deep channels), distance to the ocean (~ 6 km), tidal range (~ 1 m), substrate (sand, mud, shell hash occupied by patchy algae, sponges, tunicates, and oyster bars), and perimeter to area ratio (Shirley et al., 2004; Kendall et al., 2022). Both bays are lined with a continuous fringe of red mangroves, Rhizophora mangle. Under natural conditions before human alteration of the watersheds, these bays likely received similar amounts of freshwater primarily through small tidal rivers as a single point of watershed discharge (Booth & Knight, 2021).
a–c The southwest Florida study area including the following: (a) the position of each bay and the main water flow control structures in the watersheds, (b) the telemetry array in Pumpkin Bay labeled with receiver numbers that had detections from Fig. 2, and (c) the telemetry array in Faka Union Bay. Dotted black lines on (b, c) are the fish tagging areas near the river/canal mouths and the bay centers
For many decades, however, water control structures in the watersheds feeding into these bays have resulted in contrasting quantities and timing of freshwater flow to the estuaries relative to natural conditions (Booth & Knight, 2021). In Faka Union Bay, a massive canal network installed as part of a failed suburban development in the 1960s extends far up into the watershed. The Faka Union Canal Complex (FUCC) drained > 58,000 ha of the Big Cypress Basin until year 2022 when canals were plugged as part of the Picayune Strand Restoration Program, a component of the Comprehensive Everglades Restoration Plan (CERP) (U.S. Army Corp of Engineers, 2004). Throughout this study, however, these canals quickly drained large volumes of seasonal rainwater into the estuary via the Faka Union Canal/River instead of allowing its slower passage through natural sheet flow. In Pumpkin Bay, the opposite problem exists. Canals from the FUCC, agricultural development, and Highway US41 has resulted in much less fresh water reaching the estuary from a smaller watershed (< 5,000 ha) than occurred pre-development. This has resulted in an overall much more saline environment in Pumpkin Bay. In fact, freshwater flow to Pumpkin is only ~ 1% of the volume compared to Faka Union (Booth & Knight, 2021). This dichotomy in altered flow regimes provides an ecosystem-scale opportunity to investigate the effects of altered freshwater flow on movements of estuarine fish in two bays that are otherwise physically similar.
Rainfall is highly seasonal in the study area. Beginning in May or June, almost daily rainfall occurs (Misra et al., 2017) resulting in a surge of watershed runoff that marks the onset of the wet season (defined as June–Nov) and suddenly lower salinity in the estuaries (Kendall et al., 2022; NOAA NERRS, 2022). Salinity drops from 35 ppt in both bays to 25 ppt in Pumpkin Bay and 15 ppt in Faka Union over a span of just a few days then declines more gradually as the wet season progresses. This daily rainfall typically ends abruptly in October, although drainage from accumulated rain in the watersheds continues for some weeks. Winter and spring months are the dry season (Dec–May) and are characterized by cooler water temperatures (20°C ± 5), much less rainfall, and higher, more stable salinity in both bays (30–35 ppt).
Telemetry array design and evaluation
From March 2018 to September 2020, acoustic receivers (Innovasea Inc., model VR2W) were strategically placed to track fish movement throughout the bays, into rivers, and their emigration pathways (n = 19 receivers for Pumpkin Bay, n = 22 for Faka Union Bay) (Fig. 1). Receivers were spaced approximately evenly (~ 500 m apart) along the mangrove fringe or in the sand/mud flat areas in the middle of the bays. Receivers for monitoring upstream movements were placed as far north as possible with the navigational constraints in Pumpkin River and Faka Union River/Canal. Receivers for monitoring emigration from the bays were placed just outside them in the narrow channels and passes leading offshore or to adjacent bays. There are three passes to exit Pumpkin Bay; a main pass in the southeastern part of the bay, a narrow channel along the western shore, and a small cut on the southwest side of the bay (Fig. 1b). Faka Union Bay has more exit options including the main channel, a narrow pass to the southeast, one in the southern part of the bay, and another two along the fringing mangroves to the west (Fig. 1c).
Detection range was evaluated by repeated deployments of a range test tag which had the same specifications as the transmitters used in the fish but a 10-s ping interval. The range test tag was deployed for a minimum of 15 min at multiple distances from the receivers at 100 locations throughout the bays during high tide ± 2 h. Range was defined as the distance at which 50% of the expected pings were detected based on a binomial generalized linear model with a logit link function.
Fish tagging
The three target species were caught using hook and line or fish traps in March 2018, October–December 2018, and April 2019. Capture locations included areas near the river/canal mouth as well as more centrally in each bay (Fig. 1b, c). Coded acoustic transmitters (Innovasea Inc., model V8-4L with random ping interval of 130–230 s and 324 or 376-day battery life [depending on the manufacturing year], or model V9 with 130–230-s ping interval and 487-day battery life) were implanted into the body cavity of fish > 20 cm total length (TL) (Reese Robillard et al., 2015) and then released at the point of capture. Tag weight never exceeded 1% of the body weight of fish at the time of tagging. Unlike smaller fish, goliath groupers over 60 cm TL were tagged with larger transmitters which had longer battery life (Innovasea Inc., model V16 with 90–160-s ping interval and 2435-day battery life) and could track multiyear movements of those fish for a separate study.
Fish were categorized as juvenile or adult at the time of tagging based on published length at maturity. Maximum size thresholds for juveniles were 21 cm TL for gray snapper (Starck, 1971), 53 cm TL for red drum (Murphy & Taylor, 1990), and 115 cm TL for goliath grouper (Bullock et al., 1992). Sexes are separate for all three species but are not distinguishable based on external characteristics. Size at maturity is larger for females in red drum and goliath grouper; however, the minimum size for male adults for these two species was never exceeded.
Environmental data
Temperature and salinity were recorded every 15 min at automated monitoring stations located near the river/canal mouth leading into each bay (Fig. 1) (NOAA NERRS, 2022). Daily mean values were plotted for salinity and temperature during the same time period that fish were tracked to compare fish movement events to environmental values. In addition to daily salinity and temperature, it was also of interest to determine if fish movements were related to changes in those variables. For this, the change in temperature and salinity was calculated relative to 7 days earlier. This smoothed out small environmental fluctuations that occur on a daily or sub-weekly basis, while preserving the direction and magnitude of the more pronounced seasonal changes.
Summarizing fish movements
Array effectiveness
To demonstrate that the array was effective at monitoring fish in this complex landscape of mangrove islands and channels, we calculated an array effectiveness index (AEI) as the percentage of days that each fish was detected from their tagging date to the date when they were last detected. We also calculated the percentage of estimated battery life remaining for each tag type at the time of last detection for each fish to determine if they had likely emigrated or their transmitter batteries had died while residing within the array.
