Abstract
Phenotypic plasticity, the ability of an organism to express multiple phenotypes in response to the prevailing environmental conditions without genetic change, may result in a response to anthropogenic environmental change. Given that increasing climate variability is predicted to pose a greater risk than directional climate change, we tested the effect of a water temperature differential of 4 °C on the Arctic charr phenotypic within a single generation. We demonstrate that Arctic charr phenotype can respond rapidly and markedly to an environmental temperature cue. The plastic response to different temperature regimes comprised a shift in the mean expressed phenotype but also coupled with a reduction in the between-individual phenotypic variation in the expressed head shape. The magnitude of shape difference between temperature conditions was cumulative over time but the rate of divergence diminished as fish became larger. Overall, individuals raised in the elevated temperature treatment expressed a phenotype analogous to a benthivorous ecotype of this species, rather than that of the parental pelagic feeding form. The response of cold-water freshwater species to temperature change is likely to be an interaction between the capacity of the organism for phenotypic plasticity, the mean speed of change in the environment, and the degree of short interval variation in the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In nature, the expression of intraspecific, discrete, and alternative phenotypes can result from modulation by the environment through plasticity (Adams et al. 2003) or from the emergence of evolutionary groups with diverging gene pools (Wu and Ting 2004), or a combination of both (Nosil 2012). Phenotypic plasticity is the ability of a single genotype to express multiple alternative phenotypes in response to different environmental conditions (Pigliucci and Preston 2004). Plasticity itself is a trait, present across a broad range of taxa (Berg and Ellers 2010; Corno and Jürgens 2006; Lubchenco and Cubit 1980). It can be instantaneous, anticipatory or delayed, permanent or reversible, adaptive or non-adaptive, passive, discrete, continuous, and generational (Whitman and Agrawal 2009). It is known to facilitate the expression of novel phenotypic traits (Skúlason et al. 2019) upon which selection may then act (Ghalambor et al. 2007). Thus, phenotypic plasticity has an important role in the evolutionary processes of organisms that exhibit plasticity because variation fuels evolution (Schoener 2011).
Identifying pathways along which phenotypic plasticity can arise is still a major topic for inquiry in evolutionary ecology, since the patterns and processes that underlie variation are multifaceted and highly variable. Despite the complex nature of phenotypic plasticity, there are some abiotic factors that are known to be ubiquitous in driving variation in ecosystems, one of which is temperature (McPhee et al. 2012; Noble et al. 2018). Many plants and animals have the capacity for a plastic response to temperature, and it is unlikely that rapid environmental change would induce an equally rapid genetic response (Merilä and Hendry 2014; O’Dea et al. 2016). A rapid change in temperature could induce greater levels of phenotypic variation among individuals within a population by exposing previously hidden (cryptic) genetic variation or by inducing new epigenetic changes (Merilä and Hendry 2014; O’Dea et al. 2016; Orizaola and Laurila 2008). Accordingly, the response of an organism to temperature can include variation in the mean expressed phenotype but it can also include change in the variance of phenotypes expressed in a population (O'Dea et al. 2019).
As ectotherms, fish are particularly sensitive to change in temperature (Neuheimer et al. 2011). A phenotypic trait in fish that is strongly linked with temperature is growth (Kingsolver et al. 2004; van der Have and de Jong 1996). However, the subsequent effect of temperature and induced growth rate heterogeneity (e.g. early developmental and juvenile growth) on the modulation of morphology is not a mechanism fully understood, despite being observed to affect morphology modulation in a number of species (Heino 2014; Jacobson et al. 2015; Olsson et al. 2006). A few studies have looked at the potential for temperature change to result in heterochrony in the developmental and ontogenetic processes that modulate the expression of functional traits (Charmantier et al. 2008; Parmesan 2006), while others have attempted to predict the morphological outcome of temperature-induced plasticity (McPhee et al. 2012). However, the ecological consequences that result from temperature-induced phenotypic change have received little attention in ectotherms at higher latitudes (Burggren 2018; Ramler et al. 2014). As the expression of some discrete functional phenotypes in some species is plastic, and by definition, modulated by variation in the natural environment, it is presumed that they will also respond to anthropogenic modifications of that environment. The most obvious and far-reaching anthropogenic modification of the contemporary environment is that of climate change. The northern regions are warming at double the rate of the rest of the planet and given that a rise in water temperature is predicted to occur, cold-water freshwater species will be foremost among those affected by climate change (Heino et al. 2009; Poesch et al. 2016; Solomon 2007). Change in the variability of climate is known to occur at different temporal scales (e.g. diurnally, intra-seasonally, or inter-annually), whereas temperature extremes are now occurring more regularly and are predicted to increase even more in frequency in the future (Scheepens et al. 2018).
The Arctic charr (Salvelinus alpinus (L. 1758)) has a circumpolar distribution, it is the most northern species of freshwater fish and displays highly variable phenotypes (Klemetsen 2013). The species is suitable to explore how phenotypic diversity is realised within and among generations as it expresses a high degree of phenotypic plasticity and rapid intra-specific divergence. As a result of its range and habitat use, it experiences diverse selective environments, which facilitates tracking the link between ecological processes and evolutionary outcomes (Adams et al. 2003; Chavarie et al. 2010; Elmer 2016; Garduño-Paz and Adams 2010). Worldwide, the main described phenotypes of Arctic charr in lacustrine systems, either found in allopatry or sympatry, are benthic and pelagic ecotypes. Benthic ecotypes usually specialise to forage in the littoral zone on benthic macro-invertebrates and are typically characterised by a robust head and jaw structure, a deep and wide body, blunt snout, and smaller eyes. In contrast, pelagic ecotypes specialise in feeding on zooplankton or fish in the limnetic zone and are usually more fusiform in body shape, with delicate mouth and head structures, and larger eyes (Chavarie et al. 2021; Schluter 2000; Skulason and Smith 1995).
