Abstract
Phenotypic variation in populations of fishes that inhabit postglacial lakes is often associated with trophic specialisations. A common sympatric foraging divergence seen in Arctic charr is into either plankton or littoral-zoobenthos feeding specialisms. In this study, we report a sympatric polymorphic Arctic charr population which is not centred on this divergence but instead manifests as a plankton (pelagic)—profundal zoobenthos foraging specialisms. The head shape of profundal fish was round and robust, the body thick set and pectoral fins long and wide. In contrast, the head of pelagic fish was pointed and slender, the body fusiform in shape and with short, narrow pectoral fins. There was no difference between profundal and pelagic fish in gill raker number. Body lipid content was significantly higher in pelagic fish as were the number or Diphyllobothrium cysts. The carbon isotope ratio was more heavily depleted in profundal fish. There was no dietary overlap in the prey items recovered from stomach contents of profundal and pelagic fish. We suggest the proximate driver behind the sympatric divergence was the successful exploitation of the profundal zone. The consequences of this have led to the development of adaptations in morphology and behaviour to support and maintain this divergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In some taxonomic groups, intraspecific genetic and phenotypic structuring within a population is common (Skúlason & Smith, 1995; Smith & Skúlason, 1996). This is particularly true for fishes in postglacial lakes (Taylor & McPhail, 1999; Jonsson & Jonsson, 2001; Østbye et al., 2006) and results in alternative phenotypes living in sympatry within a single lake (Knudsen et al., 2006). This is seen in Arctic charr, Salvelinus alpinus (Linnaeus 1758) in which the structuring is based on the adaptation of foraging specialisms to alternative food resources (Malmquist et al., 1992; Adams et al., 1998; Amundsen et al., 2008; Garduño-Paz et al., 2010). Referred to as resource polymorphisms, they are frequently identified by the expression of different morphological phenotypes, foraging ecology and differences in diet (Smith & Skúlason, 1996).
Arctic charr exhibit phenotypic variability in head and body morphology (Skúlason et al., 1989; Adams et al., 1998, 2003; Jonsson & Jonsson, 2001; Adams & Huntingford, 2002; Klemetsen et al., 2003), differences in growth (Jonsson et al., 1988; Adams et al., 1998), reproduction (Jonsson & Hindar, 1982; Jonsson et al., 1988; Klemetsen et al., 2003; Corrigan et al., 2011; Garduño-Paz et al., 2012), habitat use (Hindar & Jonsson, 1982; Jonsson et al., 1988; Klemetsen et al., 2003) and behaviour (Jonsson & Jonsson, 2001; Klemetsen et al., 2003). Arctic charr sometimes exhibit clearly defined, discrete and alternative phenotypes, each adopting a different foraging specialism while living in sympatry, e.g. Lake Thingvallavatn (Iceland) (Malmquist et al., 1992), whereas elsewhere the difference in phenotype may be more subtle, e.g. Loch Tay (Scotland) (Adams et al., 2003; Garduño-Paz et al., 2010). The most commonly reported foraging divergence seen in sympatric populations of Arctic charr is that of a divergence into planktonic and littoral-zoobenthos feeding (Malmquist et al., 1992; Adams et al., 1998, 2003; Adams & Huntingford, 2002; Amundsen et al., 2008, Corrigan et al., 2011; Garduño-Paz et al., 2012).
Parallelism in body shape associated with prey specialisation and associated habitat use (Malmquist et al., 1992; Adams et al., 1998; Jonsson & Jonsson, 2001; Klemetsen et al., 2003; Knudsen et al., 2006) is almost exclusively seen as either adaptations to planktonic or benthic foraging. Functional adaptations to feeding on planktonic prey in the pelagic zone results in a more streamlined body with a narrow, more pointed and delicate head and mouth structure, often with dorso-ventral countershading. Benthic foraging adaptations in the littoral or sub-littoral zones, often in deeper waters, result in thicker set bodies with more robust deeper heads that aid consumption of larger macro invertebrates (Skúlason et al., 1989; Malmquist et al., 1992; Adams et al., 1998, 2003; Jonsson & Jonsson, 2001; Klemetsen et al., 2003; Knudsen et al., 2006; Garduño-Paz et al., 2012).
When sympatric populations occur, they provide models which help to elucidate the mechanisms that lie behind ecologically driven divergence and speciation (West-Eberhard, 1989; Bolnick & Fitzpatrick, 2007). Understanding the interaction between genetic, morphological, ecological, physiological and behavioural drivers that can be observed in sympatric polymorphisms increases our ability to understand some of the causes and effects of divergence and thus the speciation process when it occurs in sympatry. In this study, we report a previously undescribed and rare sympatric polymorphism in an Arctic charr population which is not centred on the usual divergence into planktivorous and littoral-zoobenthos foraging specialisms. We examine variation in the foraging ecology of individuals, relate this to head and body morphology and quantify the effect of different foraging specialisms (supported by stable isotope and stomach content analysis) on body lipid content, habitat use and parasite loadings.