Duration of home range stays and dates of shifts
Home range was defined as a period of consistent detections on one or more adjacent receivers lasting weeks or months. A change in home range was defined as a switch in the receiver or receivers where a fish was detected that lasted more than a few days. Home ranges were therefore defined by timing of location shifts (Crook, 2004; Börger et al., 2008) rather than tag battery life or other array parameters as is the case in many telemetry studies. Often these home range shifts were marked by an abrupt cessation of detections on one set of receivers and an immediate new pattern of detections at a different set (Fig. 2). The date of the shift was recorded for each fish, and when possible, the duration (number of days) when the fish was detected at a given home range was also calculated.
Example abacus plot of detections for gray snapper ID87 from Pumpkin Bay by receiver number (see Fig. 1) and date of detections. Symbols denote daytime (+), dawn or dusk (X), and nighttime (●) detections. Arrows indicate: (1) a home range shift from occupancy around the river mouth at receiver 6 to the western side of the bay, (2) a second shift to a home range farther south excluding receiver 7, and (3) an exit detection during the day in the small channel on the western side of the bay
It is important to note that the home range relocations reported here are all within the same habitat type (e.g., Crook, 2004) since the entire study domain is composed of mangrove lined bays and channels. These home range shifts, while not temporary, are not equivalent to the more typically described ontogenetic shifts by major life stage from one habitat type to another (e.g., mangrove bays to offshore reefs) (Sadovy & Eklund, 1999; Luo et al., 2009; Koenig et al., 2011; Huijbers et al., 2015; Kendall et al. 2021).
Home range sizes or edges were not calculated for two reasons. First, because many home ranges consisted of detections on just one receiver, it was not known how much of the receiver’s detection range the fish may have been utilizing. It was not assumed that the fish were using the entire detection area of a receiver, as is done in some studies, nor did we assume that fish couldn’t be using some adjacent but undetectable space in between receivers. In this sense, we are assuming only that the fish is using a part of its home range. Second, other studies suggest that at least for goliath grouper and gray snapper, individuals are strongly associated with only the linear fringe of mangroves, making typical home range area estimation inappropriate (Thayer et al., 1987; Frias-Torres, 2006; Koenig et al., 2011).
Direction and distance of home range shifts
To determine if fish moved randomly within bays or moved more consistently in a specific direction (e.g., offshore), for each home range shift, we determined the direction (‘landward’ or ‘seaward’) relative to the mouth of the primary river/canal leading into each bay as well as the heading (in degrees) of the shift. Occasionally, a fish clearly shifted its home range since detections ceased on one set of receivers, but moved to a deaf spot in our array (i.e., no detections until they shifted again within range of a new receiver), and it was not possible to determine the direction in such cases. When possible, we also measured the distance of home range shifts using a geographic information system of receiver coordinates.
Direction and distance of emigration from bays
The date of departure or emigration from the study bays was also noted. Emigration dates were defined as when a fish’s detections ceased within the entire array before the end of expected battery life of transmitters. In many cases, the actual exit track was discernible on one or more receivers such that it was possible to identify if the main channel or a smaller exit pass was utilized. This was often shown as a few detections on a string of receivers along the exit route until the fish’s disappearance on a receiver located just outside the bays (e.g., Fig. 2). Time of day that fish emigrated from the study bays was categorized as occurring during one of four time periods: dawn (05:00 to 07:00), day (07:00 to 17:00), dusk (17:00 to 19:00), or night (19:00 to 05:00 the following morning).
Statistical analyses
Three groups of analyses were performed to quantify patterns in the fish movement data. These included the following: (1) significance tests to determine if basic movement parameters (e.g., home range duration, route of emigration) differed between the bays or with fish age, (2) a generalized additive model (GAM) to relate the likelihood of fish movement to environmental and other predictor variables, and (3) a random-movement simulation to determine if observed patterns of fish emigration from the bays could be reproduced independent of environmental variables. For this analysis, each home range shift or emigration event was considered an independent observation. All analyses were performed separately for each species.
Comparison of movement parameters between bays
For continuous variables (i.e., duration of home range stays, distance of home range shifts), we used a linear model to determine if mean values differed significantly between bays and across fish ages, for each species. We used a t-test to determine if AEI values differed between bays. In cases where no difference was found between bays, a mean value among all fish of the same species was reported. For categorical variables (i.e., landward vs. seaward direction of home range shifts, and main channel vs. small pass in mangroves as emigration routes), we used a heterogeneity chi-square analysis (Zar, 1999) to determine if there was a difference in shift direction or emigration route compared to random. For example, if fishes were relocating their home range randomly, it would be expected that landward and seaward shifts would occur in roughly equal proportions. If there was no difference in bays, data were pooled and a chi-square was performed with Yates correction for continuity (Zar, 1999) to test the null hypothesis that the direction of home range shifts or emigration route was random (i.e., 50:50, landward:seaward). If the samples could not be pooled, chi-square analyses with the Yates correction were performed separately on each bay.
The time of day that gray snapper emigrated was evaluated similarly. Based on the length of the time-of-day categories, if fish were emigrating at random times of the day, the expected ratio of dawn:day:dusk:night departures would be 1:5:1:5. Preliminary analysis revealed that both bays and years had similar patterns; therefore, all data were combined for analysis. We used a chi-square test (Zar, 1999) to determine if the observed ratio of emigration times differed from that expected if they occurred at random. Only those fish with a well-defined exit path along multiple receivers that could be assigned to one of the time-of-day categories were used for this analysis. The other two species lacked sufficient emigration data to evaluate the time of day.
Potential influences on timing of home range shifts and emigration
To understand the relationship between the probability of a shift (either home range or emigration) and potential explanatory variables, we used a binomial GAM with presence/absence of a movement on any given day during the study as the response variable. Potential explanatory variables included environmental factors such as bay, mean salinity and temperature on the day of fish movements, and the 7-day change in salinity and temperature prior to each day of the tracking period. We also considered temporal variables including month to account for possible seasonal or photoperiod cues to movement, and fish age to account for differences in movement based on fish size and maturity. GAMs were fit with all possible combinations of explanatory variables using the mgcv package (Wood, 2017) in R Version 3.6.1 (R Core Team, 2019) which yielded 128 possible combinations. Thin plate regression splines were used to describe the potentially non-linear relationships between the probability of a fish movement, mean salinity and temperature, and temperature and salinity change. Cyclic cubic regression splines were used to describe the relationship with month because they best represent seasonal cycles. Individual fish ID was included in all models as a random effect to account for repeated measures on an individual fish. Akaike’s Information Criterion (AIC) scoring was used to determine which combination of variables best explained patterns in fish movements wherein models within 2 of the model with the lowest AIC score were all considered equally suitable (Burnham & Anderson, 2002). Once the top models were identified, the relationship between probability of a fish movement and each important explanatory variable in isolation was visualized in effects plots. For these, the probability of fish movement was expressed relative to the range of values for each important explanatory variable individually, while all other continuous variables were held at their mean level, with month fixed as June and Bay as Faka Union since they had the most movements. Lastly, we reported the percent deviance explained by the best model, which quantifies the ability of the explanatory variables to describe the presence/absence of a fish movement.