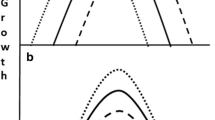
Given that increasing climate variability is predicted to pose a greater risk than directional climate change (Vasseur et al. 2014), we tested the effect of an elevated water temperature of ~ 4 °C during a period of early development on the short-term, plastic, phenotypic expression in an Arctic charr population that displays continuous variation of a pelagic only phenotype. The induced temperature variation in this study is comparable in magnitude to that of year to year extreme temperature fluctuations predicted by climate change models that Arctic charr populations are likely to experience (an interannual 4 °C temperature difference is within the predicted range for the near future in northern areas; Alley et al. 2003; Heino et al. 2009; Woelders et al. 2018). We predict that Arctic charr individuals reared in warmer water will express greater phenotypic variation than fish reared at temperatures typically experienced by populations at temperate latitudes (see Fig. 1C) but that they will not display a significant shift in the mean phenotype expressed as a result of the short-term exposure to elevated temperatures (Fig. 1B and D) (O'Dea et al. 2019). Overall, we aimed to (1) quantify the among-individual morphological variation of the head of a unimodal Arctic charr population exposed to different temperature regimes during early development, (2) determine the allometry trajectories that result in any differences in expressed phenotype as evidence of differing developmental pathways in groups exposed to different temperature, and (3) examine if at different temperature treatments, head shape among individuals is more analogous to the expressed phenotypes of plankton feeding (the parental phenotype) or macro-benthos feeding ecotypes in the wild (an alternative phenotype).
Hypothetical expressed phenotype frequency distributions shown as reaction norms resulting from an environmental change, such as temperature. Black lines represent expressed phenotypes in a population subjected to exisiting conditions and grey line represent population expression of phenotype subjected to the new environmental conditions. In panel (a), the population does not express any change in phenotypic mean or in phenotypic variation when exposed to the new environmental conditions. In panel (b), the population shows a shift in the expressed phenotypic mean but not in phenotypic variation when exposed to the new environmental regime. In panel (c), the phenotypic mean stays the same after the environmental condition changes, but the variation in expressed phenotypic increases in the population exposed to the new environmental regime. In panel (d), the population (ecotype) subjected to the new environmental conditions shifts both in phenotype mean and in phenotypic variation. Direction and magnitude of variation in panel b–d are not absolute and will be species and location dependent as well of the strength and time of exposure to the environmental variables
Materials and methods
Fish collection and rearing
Eggs from nine separate female Arctic charr were each fertilised by a single male to create nine full sibling crosses, from a morphologically unimodal, plankton feeding population that inhabits Loch Clair, Scotland. Fish were caught during spawning time from the in-flowing River Coulin (57°32.648′N, 5°19.125′W). Fertilised eggs were water hardened and transferred to incubation facilities at the Scottish Centre for Ecology and the Natural Environment, Loch Lomond. Eggs were acclimatised over a 2-h period to the new water supply and placed in mesh baskets suspended in a holding tank in a constant temperature room maintaining a water temperature of 4 °C (± 0.5 °C). Fertilisation success was greater than 95% for all family groups, which were allocated equally through temperature treatments.
Experimental procedure
To control for differences in development due to different temperatures, the number of degree-days (dd) were used as a measure of developmental rate (Fig. 2). Degree-days are the cumulative count of the water temperature for a known period of time in days. Embryos reached the eyed stage after 212dd at which point they were raised to a temperature of 6 °C (± 0.5 °C; to mimic the natural shift from winter (4 °C) to spring temperature (6 °C)) for a further 89dd, before being separated into two temperature treatment groups (N = 480 per group), an ambient and an elevated temperature treatment. Developing embryos were held in equal numbers in eight replicate tanks per treatment group (N = 60 per tank). Since Arctic charr embryo viability is greatly affected by temperature (Janhunen et al. 2010; Jeuthe et al. 2016), to reduce the risk of mortality, different temperature regimes were not induced until embryos had reached the eyed stage of development. Water temperatures were then either lowered from 6 °C by 2 to 4 °C or raised by 2 to 8 °C (± 0.5 °C) respectively, these temperatures being within the range that is unlikely to result in mortality in embryos. Embryos exposed to the 4 °C treatment began hatching after 367dd, and the hatching period lasted 73dd (total developmental time to 100% hatch 440dd). Those exposed to the elevated temperature (8 °C) began hatching after 388dd, and the hatching period lasted 50dd (total incubation time to 100% hatch 438dd). There was no significant difference in hatching rate between temperatures or replicates. When hatching was complete, temperatures of both treatments were raised by 3 °C; the lower (ambient) temperature treatment to 7 °C and the elevated temperature treatment to 11 °C (± 0.5 °C). Fish became partially dependant on exogenous food at 505dd for the ambient temperature treatment and 504dd for the elevated temperature treatment. When the yolk sac was fully exhausted, 689dd for the ambient temperature treatment and 686dd for the elevated temperature treatment, temperatures were raised a further 2 °C for both treatments to 9 °C (ambient temperature treatment) and 13 °C (elevated temperature treatment) to approximately mimic the expected rate of change in the ambient temperature with season. Both temperature treatments remained within ± 0.5 °C. Fish were fed four times a day to satiation at 3-h intervals (± 0.5 h) using a standard commercial 3 mm hatchery sinking pellet.
Diagrammatic representation of experimental setup for testing the effect of an elevated water temperature of ~ 4 °C on the short-term, plastic, phenotypic expression for nine families of Arctic charr of the plankton feeding population (continuous variation) that inhabits Loch Clair. All families of the nine females and male are represented by F1–F9
Data collection
Adult Arctic charr used as brood stock were killed using a Schedule 1 method (UK Home Office Licence Number PPL 70/8794), photographed in a lateral position on the left side before spawning. Lateral view photographs of juveniles killed using a Schedule 1 method and under licence were taken at 700dd (N = 130), 1000dd (N = 80), and 1400dd (N = 60) using a Cannon EOS 350D digital camera, for geometric morphometric analysis. Nine consistently identifiable landmarks on the head (Fig. 3) were digitised in two dimensions on each fish image using TPSdig2 (Rohlf, 2006a) and TPSutil (Rohlf, 2006b).
Nine landmarks were used to characterise the shape of the head; landmark (LM) 1, the tip of the nose; LM2, the most posterior part of the upper jaw; LM3, edge of cranium directly above the eye; LM4, edge of cranium at the central point between LM3 and LM1 at a 90° angle; LM5-8, most upper, posterior, lower and anterior parts of eye respectively; LM9, most posterior part of the gill operculum
Data analysis
Prior to geometric morphometric analysis, landmark data were subject to a Procrustes superimposition using MorphoJ (Klingenberg 2011) to remove variation in the data created by size, position, and orientation (Mitteroecker 2009). The mean shape configuration was then computed and the variation around this mean calculated (Dryden and Mardia 1998).
Following this, a single, pooled within-group regression of Procrustes co-ordinates on log centroid size was conducted in MorphoJ for samples collected at 700dd, 1000dd, and 1400dd. The residuals from this regression provide a measure of shape, free from allometric scaling (Klingenberg and McIntyre 1998) associated with early ontogeny. The residuals from this regression were subsequently used for all further morphometric analysis.
A single discriminant function analysis (1000 permutations) was conducted in MorphoJ to compare geometric morphometric data from three developmental stages (700dd, 1000dd, and 1400dd) to test if the magnitude of shape difference between groups changed over time. Procrustes distance and Mahalanobis distance were used as pairwise measures of the magnitude of shape difference. Within each temperature treatment (ambient and warm), the polynomial relationships between discriminant function scores and size (represented by centroid size) were used to illustrate allometric trajectories. To decide whether allometric trajectories between temperature treatments were parallel, convergent, divergent, or common (see Fig. A1), the slopes of the regressions were examined.
Using the scores generated in the discriminant function analysis as a measure of shape, a generalised linear mixed effect model, fitted by maximum likelihood (using the software R 3.1 for Windows (R Core Team 2014) and the package lme4), was used to describe the effect of temperature, exposure time (the number of dd at each developmental stage), and fish size (measured as centroid size) with replicate (tank) as a random effect, on the expression of shape. Models were simplified by removing the highest order, least significant terms, sequentially (Crawley 2012). Terms that were removed from the model without significantly increasing model deviance using likelihood ratio tests (χ2) were discarded. Post hoc pairwise comparisons using T-tests assessed if centroid size (related to body size) differed between ambient and elevated treatments at each sampling time (700, 1000, and 1400dd).
A canonical variate analysis (1000 permutations) executed in MorphoJ was used to establish the effect of the temperature treatment by comparing the head shape of individuals raised in ambient or elevated temperature conditions in the laboratory to their parents.
A variance ratio test was conducted using the software R 3.1 for Windows on all raw Procrustes coordinates and used to compare within-group phenotypic variation between groups.
To establish if, and by how much, exposure to a temperature regime had stimulated expression of head shape similar to that of a typical plankton or macro-benthos feeding specialist from the wild (the most common alternative phenotypes found in the wild), head shape of juveniles at 1400dd was compared against the size-corrected head shape of two known ecologically divergent, sympatric polymorphic groups of Arctic charr that show morphological and dietary specialisations on either plankton or macro-benthos feeding resources, one from Loch Rannoch (Perthshire, Scotland) (see Adams et al. 1998 for more details) and one from Loch Dughaill (Strathcarron, Scotland) (see Hooker et al. 2016 for more details) using a canonical variate analysis (1000 permutations) in MorphoJ. The same nine consistently identifiable landmarks used for analysing laboratory raised individuals were digitised in two dimensions on existing images of the fish from Loch Rannoch and Loch Dughaill (described in Adams et al. (1998) and Hooker et al. (2016) respectively).
Results
Discriminant function analysis (DFA) showed that head shape differed between ambient and elevated temperature exposed fish across all sampling periods (Procrustes distance = 0.028; P < 0.01). The magnitude of shape differences, however, increased over time (at 700dd, Procrustes distance = 0.036; P < 0.01; at 1000dd, Procrustes = 0.040; P < 0.01; at 1400dd, Procrustes distance = 0.061; P < 0.01; Fig. 4).
Individual discriminant function scores (DF1) for head shape of Arctic charr of different sizes (represented by centroid size). Three sample periods are given, 700dd are represented by circles, 1000dd by triangles and 1400dd by squares. Ambient exposed fish are denoted with open symbols; elevated temperature fish are denoted with closed symbols. Polynomial regressions for both temperature treatments are showing allometric trajectories toward phenotypic divergence of head shape changes (see Fig. A2)
Discriminant function analysis of shape only, correctly assigned 73.8% of ambient temperature fish and 82.8% of elevated temperature fish (from all sampling periods combined) to the correct temperature exposure. In general, fish exposed to elevated temperature had a more rounded head and sub-terminal mouth than ambient temperature fish (Fig. A2). Individuals in both temperature treatments displayed allometric trajectories with polynomial relationships of head shape on size differing from 0 (P < 0.05); centroid size explained 55% of variation in discriminant function score of head shape in the ambient temperature treatment group (R2 = 0.55) and 57% in the elevated temperature treatment group (R2 = 0.57). Regression slopes indicated that allometric trajectories were divergent (Figs. 4 and A1).
Using discriminant function scores as a measure of shape in a mixed model, temperature had an effect on head shape (likelihood ratio χ2 = 39.81, P < 0.01) (Table 1). There was a significant interaction between exposure time and centroid size (likelihood ratio χ2 = 7.23, P = 0.01) (Table 1). The interaction between exposure (number of degree-days) and fish size (centroid size) was negative. Thus, the difference in morphology between fish raised on each temperature regime was greater in larger fish, but the rate of divergence decreased as fish became larger, indicating that speed of divergence caused by different temperature regimes is greater during early ontogenetic stages. Pairwise comparisons of centroid size between temperature treatments at each sampling time showed a difference in size at 700dd (t = 1.98, P < 0.01) and at 1400dd (t = 2.0, P < 0.01), but no size differences were found at 1000dd (P < 0.05). At 700dd and 1400dd, individuals raised at ambient temperature were larger than individuals exposed to elevated temperature.