Random-movement simulation
To determine if the observed timing of emigration for gray snapper could have arisen in the absence of environmental cues, we conducted a random-movement simulation and then compared the observed emigration curve to the simulated one (Crook, 2004; Börger et al., 2008; Cramer et al., 2021). The emigration curve was a plot of the percentage of gray snapper remaining in each bay from the time when all fish were tagged (100%) to the time at which the last fish departed (0%). In the simulation, virtual fish started at the center of each of the fishing areas depicted in Fig. 1b and c. The number of fish in each bay and fishing area matched the number that was actually tagged and released there in each year. Fish were then moved within the landscape following the home range shift pattern observed for real fish. Three key parameters controlled movements in the simulation: home range duration, movement direction, and movement distance. During the simulation, parameter values were drawn from ‘random’ distributions that were independent of time and environmental conditions. Parameter distributions were chosen in an attempt to replicate the observed emigration curve while maintaining realistic values that fell within the range of observed values. Home range duration was assumed to be normally distributed with a mean of 60 ± 10 (SD) days. Direction of a home range shift was also assumed to be normally distributed with a mean of 200 ± 40° (azimuth). Movement distance was assumed to follow a (negative) half-normal distribution with means/maximums of 2400 ± 750 m and 2600 ± 1250 m for Pumpkin and Faka Union Bays, respectively. Duration and distance were enforced to be ≥ 1 and ≥ 0, respectively, and direction was enforced to range between 0 and 360, resulting in realized distributions that differed slightly from true normal and half-normal distributions. The simulation proceeded by generating a random home range duration for each fish, followed by a movement with a random direction and distance, followed by a new home range duration, etc. If a simulated movement resulted in the fish being on land according to the digital shoreline, a new random direction and distance were generated. The land constraint altered the realized distributions of movement parameters, especially distance, with more frequent shorter distances than specified by the half-normal distributions. The simulation continued until all fish had crossed the boundary beyond the southern edge of the bay at which point they were considered to have emigrated. The simulation was conducted for each bay and year 100 times to generate an average emigration curve with a 95% probability interval. The observed and simulated curves for gray snapper were compared visually. The other two species lacked sufficient emigration events to compare with the simulated curves.
Results
Salinity and temperature
Salinity reached a peak > 35 ppt in both bays and years in May, which corresponded with the end of the dry season (Fig. 3). The start of the wet season was marked in both years and bays with an abrupt decline in salinity; however, this decline occurred earlier in 2018 (beginning May 13) than in 2019 (June 5). The rapid declines in salinity continued for 15–20 days before leveling off to less saline but still variable (compared to the dry season) salinity for the rest of the summer. The main difference between the bays was that Faka Union was 10–15 ppt lower than Pumpkin Bay during the wet season and the magnitude of the summer fluctuations in salinity in Faka Union were typically twice as large as those in Pumpkin.
a–f Total number of daily fish movements in Pumpkin and Faka Union Bays for gray snapper (a), red drum (b), and goliath grouper (c), with mean daily salinity. Daily fish movements for gray snapper (d), red drum (e), and goliath grouper (f), with mean daily temperature. Gray shading within each plot denotes the dates outside the tracking intervals for each species based on tagging date and expected battery life. There are two sets of lines for gray snapper, one for each tagging year
Both the timing and magnitude of daily changes in temperature followed similar patterns in both bays and years, although Faka Union was often 1–2°C cooler than Pumpkin Bay (Fig. 3). On a weekly basis, temperature fluctuated 1–2°C. In both years, water temperature increased by ~ 10°C from a low in January through June. June temperature did not correspond in timing with the decline in salinity (i.e., wet season started earlier in 2018). Highest temperatures, occasionally exceeding 31–32°C, occurred after salinity dropped, and were sustained throughout the summer until waters began to cool in October. Annual temperature minima were observed on one day in January of each year with values reaching 17°C (2018) and 16°C (2019).
Comparison of movement parameters
A combined total of 76 Gy snapper were tagged in 2018 and 2019 (Supplementary Tables S1–S3). Seventeen red drum and 22 goliath grouper were tagged in 2019. Based on length at maturity and growth data (Starck, 1971; Murphy & Taylor, 1990; Bullock et al., 1992), it is likely that all of the gray snapper had already reached maturity or would do so during the tracking period, whereas all of the tagged red drum and goliath grouper were juveniles throughout the study.
Average tracking span was 102 days for gray snapper, 214 days for red drum, and 270 days for goliath grouper. Overall detection range was estimated to be 150 to 200 m which suggests that our array covered ~ 50% of both bays and all of the possible emigration routes. There was no difference in AEI between bays for gray snapper and red drum with mean values > 75% indicating that the array effectively monitored daily fish locations (Table 1a–c). There was a significant difference in AEI between bays for goliath grouper (P < 0.04; 41% in Pumpkin vs. 69% in Faka Union); however, sample size of tagged goliath groupers in Pumpkin Bay was low and specific tagging locations of goliath grouper in that system were in an area of especially patchy mangrove islands that may have obstructed transmitter detections.
All but one of the gray snapper left the array before the end of their transmitter battery life whereas five red drum and three goliath grouper were still being detected when their batteries expired or the study was concluded (i.e., the 5-year transmitters in large goliaths extended beyond our study time frame) (Supplementary Tables S1–S3). Also of note, seven goliath grouper (32% of tagged fish) stopped being detected before the study ended or tags expired, but there was no evidence of emigration through any channel. These fish either settled in locations in these narrow waterways that were obscured from the array, slipped past exit receivers undetected, or were illegally harvested.
For gray snapper, there was no significant difference in home range duration (mean = 54 ± 6 days), or distance between home range locations (mean = 1.3 ± 0.1 km) in Pumpkin Bay compared to Faka Union Bay (Table 1a). Fish age also had no significant effect on any of those parameters for gray snapper.
The heterogeneity chi-square test comparing the landward vs. seaward shifts in home range of gray snapper indicated that the samples from both bays should be pooled. Analysis of the pooled data indicated that there was a significant difference in the direction of home range shifts compared to random landward or seaward shifts (χ2(0.05, 1) = 20.9, P < 0.001). Home range shifts occurred in the seaward direction in 39 out of 47 cases (83%) where the direction could be determined. Specifically, movements were on average toward the compass heading of 200°–250°, or roughly southwestward.
The heterogeneity chi-square test comparing the use of the main channel vs. other exits by gray snapper indicated that the bays had different emigration route ratios and should not be pooled. Therefore, bays were analyzed in separate chi-square analyses. There was no significant difference in the route of emigration (main channel vs other exits) for gray snapper in Pumpkin Bay (χ2(0.05, 1) = 0.07, NS) where 14 fish used the main channel and 16 used a different pass. In contrast, there was a significant difference in the route of emigration for gray snapper in Faka Union Bay (χ2(0.05, 1) = 15.1, P < 0.001). In Faka Union, only 5 fish used the main channel to exit the Bay, and the remaining 27 utilized one of the smaller exit routes with the majority of fish (n = 19) using the pass on the western edge of the mangroves.