The variance ratio test found that the phenotypic variation within treatment groups was higher in ambient temperature fish compared with elevated temperature fish (P < 0.01). For ambient temperature, variance in Procrustes scores (SS distance/(n-1)) was 0.0046 at 700dd, 0.0032 at 1000dd, and 0.0018 at 1400dd. For elevated temperature, variance of Procrustes scores (SS distance/(n-1)) was 0.0038 at 700dd, 0.0039 at 1000dd, and 0.0016 at 1400dd.
Canonical variate analysis (CVA) found that the head shape of fish exposed to the ambient temperature treatment was not significantly different to that of their parents (Procrustes distance = 0.014; P = 0.38); however, fish exposed to elevated temperature were significantly different from their parents (Fig. A3; Procrustes distance = 0.044; P ≤ 0.001).
Using a CVA to compare experimental (Coulin) charr from both temperature groups to benthic and pelagic foraging specialists from Loch Rannoch and Loch Dughaill, we found that ambient temperature exposed fish expressed a phenotype closer to the pelagic ecotype and fish exposed to elevated temperature displayed a phenotype closer to benthic ecotype for both comparisons (Fig. 5 and Table 2). CV1 mostly captures shape differences between fish of different origin, experimental charr and Loch Rannoch charr (Fig. 5A), and experimental and Loch Dughaill charr (Fig. 5B). CV2, however, captures within group shape differences between fish raised at ambient or elevated temperatures, benthic and pelagic ecotypes from Loch Rannoch (Fig. 5A), and benthic and pelagic ecotypes Loch Dughaill (Fig. 5B).
Canonical variate analysis of 1400dd ambient and elevated temperature exposed Arctic charr and sympatric polymorphic populations of Arctic charr from; A Loch Rannoch, B Loch Dughaill. Elipses represent 95% confidence limits for means. Polymorphic charr populations are represented by squares and experimental Arctic charr by circles. Open symbols denote wild plankton feeding Arctic charr and Arctic charr raised at an ambient temperature. Closed symbols denote benthic feeding Arctic charr and Arctic charr raised at an elevated temperature. X and Y axis percentages denote the amount of variation for each canonical variate. Wire frames on CV1 are scaled at − 8 and + 8, wire frames for CV2 are scaled at − 4 and + 4. C and D show mean (± 95% CL) for CV2 scores
Discussion
Variation in climate can occur at different temporal scales (e.g. diurnally, intra-seasonally, or inter-annually), and extreme temperature anomalies are now occurring more regularly and are predicted to increase in frequency in the future (Scheepens et al. 2018). Accordingly, in this study, we demonstrate differences of an environmentally contingent expressed phenotype in an ectotherm subjected to a severe climate event. Our results demonstrate an influence of temperature on the direction, magnitude, and variation in the expression of phenotypic plasticity in a cold-water specialist species where the population in the wild displays continuous variation (i.e. unimodal phenotype; Klemetsen 2010, 2013). Elsewhere studies on plasticity during development have shown responses to alternative environmental inputs, resulting in morphological differences among individuals (Morris 2014; West-Eberhard 2003). Here, offspring of wild Arctic charr raised at elevated temperatures expressed a mean phenotype different to their siblings raised at an ambient temperature but also to that of their parents. The magnitude of this morphological response mimicking the level of divergence observed in wild Arctic charr populations that express divergent sympatric foraging specialist ecotypes (polymodal phenotype distribution; Skulason and Smith 1995). When compared to polymorphic populations that show distinct and stable foraging specialisms, individuals raised in the elevated temperature treatment expressed a phenotype analogous to a benthivorous ecotype rather than that of their parental pelagic form (Adams et al. 1998; Hooker et al. 2016). The head of fish exposed to elevated temperature was shorter, more robust, with a rounder snout; features that are typical of littoral macro-benthos feeding specialists (Fig. A3) in several post-glacial fishes (Bolnick and Lau 2008; Robinson and Parsons 2002). In contrast, ambient temperature exposed fish expressed a phenotype which comprised an elongated and pointed head, which is more typical of fish showing pelagic planktivory in the wild (Schluter 2000; Taylor 1999).
Early development represents a critical life-stage for the emergence of phenotypic variation. Heterochrony can disrupt the onset and termination of developmental processes and the rate at which these processes occur. Developmental pace in structurally important features, such as bone metabolism, is fundamentally linked to the form of morphological plasticity examined in this experiment (Campinho et al. 2004; Sfakianakis et al. 2004). As a result during early ontogeny, the environment is likely to have a greater influence on a range of traits and the magnitude of the variation of expression in a population (Ackerly and Ward 2016; Gillooly et al. 2001; Imsland et al. 2005; Parsons et al. 2010). The thermal history experienced by an individual during early development is known to be a key factor defining adult phenotype (Angilletta et al. 2004; Georga and Koumoundouros 2010; Ramler et al. 2014). It is particularly during early development that environmental signals can influence developmental processes through epigenetic mechanisms, influencing the expressed phenotype (Duclos et al. 2019). This observed effect on the phenotype in this study was cumulative, with divergence between treatment groups increasing over time. However, the rate of divergence decreased with time, suggesting that the effect of temperature on the expression of phenotypic differences occurred predominant at early life stages and that there was a decrease in divergence rate as size increases and presumably the pace of ontogenetic change slows. This indicates that the timing of exposure to different environmental conditions by individuals is important in the initiation of a plastic response and its magnitude.
Temperature exposure in early life is known to result in epigenetic effects on traits such as growth, with consequences that can range from short-term to transgenerational (Burton and Metcalfe 2014). Our results suggest that temperature-induced variation in developmental pathways results in divergent plastic allometric trajectories in the early development of Arctic charr. Allometry can been seen as a canalising process and an interacting agent that can steer morphological variation (Franklin et al. 2018; Simonsen et al. 2017). However, the opposite is also true, whereas allometric trajectories can deviate from the “usual” shape-size relationship (e.g. whereas size increases, shape changes under a fixed allometric relationship), deviation from this fixed allometry relationship could serve as additional measure of development instability (Lazić et al. 2015). Consequently, growth trajectories manifested as allometric scaling provide the potential to express different phenotypes, which increase the potential for novelty (Frankino et al. 2005). Altogether, both heterochrony and allometry are involved in determining the degree of phenotypic variation generated during growth and morphological development. In this study, the expression of phenotypic variation observed appears to be influenced by temperature with concomitant developmental effects (Lazić et al. 2015; Westneat et al. 2015).
The potential of Arctic charr to display phenotypic variation is considerable, whether expressed variation is continuous (e.g. unimodal) or discontinuous (e.g. polymodal) (Klemetsen 2013). Populations that express a high level of variability in natural conditions show higher means and greater variance in the plastic expression of phenotype in response to different temperatures (O'Dea et al. 2019; Reed et al. 2010). Although our study also showed a significant plastic response resulting in a shift in the mean phenotype expressed, there was also a decrease in variation of the phenotypes expressed among individuals raised at higher temperature treatment, which contrasts with previous work. Increased phenotypic variance in response to elevated temperatures has been suggested to be the reflection of a reduction in genotype precision (e.g. canalization and developmental stability), and this lack of precision results in a spreading of reaction norms (Campbell 2019; O'Dea et al. 2019). Our opposing result could be the result of a non-linear reaction norm of head shape on size. In this scenario, a specific range of temperature exposures (either colder or warmer) has a different magnitude effect on phenotypic mean and variation (see description of how this may occur in Fig. 1 in Ramler et al. 2014). Another, not mutually exclusive possibility, is that despite both treatments being sampled after the same number of degree-days (a proxy for growth opportunity; Neuheimer and Taggart 2007) had elapsed, individual responses in the two temperature groups were partly the result of a size effect. For an unknown reason, in our experiment, growth opportunity and the actual realised body growth were uncoupled. Contrary to the predictions of Neuheimer and Taggart (2007), the effects of time and temperature are thus not necessary equal in the promotion of body size and anatomical structures (e.g. heterochrony and allometry). Yet, the metabolic theory of ecology predicts that smaller body size could result when individuals are exposed to higher temperature (Clarke 2006; Riemer et al. 2018). Consequently, if variance is cumulative with the increase of size (Gillooly et al. 2002), this could partly explain the discrepancies between our predictions and findings because ambient temperature treatment individuals were generally larger by the end of the experiment.
Broader implications
Environmentally mediated variation in phenotypic expression is a fundamental attribute of many organisms, which can result in both short- and long-term ecological evolutionary consequences for species that display plasticity. The disjunctive nature of the freshwater environment that lacustrine Arctic charr occupy means that, in the absence of migratory routes for range adjustment, this species particularly will need to cope to abrupt changing novel conditions (Collins et al. 2013; O’Dea et al. 2016). High levels of plasticity are one way in which they might do this. Yet, the role of phenotypic plasticity as a potential mitigator of climate change is contentious, with both positive and negative effects possible. We demonstrate that the expressed phenotypic mean in Arctic charr can respond to an environmental cue rapidly and with a magnitude that is highly likely to have functional consequences (i.e. temperature-mediated shift from a pelagic-like towards a benthic-like phenotype). In the context that even subtle differences in individual’s morphology can influence feeding efficiency and specialisation (Garduño-Paz and Adams 2010), the potential of a fitness-buffering effect of plasticity can easily fluctuate, depending if environmental cues and selective filters become decoupled or not (O’Dea et al. 2016; Reed et al. 2010). Consequently, important questions arise when extrapolating our results into the wild. Would such plastic phenotypic change result in an “overshoot” of the optimum phenotype (e.g. would such processes produce phenotype-environment mismatches) or would it buffer against rapid fluctuations in the environment?
While a plasticity response occurs within a single cohort, another important question at hand is what would be the consequences to subsequent generations (e.g. transgenerational plasticity effects)? The high magnitude of the short temporal environmental oscillations, for instance extreme environmental events, can induce a greater set of phenotypic responses than a small or slower change (e.g. our tested 4 °C variation scenario; Donelson et al. 2018). More gradual or stepwise change across generations have the potential to produce different phenotypic results compared to a single large change within a cohort (e.g. reproductive capacity of individuals subjected to a 3 °C elevated temperature within a generation compared with a 1.5 °C elevation over two generations; Donelson et al. 2016). In reality, the probability of occurrence of alternative effects of phenotypic plasticity (positive or negative) on responses to temperature change is likely to be an interaction between the capacity of the organism for phenotypic plasticity (both within-generation and transgenerational plasticity), the speed of mean change in the environment (e.g. temperature), and the degree of short interval variation in the environment (Donelson et al. 2018; Reed et al. 2010).
The last component emerging from this experiment with evolutionary consequences for a population in the face of changing selection regimes by affecting population dynamics is the phenotypic variance (Chevin et al. 2010; Reed et al. 2010). In this study, we showed a decrease in variation in expressed phenotype at elevated temperature. The decrease in the variation in the phenotypes expressed among individuals raised in the elevated temperature treatment could reduce the range of phenotypes for selection to act upon if this effect occurs in the wild. Accordingly, phenotypic variance has the potential to be a very important component providing a population with scope for recovery from rapid (possibly transient) environmental change. Consequently, a reduction phenotypic variance under climate change scenarios has important consequences at the population level which should not be underestimated as it represents a loss of diversity that could be a major threat for the population resilience.
Conclusion
The ecological and evolutionary consequence of rising temperature on Arctic charr will depend on many factors (e.g. population specific lethal limits or growth optima, interspecific dynamics, and changing disease risk; Crozier and Hutchings 2014). Here, we demonstrate the capacity of Arctic charr for a rapid plastic response to the effect of a severe temperature increases in effect over periods of less than a single year. The parental phenotype mean was shifted within a generation, but also individuals showed a marked decline in the expression of intra-population phenotypic variation. Such variation has been recently shown to have multi-generational effects on survival in another ectotherm the minnow Phoxinus phoxinus (L. 1758) (Raffard et al. 2019). Temperature varies in a stochastic manner resulting in year-to year variation around a coarse-scale temporal trend. Inter-annual temperature variation can modify a cohort’s phenotype in a population, consolidating the role of plasticity in species resilience to rapid environmental change, as genetic changes are not expected to be capable of responding over such short time scales (Merilä and Hendry 2014).