Emigration time of day could be determined with confidence for 54 of 79 Gy snapper emigration events. Of those, the time of day for emigration was significantly non-random (χ2(0.05, 3) = 27.7, P < 0.001) with 60% of fish departing during day time, 22% at dusk, 6% at dawn, and only 13% at night.
For red drum, there was also no significant difference in home range duration (mean = 132 ± 39 days) or distance between home range locations (mean = 1.2 ± 0.3 km) in Pumpkin Bay compared to Faka Union Bay (Table 1b). Fish age also had no significant effect on any of those parameters for red drum.
For goliath grouper, there was no significant difference in home range durations between bays (mean = 226 ± 63 days) (Table 1c). However, there was insufficient data to test for differences in distance between home range shifts for goliath grouper, because unlike the other two species, there was a significant effect of fish age on home range duration for goliath grouper. The three youngest individuals (1 to 1.5 years old) all had a home range duration shorter than 100 days, whereas, the three oldest individuals (4 to 4.5 years old) all had home range durations in the 300 to 425 day range.
Only 9 home range shift directions for red drum (5 landward and 4 seaward) and 6 for goliath grouper (2 landward and 4 seaward) were observed. Similarly, the route for emigration events was determined for only 4 red drum (3 via the main channels and 1 via a western cut in the mangroves) and 5 for goliath grouper (5 via the main channels and 1 via a western cut in the mangroves); the low number of these events is evidence for longer site fidelity for these species compared to gray snapper and precluded statistical tests of trends.
Potential influences on timing of home range shifts and emigration
There were 129 home range shifts or emigration events observed for gray snapper, 26 for red drum, and 29 for goliath grouper (Fig. 3a–c). Only gray snapper had a sufficient number of movements for the analysis with the GAM given the much greater site fidelity of the other two species. Of the 128 possible combinations of explanatory variables that were considered in the GAMs, there were 9 combinations that explained the presence/absence of gray snapper movement equally well based on AIC scoring (Supplementary Table S4). All nine of these top models included mean daily salinity and month. Mean daily temperature and weekly change in salinity were present in 7 of the 9 top models. Fish age was present in 5 of the top 9 models, while bay and weekly change in temperature were present in only 2 and 1 of the top models, respectively.
Effects of these variables were small and were plotted for explanatory variables that were present in more than two of the top models (Fig. 4a–e). Probability of fish movement was only slightly higher when weekly salinity trends were declining (5–10 ppt decrease), at moderate values of daily salinity (15–22 ppt), at moderate values of daily temperature (25–29°C), and during late summer and fall months. Older, larger fish were more likely to move, but only marginally so.
a–e Effects plots of the probability of gray snapper movement relative to important variables in the GAM including weekly salinity change (a), mean daily salinity (b), mean daily temperature (c), fish age (d), and month (e). Gray shading represents the 95% confidence interval. Plots here are from the model with the lowest AIC score and are representative of effect curves in all top models
Of note, the percent deviance explained by even the best fitting models was quite low, accounting for only 11–12% of the observed pattern in fish movements. All other combinations of variables or individual variables explained even less of the pattern in fish movements. This indicates that some other variables, cues, or even random processes not considered in the GAM account for a much greater share of the pattern observed in fish movements. Although red drum and goliath grouper had site fidelity too high to enable statistical analysis with the GAMs, movements for both species occurred evenly throughout the year (Fig. 3b, c, e, f) and in all observed temperatures and salinity conditions in both bays.
Random-walk simulation
The simulated emigration curves for gray snapper were similar to the observed emigration curves for both Pumpkin and Faka Union Bays, with the simulated 95% probability intervals largely containing the observed emigration curves (Fig. 5). This similarity was somewhat expected since the simulation parameters were intentionally chosen to see if a match could be achieved through random movements. It is important to emphasize, however, that the simulation parameters did not incorporate any change in movement parameters over time as exhibited by the actual fish, nor did simulation parameters include any aspects of actual environmental conditions in the bays including salinity and temperature. In other words, the observed pattern of emigration can be achieved in the absence of salinity and temperature cues. The minimum simulated home range duration was important for replicating the initial ‘shoulder’ of the emigration curve. If home ranges were allowed to be very short, the emigration curve simply declined with days since tagging. Movement direction and distance had predictable effects on the simulated emigration curve with longer movements, and movements more directed toward the mouth of the bay, resulting in a steeper emigration curve.
Discussion
We implanted uniquely coded acoustic transmitters into 3 species of fish to track their movements in 2 sub-estuaries in SW Florida, one estuary had unnaturally high freshwater flow and one had unnaturally low freshwater flow. There were few differences in home range parameters between the two bays for any of the three species. Furthermore, although some environmental variables appeared slightly related to the probability of gray snapper moving (e.g., moderate salinity), the main conclusion was that all of the environmental variables tested here, either alone or in any combination, explained only a small percentage of the observed movement patterns. This is generally supported by experimental studies that indicate gray snapper can tolerate 100% freshwater (Serafy et al., 1997) and become less active during salinity extremes (Serrano et al., 2010). Evidence for red drum and goliath grouper was similar, wherein movements were observed for both those species evenly throughout the year in both bays and across all temperatures and salinity conditions. Although the number of home range shifts and emigration events was limited for red drum and goliath grouper, the actual numbers of fish tracked were robust.
Collectively, these results suggest that salinity and temperature may not be important drivers for movement of these species at the levels encountered in these bays despite highly modified watersheds and in contrast to observations of salinity influence in other locations (Adams & Tremain, 2000; Bachelor et al., 2009; Dance & Rooker, 2015). The lack of a clear relationship between fish movement and environmental variables (i.e., a negative result) for any species may at first seem disappointing, but is actually very important information to watershed managers concerned about impact from this component of the CERP restoration. Based on this evidence, it is not anticipated that greater parity in salinity levels between bays expected after watershed restoration will strongly affect fish movements.
Movements of these species have been investigated in other locations but never by partitioning home range space as described here. For example, Hammerschlag-Peyer & Layman (2010) documented movements of 1 to 7-year-old gray snapper for one month in a Bahamian estuary using primarily manual tracking. Two distinct home range behaviors were observed that were independent of fish size. Some fish utilized a restricted home range area (100–300 m distance moved) (Hammerschlag-Peyer & Layman, 2010) which is broadly consistent with observations in our study, wherein fish were typically detected on just one or two adjacent receivers. Other fish in the Bahamian study used a much larger space (400–600 m), potentially corresponding to the home range shifts documented in our study. In fact, tracking in the Bahamas spanned only one month and it is unknown if that larger space could be partitioned in time into two or more smaller but discrete home range areas.