Phenotypic plasticity is thus likely to be particularly important for population persistence in new and changing environments and where the pace of change is relatively rapid (Morris 2014). Contemporary temperature-driven divergence in Nordic freshwater fishes have been reported (Kavanagh et al. 2010), whether this flexible response will be common within and among cold-water species is unknown. Nonetheless, the fact remains that the majority of landlocked Arctic charr populations are currently planktivorous (Maitland and Adams 2018). By confirming the potential for a rapid and marked change in morphology with higher temperature, we provide evidence that an Arctic charr population expressed a more benthic-like ecotype morphology and showed a loss of phenotypic variance under climate change scenarios. A similar effect may also occur in other Arctic charr populations worldwide, salmonids and other cold-water species.
Data availability
Upon acceptance, data will be available in dryad.
References
Ackerly KL, Ward AB (2016) How temperature-induced variation in musculoskeletal anatomy affects escape performance and survival of zebrafish (Danio rerio). J Exp Zool 325(1):25–40. https://doi.org/10.1002/jez.1993
Adams CE, Fraser D, Huntingford FA, Greer RB, Askew CM, Walker AF (1998) Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. J Fish Biol 52(6):1259–1271. https://doi.org/10.1111/j.1095-8649.1998.tb00970.x
Adams CE, Woltering C, Alexander G (2003) Epigenetic regulation of trophic morphology through feeding behaviour in Arctic charr, Salvelinus Alpinus. Biol J Linn Soc 78(1):43–49. https://doi.org/10.1046/j.1095-8312.2003.00126.x
Alley RB et al (2003) Abrupt climate change. Science 299(5615):2005. https://doi.org/10.1126/science.1081056
Angilletta MJ Jr, Steury TD, Sears MW (2004) Temperature, growth rate, and body Size in ectotherms: fitting pieces of a life-history puzzle. Integr Comp Biol 44(6):498–509. https://doi.org/10.1093/icb/44.6.498
Berg MP, Ellers J (2010) Trait plasticity in species interactions: a driving force of community dynamics. Evol Ecol 24(3):617–629. https://doi.org/10.1007/s10682-009-9347-8
Bolnick DI, Lau OL (2008) Predictable patterns of disruptive selection in stickleback in postglacial lakes. Am Nat 172:1–11. https://doi.org/10.1086/587805
Burggren W (2018) Developmental phenotypic plasticity helps bridge stochastic weather events associated with climate change. J Exp Zool 221(9):jeb161984. https://doi.org/10.1242/jeb.161984
Burton T, Metcalfe NB (2014) Can environmental conditions experienced in early life influence future generations? Proc Royal Soc B 281(1785):20140311. https://doi.org/10.1098/rspb.2014.0311
Campbell CS (2019) The effects of climate change on phenotypic plasticity in Arctic charr. University of Glasgow, Glasgow, UK
Campinho MA, Moutou KA, Power DM (2004) Temperature sensitivity of skeletal ontogeny in Oreochromis mossambicus. J Fish Biol 65.4:1003–1025
Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC (2008) Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320(5877):800–803. https://doi.org/10.1126/science.1157174
Chavarie L, Dempson JB, Schwarz C, Reist J, Power G, Power M (2010) Latitudinal variation in growth among Arctic charr in eastern North America: evidence for countergradient variation? Hydrobiologia 650(1):161–177. https://doi.org/10.1007/s10750-009-0043-z
Chavarie L, Adams CE, Swanson HK, Ridgway MS, Tonn WM, Wilson CC (2021) Ecological diversity. In: Muir AM, Krueger CC, Hansen MJ, Riley SC (eds) The Lake Charr Salvelinus namaycush: Biology, Ecology, Distribution, and Management. Springer International Publishing, Cham, pp 69–117
Chevin L-M, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8(4):e1000357. https://doi.org/10.1371/journal.pbio.1000357
Clarke A (2006) Temperature and the metabolic theory of ecology. Funct Ecol 20(2):405–412. https://doi.org/10.1111/j.1365-2435.2006.01109.x
Collins M et al (2013) Long-term climate change: projections, commitments and irreversibility Climate Change 2013-The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, p 1029–1136
Corno G, Jürgens K (2006) Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl Environ Microbiol 72(1):78–86. https://doi.org/10.1128/AEM.72.1.78-86.2006
Crawley MJ (2012) The R book. John Wiley & Sons, Oxford, UK
Crozier LG, Hutchings JA (2014) Plastic and evolutionary responses to climate change in fish. Evol Appl 7(1):68–87. https://doi.org/10.1111/eva.12135
Donelson JM, Wong M, Booth DJ, Munday PL (2016) Transgenerational plasticity of reproduction depends on rate of warming across generations. Evol Appl 9(9):1072–1081. https://doi.org/10.1111/eva.12386
Donelson JM, Salinas S, Munday PL, Shama LNS (2018) Transgenerational plasticity and climate change experiments: where do we go from here? Global Change Biol 24(1):13–34. https://doi.org/10.1111/gcb.13903
Dryden IL, Mardia KV (1998) Statistical shape analysis: Wiley series in probability and statistics. John Wiley and Sons, New York
Duclos KK, Hendrikse JL, Jamniczky HA (2019) Investigating the evolution and development of biological complexity under the framework of epigenetics. Evol Dev 21(5):276–293. https://doi.org/10.1111/ede.12301
Elmer KR (2016) Genomic tools for new insights to variation, adaptation, and evolution in the salmonid fishes: a perspective for charr. Hydrobiologia. 191–208. https://doi.org/10.1007/s10750-015-2614-5
Frankino WA, Zwaan BJ, Stern DL, Brakefield PM (2005) Natural selection and developmental constraints in the evolution of allometries. Science 307(5710):718–720. https://doi.org/10.1126/science.1105409
Franklin OD, Skúlason S, Morrissey MB, Ferguson MM (2018) Natural selection for body shape in resource polymorphic Icelandic Arctic charr. J Evol Biol 31(10):1498–1512. https://doi.org/10.1111/jeb.13346
Garduño-Paz MV, Adams CE (2010) Discrete prey availability promotes foraging segregation and early divergence in Arctic charr, Salvelinus alpinus. Hydrobiologia 650(1):15–26. https://doi.org/10.1007/s10750-009-0055-8
Georga I, Koumoundouros G (2010) Thermally induced plasticity of body shape in adult zebrafish Danio rerio (Hamilton, 1822). J Morphol 271(11):1319–1327. https://doi.org/10.1002/jmor.10874
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21(3):394–407. https://doi.org/10.1111/j.1365-2435.2007.01283.x
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251. https://doi.org/10.1126/science.1061967
Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH (2002) Effects of size and temperature on developmental time. Nature 417(6884):70–73. https://doi.org/10.1038/417070a
Heino J, Virkkala R, Toivonen H (2009) Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions. Biol Rev 84(1):39–54. https://doi.org/10.1111/j.1469-185X.2008.00060.x
Heino M (2014) Quantitative traits In: Stock identification methods. Academic Press, p 59–76
Hooker OE et al (2016) Morphological, ecological and behavioural differentiation of sympatric profundal and pelagic Arctic charr (Salvelinus alpinus) in Loch Dughaill Scotland. Hydrobiologia 783(1):209–221. https://doi.org/10.1007/s10750-015-2599-0
Imsland AK, Foss A, Folkvord A, Stefansson SO, Jonassen TM (2005) The interrelation between temperature regimes and fish size in juvenile Atlantic cod (Gadus morhua): effects on growth and feed conversion efficiency. Fish Physiol Biochem 31(4):347–361. https://doi.org/10.1007/s10695-005-4244-8
Jacobson B, Grant JWA, Peres-Neto PR (2015) The interaction between the spatial distribution of resource patches and population density: consequences for intraspecific growth and morphology. J Anim Ecol 84(4):934–942. https://doi.org/10.1111/1365-2656.12365
Janhunen M, Piironen J, Peuhkuri N (2010) Parental effects on embryonic viability and growth in Arctic charr Salvelinus alpinus at two incubation temperatures. J Fish Biol 76(10):2558–2570. https://doi.org/10.1111/j.1095-8649.2010.02648.x
Jeuthe H, Brännäs E, Nilsson J (2016) Effects of variable egg incubation temperatures on the embryonic development in Arctic charr Salvelinus alpinus. Aquac Res 47(12):3753–3764. https://doi.org/10.1111/are.12825
Kavanagh KD, Haugen TO, Gregersen F, Jernvall J, Vøllestad LA (2010) Contemporary temperature-driven divergence in a Nordic freshwater fish under conditions commonly thought to hinder adaptation. BMC Evol Biol 10(1):350. https://doi.org/10.1186/1471-2148-10-350
Kingsolver JG, Izem R, Ragland GJ (2004) Plasticity of size and growth in fluctuating thermal environments: comparing reaction norms and performance curves. Integr Comp Biol 44(6):450–460. https://doi.org/10.1093/icb/44.6.450
Klemetsen A (2010) The charr problem revisited: exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshw Rev 3:49–74. https://doi.org/10.1608/frj-3.1.3
Klemetsen A (2013) The most variable vertebrate on earth. J Ichthyol 53(10):781–791. https://doi.org/10.1134/s0032945213100044
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11(2):353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
Klingenberg CP, McIntyre GS (1998) Geometric morphometrics of develpmental instability: analyzing patterns of fluctuating asymetry with procrustes methods. Evolution 52(5):1363–1375. https://doi.org/10.1111/j.1558-5646.1998.tb02018.x
Lazić MM, Carretero MA, Crnobrnja-Isailović J, Kaliontzopoulou A (2015) Effects of environmental disturbance on phenotypic variation: an integrated assessment of canalization, developmental stability, modularity, and allometry in lizard head shape. Am Nat 185(1):44–58. https://doi.org/10.1086/679011
Lubchenco J, Cubit J (1980) Heteromorphic life histories of certain marine algae as adaptations to variations in herbivory. Ecology 61(3):676–687. https://doi.org/10.2307/1937433
Maitland PS, Adams CE (2018) Arctic charr in the Llochs of Scotland: an assessment of distribution and status. Fast-Print Publishing, Peterborough, UK
McPhee MV, Noakes DL, Allendorf FW (2012) Developmental rate: a unifying mechanism for sympatric divergence in postglacial fishes? Curr Zool 58(1):21–34
Merilä J, Hendry AP (2014) Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol Appl 7(1):1–14. https://doi.org/10.1111/eva.12137
Mitteroecker P (2009) The developmental basis of variational modularity: insights from quantitative genetics, morphometrics, and developmental biology. Evol Biol 36(4):377–385. https://doi.org/10.1007/s11692-009-9075-6
Morris MR (2014) Plasticity-mediated persistence in new and changing environments. Int J Evol Biol 2014:1–18. https://doi.org/10.1155/2014/416497
Neuheimer AB, Taggart CT (2007) The growing degree-day and fish size-at-age: the overlooked metric. Can J Fish Aquat Sci 64(2):375–385. https://doi.org/10.1139/f07-003
Neuheimer AB, Thresher RE, Lyle JM, Semmens JM (2011) Tolerance limit for fish growth exceeded by warming waters. Nat Clim Change 1(2):110–113. https://doi.org/10.1038/nclimate1084
Noble DW, Stenhouse V, Schwanz LE (2018) Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biol Rev 93(1):72–97. https://doi.org/10.1111/brv.12333
Nosil P (2012) Ecological speciation. Oxford University Press, Oxford, UK
O’Dea RE, Noble DW, Johnson SL, Hesselson D, Nakagawa S (2016) The role of non-genetic inheritance in evolutionary rescue: epigenetic buffering, heritable bet hedging and epigenetic traps. Environ Epigenet 2(1):1–12. https://doi.org/10.1093/eep/dvv014
O’Dea RE, Lagisz M, Hendry AP, Nakagawa S (2019) Developmental temperature affects phenotypic means and variability: a meta-analysis of fish data. Fish Fish 20(5):1005–1022. https://doi.org/10.1111/faf.12394
Olsson J, Svanbäck R, Eklöv P (2006) Growth rate constrain morphological divergence when driven by competition. Oikos 115(1):15–22. https://doi.org/10.1111/j.2006.0030-1299.14965.x