A large majority of home range shifts by gray snapper were southwestward which resulted in a gradual seaward movement over a few months as fish matured. This seaward movement makes their ultimate shift to adult habitat on reefs a shorter distance than had they remained far up in estuaries. Splitting the trip into shorter segments may carry an advantage in energy replenishment, foraging, or minimizing predation exposure compared to making the entire migration all at once. Gray snapper spawn on reefs offshore near full moons in June–August (Starck, 1971; Rutherford et al., 1983), which coincides with the time that we observed movements begin to increase. When gray snapper finally did exit the bays, they did so using smaller passes and cuts just as often, if not more so, than the main channel. This was somewhat surprising, given that the main channel often has the greatest current speed (which may offer energetic savings from swimming effort) and the most direct route out of the estuary (which reduces the transit distance). That other exit corridors were used equally as often or, in the case of Faka Union Bay, significantly more often than the main channel, suggests that some migration corridors are more important than others.
Also of note, a large majority of gray snapper (82%) emigrated from the bays during the daytime or dusk. This is in contrast to other studies that have identified offshore ontogenetic movements of snapper and other species from mangroves to locations farther offshore as primarily occurring at nighttime (Luo et al., 2009; Huijbers et al., 2015; Kendall et al., 2021). The reason for the observed difference may be that in the other studies, offshore movements were from mangroves to reefs, whereas in this study the movements may not have been all the way to the reef, and instead just seaward toward another location within the Ten Thousand Islands.
Movement cues and habitat use in sub-adult red drum have been investigated by many researchers throughout their range with sometimes inconsistent results. Differences in methodology prevent comparison of our results on home range shifts to those other studies, either due to differing telemetry array designs (i.e., high-resolution telemetry systems; Dance & Rooker, 2015; Fodrie et al., 2015), timescale of relocation (Bachelor et al., 2009), or failure to separate home range relocations into smaller subsets within the same habitat as we have done. In some studies, movements of red drum have been more related to temperature or salinity change with the landward/seaward direction of movement influenced by the sign of the change or threshold values (Adams & Tremain, 2000; Bachelor et al., 2009; Dance & Rooker, 2015). For example, red drum in Texas may be more likely to make bay-scale relocations akin to our home range relocations when temperatures drop below 14°C, when salinity is higher than 26 ppt, and is decreasing (Dance & Rooker, 2015). However, such low temperatures were observed on only a few days during the present study (NOAA NERRS, 2022), and fish in our bays were simply not observed to move more often on days with those temperature or salinity characteristics. In a movement study on red drum from a North Carolina estuary, no link was found between emigration and salinity; however, increasing monthly salinity (i.e., by 4–6 PPT) resulted in more fish moving farther upstream over distances similar to our home range relocations, whereas decreasing salinity (4–6 PPT) actually resulted in movement downstream (Bachelor et al., 2009). Although the Pumpkin and Faka Union Bay study area experienced many such incidences of salinity change in excess of those magnitudes in both directions, observed movements of red drum were not obviously related to salinity patterns.
Duration of site fidelity for red drum also varies among studies and results may depend more on temporal resolution of the study (e.g., Fodrie et al., 2015) or the methodology used such as simple mark-recapture (Adams & Tremain, 2000), high-resolution (~ 1 m) positioning systems from telemetry (Dance & Rooker, 2015; Fodrie et al., 2015), or broad-scale telemetry arrays (Dance & Rooker 2015). Collectively, these studies of juvenile red drum suggest that the environmental factors that influence movements do not necessarily operate in the same ways across estuaries with different conditions. Indeed, red drum are estuarine generalists capable of occupying most coastal conditions throughout the southeastern US from Texas through North Carolina.
Unlike for gray snapper and red drum, the only estimate of home range size available for juvenile goliath grouper is derived from manual tracking with a directional hydrophone and suggests that they utilize ~ 170 to 586 linear m of mangrove shoreline for up to 34 months (Koenig et al., 2011). Unfortunately, the number and frequency of relocations, uniformity (or lack thereof) of detections within those estimated ranges, and size of the area monitored for relocating fish were not reported (Koenig et al., 2011), making comparison with the current study problematic. However, those values are broadly consistent with our observations wherein individual fish were typically only detected on one or two adjacent receivers in our fixed arrays. Given that our receiver detection range was ~ 150–200 m, the magnitude of home ranges for juvenile goliath groupers reported here is similar to that from the previous study. In contrast to our findings; however, the Koenig et al. (2011) study and another study elsewhere in the Ten Thousand Islands (Frias-Torres, 2007) did not explicitly report any home range shifts within the estuary, likely due to different methodologies and smaller array coverage than was used here. Also of note, our results indicate that home range duration may be size dependent with 1-year-olds using a home range for only ~ 100 days whereas 4-year-olds remain in place for ~ 300 days. This has important considerations for the limited-take fishery that may cause lasting ecosystem gaps when fish are harvested.
There are several possible explanations for the lack of a strong relationship between probability of fish movement and environmental cues. Many other variable(s) not considered here may be more responsible for prompting movements. Density dependence (e.g., can be repulsive as in overcrowding, or attractive as in schooling or spawning aggregations) or interspecific competition causes some fish to relocate (Rose et al., 2002; Hammerschlag-Peyer & Layman, 2010; Grüss et al., 2011; Koenig et al., 2011). Local depletion of prey may or influx of predators can push fish to seek new home range areas (Werner et al., 1983; Sims et al., 2006; Grüss et al., 2011; Livernois et al., 2021). Reproductive imperatives are also well-known drivers of fish movement (Trotter et al., 2012; Massie et al., 2022), although only gray snapper achieved maturity during our study (Serrano et al., 2010; Koenig et al., 2011). Even discrete weather events can correlate with relocation (Sadovy & Eklund, 1999; Heupel et al., 2003; Massie et al., 2020). Each of these factors may result in fish relocating their home range at different times and situations independently of environmental influences such as temperature and salinity, confounding efforts to identify a discrete set of environmental parameters as the main drivers of fish movement.
Interestingly, based on the results of the random-movement simulations, we cannot exclude the possibility that the observed patterns in fish emigration were merely due to ‘random’ movements unrelated to time or environmental conditions. The simulations resulted in fish leaving the estuaries at similar rates to those observed for actual fish. In these simple simulations, movements were independent of any temperature, salinity, seasonal, or other environmental cues and were instead controlled by random draws of only three parameters (i.e., home range duration, movement distance, and movement direction). The simulation results do not prove that movements and emigration are random, but instead demonstrate that the observed emigration patterns could have resulted from random processes independent of time and environmental conditions (Börger et al., 2008; Cramer et al., 2021). Indeed, there were no strong relationships between fish movement and any environmental variables in the GAMs; thus, it is possible that home range relocations are the result of such a diverse suite of cues (Crook, 2004) that they resemble random movements. Such seemingly random movements (i.e., home range shifts) will, if carried through enough steps, eventually result in fish leaving the bays to the southwest as was observed for the real fish.
There were clear differences in home range duration among species in our study. On average, the later maturing-, longer-lived, and larger the species, the longer their home range durations and overall residence time in the mangrove bays. Gray snapper reach maturity ~ 2.5–3 years of age with a maximum age of ~ 25 years (Starck, 1971; Burton, 2001). In contrast, red drum reach maturity between ~ 3 and 4 years of age (Murphy & Taylor, 1990), and goliath grouper take even longer, not reaching maturity until ~ 5–7 years and living at least into their mid 30’s (Bullock et al., 1992). Although some fishes show a positive relationship between fish age or size (i.e., larger individuals of the same species) and some of the parameters tested here (Welsh et al., 2013; Huijbers et al., 2015; Daly et al., 2021), we detected few such patterns for any of the three species evaluated, with the exception of longer home range duration for older goliath grouper.
Despite the potential for fish to disappear in telemetry detection shadows in an area known for its convoluted maze of islands and dead-end coves, the AEI analysis suggested that for most fish, our telemetry array was effective at monitoring their presence in the bays. Fish tagged in this study were detected with an average AEI of 60–73% depending on the species, which indicates that they were monitored on a majority of days that they were present in the array before emigrating. Only 12 of the 76 (16%) gray snapper had AEI values < 25%; however, nearly all of these were tracked intermittently in the array for 65 to 183 days suggesting they still resided in the bays but were using a home range that was mostly in detection shadows. All but two gray snapper had a clear exit track, further suggesting that fish with low AEI values were residing somewhere in the bays until they departed. Only one red drum had an AEI under 25%, yet it was detected over a 187 days span and the low AEI was due to it temporarily leaving the study bays for several months. Four of the goliath grouper tagged in the Faka Union canal had an AEI under 25%; however, intermittent detections over several months suggested they too were still in the canal. Also of concern for goliath grouper were the nearly 1/3 of tagged individuals that simply disappeared from the array with no exit track. We suspect that these fish were caught by fishermen, as illegal poaching of this protected species is well known to occur throughout Florida waters (Ellis pers. comm.). Overall, despite the potential for fish to hide in spots undetectable to our receivers, evidence indicates that the arrays functioned well for detecting occupancy and emigration patterns for all three focal species.
Although fish labeled as emigrating undertook directed movements leaving the bays that were unlike their typical home range shifts, we cannot say conclusively that they did not return after the batteries died in their transmitters. However, larger size classes were never caught during sampling and only a single tagged fish undertook a directed emigration and then returned to the array after a 3-month absence. With no other fish exhibiting this behavior, we suspect that particular fish may have been preyed upon by a larger mobile predator such as a shark. We also did not determine how far out of the bays these fish may have traveled, if they merely shifted to more seaward parts of the Ten Thousand Islands, or if they departed the estuary entirely and took up residence in offshore habitats. Additional receivers strategically deployed further along channels and passes offshore would be required to document those shifts.
By defining the home range based on the timing of fish behaviors rather than the typically used but arbitrary bounds imposed by transmitter battery life or array duration, this study revealed previously undescribed occupancy patterns for these three species. Despite the initial expectations that the large differences in freshwater flow into Pumpkin and Faka Union Bays would result in different home range characteristics for one or more of the species studied here, such major differences were simply not observed. Salinity and temperature conditions in the bays are currently and should continue to be within acceptable limits for all three species even after watershed restoration is completed. Because there were already few differences in fish occupancy patterns between the two bays when conditions differed (Kendall et al., 2022; Williams et al., 2023), there is no reason to suspect that patterns would be affected when restoration causes the bay environments to become more similar. Results here and elsewhere highlight the resiliency of these species as estuarine generalists capable of occupying a range of salinity regimes.
Data availability
Summary data are provided as Electronic Supplementary Tables. Raw telemetry data are archived at the Ocean Tracking Network and are available at no cost upon request from the authors.
References
Adams, D. H. & D. M. Tremain, 2000. Association of large juvenile red drum, Sciaenops ocellatus, with an estuarine creek on the Atlantic coast of Florida. Environmental Biology of Fishes 58: 183–194.
Adams, A. J., C. P. Dahlgren, G. T. Kellison, M. S. Kendall, C. A. Layman, J. A. Ley, I. Nagelkerken & J. E. Seraphy, 2006. Nursery function of tropical back-reef systems. Marine Ecology Progress Series 318: 287–301.
Adams, A. J., R. K. Wolfe & C. A. Layman, 2009. Preliminary examination of how human-driven freshwater flow alteration affects trophic ecology of juvenile snook (Centropomus undecimalis) in estuarine creeks. Estuaries and Coasts 32: 819–828. https://doi.org/10.1007/s12237-009-9156-x.
Bachelor, N. M., L. M. Paramore, S. M. Burdick, J. A. Buckel & J. E. Hightower, 2009. Variation in movement patterns of red drum (Sciaenops ocellatus) inferred from conventional tagging and ultrasonic telemetry. Fisheries Bulletin 107: 405–419.
Beck, M. W., K. L. Heck, K. W. Able, D. L. Childers, D. B. Eggleston, B. M. Gillanders, B. Halpern, C. G. Hays, K. Hoshino, T. J. Minello, R. J. Orth, P. F. Sheridan & M. P. Weinstein, 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51(8): 633–641.
Bertoncini, A. A., Aguilar-Perera, A., Barreiros, J., Craig, M. T., Ferreira, B., Koenig, C., 2018. Epinephelus itajara (errata version published in 2019). In The IUCN Red List of Threatened Species 2018: e.T195409A145206345. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T195409A145206345.en.
Booth, A. C., Knight, T. M., 2021. Flow characteristics and salinity patterns in tidal rivers within the northern Ten Thousand Islands, southwest Florida, water years 2007–19: U.S. Geological Survey Scientific Investigations Report 2021–5028: 21. https://doi.org/10.3133/sir20215028.
Börger, L., B. D. Dalziel & J. M. Fryxell, 2008. Are there general mechanisms of animal home range behavior? A review and prospects for future research. Ecology Letters 11: 637–650. https://doi.org/10.1111/j.1461-0248.2008.01182.x.
Brownscombe, J. W., L. P. Griffin, J. L. Brooks, A. J. Danylchuk, S. J. Cooke & J. D. Midwood, 2022. Applications of telemetry to fish habitat science and management. Canadian Journal of Fisheries and Aquatic Sciences 79: 1347–1359. https://doi.org/10.1139/cjfas-2021-0101.
Bullock, L. H., M. D. Murphy, M. F. Godcharles & M. E. Mitchell, 1992. Age, growth, and reproduction of jewfish Epinephelus itajara in the eastern Gulf of Mexico. Fisheries Bulletin 90: 243–249.
Burnham, K. P. & D. A. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoric Approach, 2nd ed. Springer, New York:
Burton, M. L., 2001. Age, growth, and mortality of gray snapper, Lutjanus griseus, from the east coast of Florida. Fisheries Bulletin 99: 254–265.