Orizaola G, Laurila A (2008) Microgeographic variation in temperature-induced plasticity in an isolated amphibian metapopulation. Evol Ecol 23(6):979–991. https://doi.org/10.1007/s10682-008-9285-x
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37(1):637–669. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Parsons KJ, Skúlason S, Ferguson M (2010) Morphological variation over ontogeny and environments in resource polymorphic arctic charr (Salvelinus alpinus). Evol Dev 12(3):246–257. https://doi.org/10.1111/j.1525-142X.2010.00410.x
Pigliucci M, Preston K (2004) Phenotypic integration: studying the ecology and evolution of complex phenotypes. Oxford University Press, Oxford, UK
Poesch MS, Chavarie L, Chu C, Pandit SN, Tonn W (2016) Climate change impacts on freshwater fishes: a canadian perspective. Fisheries 41(7):385–391. https://doi.org/10.1080/03632415.2016.1180285
Raffard A, Cucherousset J, Santoul F, Di Gesu L, Blanchet S (2019) Intraspecific variation alters ecosystem and next-generation performance as much as temperature. bioRxiv:332619. https://doi.org/10.1101/332619
Ramler D, Mitteroecker P, Shama LNS, Wegner KM, Ahnelt H (2014) Nonlinear effects of temperature on body form and developmental canalization in the threespine stickleback. J Evol Biol 27(3):497–507. https://doi.org/10.1111/jeb.12311
R Core Team (2014) R: A Language and Environment for Statistical Computing http://www.R-project.org. Accessed 31 Oct 2022
Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT (2010) Phenotypic plasticity and population viability: the importance of environmental predictability. Proc Royal Soc B 277(1699):3391–3400. https://doi.org/10.1098/rspb.2010.0771
Riemer K, Anderson-Teixeira KJ, Smith FA, Harris DJ, Ernest SKM (2018) Body size shifts influence effects of increasing temperatures on ectotherm metabolism. Glob Ecol Biogeogr 27(8):958–967. https://doi.org/10.1111/geb.12757
Robinson BW, Parsons KJ (2002) Changing times, spaces, and faces: tests and implications of adaptive morphological plasticity in the fishes of northern postglacial lakes. Can J Fish Aquat Sci 59(11):1819–1833. https://doi.org/10.1139/f02-144
Rohlf FJ (2006a) tpsDig, version 2.10. http://life.bio.sunysb.edu/morph/index.html
Rohlf FJ (2006b) tpsUtil, version 1.38. http://life.bio.sunysb.edu/morph/index.html
Scheepens JF, Deng Y, Bossdorf O (2018) Phenotypic plasticity in response to temperature fluctuations is genetically variable, and relates to climatic variability of origin, in Arabidopsis thaliana. AoBP 10(4). https://doi.org/10.1093/aobpla/ply043
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford, UK
Schoener TW (2011) The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331(6016):426–429. https://doi.org/10.1126/science.1193954
Sfakianakis DG, Koumoundouros G, Divanach P, Kentouri M (2004) Osteological development of the vertebral column and of the fins in Pagellus erythrinus (L. 1758). Temperature effect on the developmental plasticity and morpho-anatomical abnormalities. Aquaculture 232(1–4):407–424
Simonsen MK, Siwertsson A, Adams CE, Amundsen P-A, Præbel K, Knudsen R (2017) Allometric trajectories of body and head morphology in three sympatric Arctic charr (Salvelinus alpinus (L.)) morphs. Ecol Evol 7(18):7277–7289. https://doi.org/10.1002/ece3.3224
Skúlason S et al (2019) A way forward with eco evo devo: an extended theory of resource polymorphism with postglacial fishes as model systems. Biol Rev 94:1786–1808. https://doi.org/10.1111/brv.12534
Skulason S, Smith TB (1995) Resource polymorphisms in vertebrates. Trends Ecol Evol 10:366–370
Solomon S (2007) Climate change 2007-the physical science basis: working group I contribution to the fourth assessment report of the IPCC, vol 4. Cambridge University Press, Cambridge, UK
Taylor EB (1999) Species pairs of north temperate freshwater fishes: evolution, taxonomy, and conservation. Rev Fish Biol Fish 9(4):299–324. https://doi.org/10.1023/A:1008955229420
van der Have TM, de Jong G (1996) Adult size in ectotherms: temperature effects on growth and differentiation. J Theor Biol 183(3):329–340. https://doi.org/10.1006/jtbi.1996.0224
Vasseur DA et al (2014) Increased temperature variation poses a greater risk to species than climate warming. Proc Royal Soc B 281(1779):1–8. https://doi.org/10.1098/rspb.2013.2612
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford, UK
Westneat DF, Wright J, Dingemanse NJ (2015) The biology hidden inside residual within-individual phenotypic variation. Biol Rev 90(3):729–743. https://doi.org/10.1111/brv.12131
Whitman DW, Agrawal AA (2009) What is phenotypic plasticity and why is it important. CRC Press, Boca Raton
Woelders L, Lenaerts JTM, Hagemans K, Akkerman K, van Hoof TB, Hoek WZ (2018) Recent climate warming drives ecological change in a remote high-Arctic lake. Sci Rep 8(1):1–8. https://doi.org/10.1038/s41598-018-25148-7
Wu C-I, Ting C-T (2004) Genes and speciation. Nat Rev Genet 5(2):114–122. https://doi.org/10.1038/nrg1269
Acknowledgements
The authors would like to acknowledge Travis van Leeuwen, Luc Bussierre, Alex Lyle, Jennifer Dodd, Martin Hughes, Peter Cunningham (Wester Ross Fisheries Trust) and The Coulin Estate where fish were collected.
Funding
Open access funding provided by Norwegian University of Life Sciences
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was conducted in accordance with UK legislation under Home Office Licence Number: PPL 70/8794.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hooker, O.E., Adams, C.E. & Chavarie, L. Arctic charr phenotypic responses to abrupt generational scale temperature change: an insight into how cold-water fish could respond to extreme climatic events. Environ Biol Fish 106, 909–922 (2023). https://doi.org/10.1007/s10641-022-01363-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01363-0