Cramer, A. N., S. Katz, C. Kogan & J. Lindholm, 2021. Distinguishing residency behavior from random movements using passive acoustic telemetry. Marine Ecology Progress Series 672: 73–87. https://doi.org/10.3354/meps13760.
Crook, D. A., 2004. Is the home range concept comparable with the movements of two species of lowland river fish? Journal of Animal Ecology 73: 353–366.
Daly, R., J. D. Filmalter, L. R. Peel, B. G. Mann, J. S. E. Lea, C. R. Clarke & P. D. Cowley, 2021. Ontogenetic shifts in home range size of a top predatory reef-associated fish (Caranx ignobilis): implications for conservation. Marine Ecology Progress Series 664: 165–182. https://doi.org/10.3354/meps13654.
Dance, M. A. & J. R. Rooker, 2015. Habitat- and bay-scale connectivity of sympatric fishes in an estuarine nursery. Estuarine Coastal Shelf Science 167: 447–457. https://doi.org/10.1016/j.ecss.2015.10.025.
Erwin, K. L., 2009. Wetlands and global climate change: the role of wetland restoration in a changing world. Wetlands Ecology and Management 17: 71–84. https://doi.org/10.1007/s11273-008-9119-1.
Fodrie, F. J., L. A. Yeager, J. H. Grabowski, C. A. Layman, G. D. Sherwood & M. D. Kenworthy, 2015. Measuring individuality in habitat use across complex landscapes: approaches, constraints, and implications for assessing resource specialization. Oecologia 178: 75–87. https://doi.org/10.1007/s00442-014-3212-3.
Frias-Torres, S., 2006. Habitat use of juvenile goliath grouper Epinephelus itajara in the Florida Keys, USA. Endangered Species Research 2: 1–6.
Frias-Torres, S., 2007. Activity patterns of three juvenile goliath grouper, Epinephelus itajara, in a mangrove estuary. Bulletin Marine Science 80: 587–594.
Gillanders, B. M. & M. J. Kingsford, 2002. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology, an Annual Review 40: 233–309.
Grüss, A., D. M. Kaplan, S. Guénette, C. M. Roberts & L. W. Botsford, 2011. Consequences of adult and juvenile movement for marine protected areas. Biological Conservation 144: 692–702. https://doi.org/10.1016/j.biocan.2010.12.015.
Hammerschlag-Peyer, C. M. & C. A. Layman, 2010. Intrapopulation variation in habitat use by two abundant coastal fish species. Marine Ecology Progress Series 415: 211–220. https://doi.org/10.3354/meps08714.
Heupel, M. R., C. A. Simpfendorfer & R. E. Heuter, 2003. Running before the storm: blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle. Journal of Fish Biology 63: 1357–1363. https://doi.org/10.1046/j.1095-8649.2003.00250.x.
Huijbers, C. M., I. Nagelkerken & C. A. Layman, 2015. Fish movement from nursery bays to coral reefs: a matter of size? Hydrobiologia 750: 89–101. https://doi.org/10.1007/s10750-014-2162-4.
Kendall, M. S., L. Siceloff, A. Ruffo, A. Winship & M. E. Monaco, 2021. Green morays (Gymnothorax funebris) have sedentary ways in mangrove bays, but also ontogenetic forays to reef enclaves. Environmental Biology of Fisheries 104: 1017–1031. https://doi.org/10.1007/s10641-021-01137-0.
Kendall, M. S., B. L. Williams, P. M. O’Donnell, B. Jessen & J. Drevenkar, 2022. Too much freshwater, not enough, or just right? Long-term trawl monitoring demonstrates the impact of canals that altered freshwater flow to three bays in SW Florida. Estuaries and Coasts. https://doi.org/10.1007/s12237-022-01107-4.
Kinch, J. C. & W. L. E. O’Harra, 1976. Characteristics of the sport fishery in the Ten Thousand Islands area of Florida. Bulletin of Marine Science 26(1): 479–487.
Koenig, C. C., F. C. Coleman & K. Kingon, 2011. Pattern of recovery of the Goliath Grouper Epinephelus itajara population in the southeastern US. Bulletin of Marine Science 87(4): 891–911. https://doi.org/10.5343/bms.2010.1056.
Krauss, K. W., A. S. From, T. W. Doyle, T. J. Doyle & M. J. Barry, 2011. Sea-level rise and landscape change influence mangrove encroachment onto marsh in the Ten Thousand Islands region of Florida, USA. Journal of Coastal Conservation 15: 629–638. https://doi.org/10.1007/s11852-011-0153-4.
Lammers, J., E. Soelen, T. H. Donders, F. Wagner-Cremer, J. Sinninghe-Damste & G. Reichart, 2013. Natural environmental changes versus human impact in a Florida estuary (Rookery Bay, USA). Estuaries and Coasts. https://doi.org/10.1007/s12237-012-9552-5.
Livernois, M. C., M. Fujiwara, M. Fisher & R. J. D. Wells, 2021. Seasonal patterns of habitat suitability and spatiotemporal overlap within an assemblage of estuarine predators and prey. Marine Ecology Progress Series 668: 39–55. https://doi.org/10.3354/meps13700.
Luo, J., J. E. Serafy, S. Sponaugle, P. B. Teare & D. Kieckbusch, 2009. Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Marine Ecology Progress Series 380: 255–269. https://doi.org/10.3354/meps07911.
Massie, J. A., B. A. Strickland, R. O. Santos, J. Hernandez, N. Viadero, R. E. Boucek, H. Willoughby, M. R. Heithaus & J. S. Rehage, 2020. Going downriver: patterns and cues in hurricane-driven movements of common snook in a subtropical coastal river. Estuaries and Coasts 43: 1158–1173. https://doi.org/10.1007/s12237-019-00617.
Massie, J. A., R. O. Santos, R. J. Rezek, W. R. James, N. M. Viadero, R. E. Boucek, D. A. Blewett, A. A. Trotter, P. W. Stevens & J. S. Rehage, 2022. Primed and cued: long-term acoustic telemetry links interannual and seasonal variations in freshwater flows to the spawning migrations of Common Snook in the Florida Everglades. Movement Ecology 10(1): 1–20.
McCawley, J., 2022. Final Rules—Goliath Grouper. Florida Fish and Wildlife Conservation Commissioners, Division of Fisheries Management. Rule 68B-14.0091 Recreational Goliath Grouper Harvest Permits; Goliath Grouper Tag Specifications; Harvest Reporting Requirements. https://myfwc.com/media/28517/9a-sm-goliathgrouper.pdf. Accessed 3 Sept 2022.
Michot, B. D., E. A. Meselhe, A. M. Ache, K. W. Krauss, S. Shrestha, A. S. From & E. Patino, 2017. Hydrologic modeling on a marsh-mangrove ecotone: predicting wetland surface water and salinity response to restoration in the Ten Thousand Islands Region of Florida, USA. Journal of Hydrologic Engineering 22(1): D4015002.
Misra, V., A. Bhardwaj & A. Mishra, 2017. Characterizing the rainy season of peninsular Florida. Climate Dynamics 51(25): 1–11. https://doi.org/10.1007/s00382-017-4005-2.
Murphy, M. D. & R. G. Taylor, 1990. Reproduction, growth, and mortality of red drum Sciaenops ocellatus in Florida waters. Fishery Bulletin 88: 531–542.
NOAA NERRS, 2022. National estuarine research reserve system, system-wide monitoring program. In Data accessed from the NOAA NERRS Centralized Data Management. http://www.nerrsdata.org. Accessed 1 Jan 2020.
R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Reese Robillard, M. M. R., L. M. Payne, R. R. Vega & G. W. Stunz, 2015. Best practices for surgically implanting acoustic transmitters in spotted seatrout. Transactions of the American Fisheries Society 144: 81–88.
Rose, K. A., J. H. Cowan Jr., K. O. Winemiller, R. A. Myers & R. Hilborn, 2002. Compensatory density dependence in fish populations: importance, controversy, understanding and prognosis. Fish and Fisheries 2: 293–327. https://doi.org/10.1046/j.1467-2960.2001.00056.x.
Rutherford, E. S., Thue, E. B., Buker, D. G., 1983. Population structure, food habits, and spawning activity of gray snapper, Lutjanus griseus, in Everglades National Park. In South Florida Research Center Report SFRC-83/02: 41.
Sadovy, Y., Eklund, A. M., 1999. Synopsis of biological information on the Nassau grouper, Epinephelus striatus (Bloch 1792), and jewfish, E. itajara (Lichtenstein 1822). In NOAA Tech Rep NMFS 146: 65.
Serafy, J. E., K. C. Lindeman, T. E. Hopkins & J. S. Ault, 1997. Effects of freshwater canal discharge on fish assemblages in a subtropical bay: field and laboratory observations. Marine Ecology Progress Series 160: 161–172.
Serrano, X., M. Grosell & J. E. Serafy, 2010. Salinity selection and preference of the grey snapper Lutjanus griseus: field and laboratory observations. Journal of Fish Biology 76: 1592–1608. https://doi.org/10.1111/j.1095-8649.2010.02585.x.
Shideler, G. S., S. R. Sagarese, W. J. Harford, J. Schull & J. E. Serafy, 2015. Assessing the suitability of mangrove habitats for juvenile Atlantic goliath grouper. Environmental Biology of Fishes 98: 2067–2082. https://doi.org/10.1007/s10641-015-0430-4.
Shirley, M., P. O’Donnell, V. McGee & T. Jones, 2004. Nekton species composition as a biological indicator of altered fresh-water inflow: a comparison of three South Florida Estuaries. In Bortone, S. (ed), Chapter 22 in Estuarine Indicators. CRC Press, Boca Raton.
Sims, D. W., M. J. Witt, A. J. Richardson, E. J. Southall & J. D. Metcalfe, 2006. Encounter success of free-ranging marine predator movements across a dynamic prey landscape. Proceeding Royal Society B 273: 1195–1201. https://doi.org/10.1098/rspb.2005.3444.
Starck, W. A., 1971. The biology of the grey snapper, Lutjanus griseus (Linnaeus), in the Florida Keys. In Starck, W. A. & R. E. Schroeder (eds), Investigations on the Gray Snapper, Lutjanus griseus. Studies in Tropical Oceanography No. 10, Rosenstiel School of Marine and Atmospheric Sciences University of Miami Press, Miami: 11–150.
Thayer, G. W., D. R. Colby & W. F. Hettler, 1987. Utilization of the red mangrove prop root habitat by fishes in south Florida. Marine Ecology Progress Series 35: 25–38.
Trotter, A. A., D. A. Blewett, R. G. Taylor & P. W. Stevens, 2012. Migrations of common snook from a tidal river with implications for skipped spawning. Transactions of the American Fisheries Society 141(4): 1016–1025.
U.S. Army Corp of Engineers, 2004. Picayune Strand Restoration Project (formerly Southern Golden Gate Estates Ecosystem Restoration): Final Integrated Project Implementation Report and Environmental Impact Statement, Jacksonville: 414.
Welsh, J. Q., C. H. R. Goatley & D. R. Bellwood, 2013. The ontogeny of home ranges: evidence from coral reef fishes. Proceedings of the Royal Society B 280: 20132066. https://doi.org/10.1098/rspb.2013.2066.
Werner, E. E., G. F. Gilliam, D. J. Hall & G. G. Mittelbach, 1983. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64: 1540–1548. https://doi.org/10.2307/1937508.
Williams, B. L., P. M. O’Donnell, M. S. Kendall, A. J. Winship & B. Jessen, 2023. How are man-made changes in freshwater flow related to the abundance of juvenile estuarine fishes? Estuaries and Coasts. https://doi.org/10.1007/s12237-023-01232-8.
Wood, S. N., 2017. Generalized Additive Models: An Introduction with R, 2nd ed. Chapman and Hall/CRC, Berlin:
Zar, J. H., 1999. Biostatistical Analysis, 4th ed. Prentice-Hall Inc., Upper Saddle River:
Acknowledgements
This work was conducted under Florida Special Activity Permits SAL-18-1871B-SRP, SAL-19-1871-SRP, SAL-20-1871-SRP, and SAL-18-1987-SR, TTI permits 41555-2018-R02 and R03, 4155-19-R03, and 4155-20-R01, and US ACE permit SAJ-2018-00319. The project was funded by NOAA/NCCOS projects 848 and 703, with major in-kind contributions from the Florida Fish and Wildlife Conservation Commission via Federal Aid for Sport Fish Restoration, US Department of Interior, US Fish and Wildlife Service, that supported purchase of transmitters for goliath grouper, acoustic receivers, and FWC staff time (FWC staff who worked on this project: M. O’Boyle, J. Ritch, P. Stevens, S. Webb, D. Westmark, and K. Williams). J. Drevenkar provided water quality data and information about its limitations. Volunteer anglers G. Kniffen, W. Rapchinski, K. Zikmanis, J. Young, M. Figueroa and C. Gervasi helped catch subject fishes. Environmental Compliance including animal care approval and permits was reviewed and approved by NOAA through the National Environmental Policy Act. Raw detection data have been archived and are available from the FACT Network. There is no conflict of interest to report. We also thank J. Christensen, C. Jeffrey, and K. Laakkonen for reviewing the drafts of this paper. Labor was provided by CSS Inc., under contract GS-00F-217CA / EA133C17BA0062.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and no relevant financial or non-financial interest to disclose.
Additional information
Handling editor: Ian Nagelkerken
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kendall, M.S., Siceloff, L., O’Donnell, P. et al. What controls home range relocations by estuarine fishes downstream from watersheds with altered freshwater flow?. Hydrobiologia 851, 223–241 (2024). https://doi.org/10.1007/s10750-023-05330-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05330-3