Materials and methods
Arctic charr were collected from Loch Dughaill, Strathcarron, Highland, Scotland (Lat 57.47°N–Long 05.34°W). Loch Dughaill has a surface area of 1.15 km2, a mean depth of 20 m and max depth of 62 m and a total volume of 10−6 m3. The littoral zone constitutes 27.3% of the surface area. It is situated at 24 m above sea level, receives no ice cover during the winter months and is oligotrophic. In addition to Arctic charr, the fish community includes brown trout, Salmo trutta (Linnaeus 1758), Atlantic salmon, Salmo salar (Linnaeus 1758), European eel, Anguilla anguilla (Linnaeus 1758), flounder, Platichthys flesus (Linnaeus 1758), three-spine stickleback, Gasterosteus aculeatus (Linnaeus 1758) and European minnow, Phoxinus phoxinus (Linnaeus 1758). Arctic charr were sampled using Nordic multipanel gill nets, consisting of 12 panels each measuring 2.5 m long and ranging from 5 to 55 mm knot-to-knot mesh. These nets select impartially across size classes in the size range of 45–495 mm fork length in salmonids (Jensen & Hesthagen, 1996). Benthic nets measuring 30 m × 1.5 m (depth) were set overnight on the bed of the lake at depths ranging from 5 to 60 m. Pelagic nets measuring 30 m × 6 m (depth) were set overnight at the water surface over water depths ranging from 18 to 55 m. For benthic set nets, the depth of each end of the net was measured by a hand-held sonar. The capture depth of each fish was estimated by interpolation of the depth of each net panel in which it was caught.
A total of 57 fish were sampled in October 2013 and a further 42 in June 2014. During the June sampling period, 30 of the 42 fish were released alive (as part of an acoustic telemetry study) with only non-lethal data collected on their morphology and ecology. For information on sample period and size for each ecological variable tested, please refer to Table 1.
All 99 fish were photographed, measured (fork length ± 1 mm) and weighed (±1 g) after which 69 of the total fish sampled were dissected.
Whole-body tissue lipid content was measured on 30 live individuals using a Distell FM 692 fat meter. This meter is pre-calibrated (factory calibration) to the fat–water relationship specific to Arctic charr. The Distell fat meter has a microstrip sensor which can measure the water content of a sample. The fat content of fish is correlated with the water content and thus the measurement of one can determine the other if the relationship between the two is known. Only live individuals were used due to the method in which the fat content is calculated. A mean was determined from four measurements, one taken on the anterior lateral surface of the body and one on the posterior lateral surface on both sides of the fish.
Lateral view photographs of fish were taken on a scale using a Canon EOS 350D digital camera to enable geometric morphometric analysis of shape for all of the 99 fish sampled. Twenty analogous landmarks (Fig. 1a) were digitised in two dimensions using the software tpsDig (Rohlf, 2006a) and tpsUtil (Rohlf, 2006b). Landmarks were carefully chosen to clearly represent both head and body shape (Fig. 1a). Procrustes superimposition was then used to remove unwanted variation created by size, position and orientation (Rohlf & Slice, 1989; Mitteroecker & Gunz, 2009).
Shape change associated with size (ontogenetic allometry) was removed (size corrected) by deriving residuals from a multivariate, pooled within-group regression of the Procrustes coordinates on the log centroid size (a robust measure of fish size) (Klingenberg, 1998). Principal Component Analysis (PCA) of these residuals was performed to explore shape differences between groups. Principal Component 2 (PC2) was dominated by unwanted non-biological lunate distortion. This type of artefact from the image collection process is frequently reported in studies that involve fish and is caused by rigor mortis of the body muscles (Siwertsson et al., 2013). This shape artefact was removed by using the residuals from a regression of the raw Procrustes coordinates on PC2. This creates a new set of Procrustes coordinates which are independent of PC2 and thus free of any shape variation associated with the lunate bending effect. Although the loss of some variation from other parts of the anatomy can occur using this method, examination showed that landmark position not associated with bending in PC2 was minimal and thus removal of bending effects did not interfere with the overall results.
Discriminant function analysis (1000 permutations) was used to test for and quantify the shape difference between fish groups (measured as Procrustes and Mahalanobis distance). Fish were assigned to one of two working class groups using data collected on their ecology; the approach used is described later in the methods. All morphometric analyses were carried out using the software MorphoJ v.1.06d (Klingenberg, 2011).
Dorsal view photographs of 20 pectoral fins from the left side were taken to compare the fin shape between fish. The pectoral fin was removed, fanned out and mounted on foam using pins and a scale reference added. Three landmarks were identified (Fig. 1b), the upper most point at the base of the fin, the tip of the longest fin ray at the leading edge of the fin, and the tip of the longest ray towards the back of the fin. Damaged fins were not used. Fin shape was then analysed as described above.
The intensity of infection by Diphyllobothrium sp. (larvae), a parasitic cestode, was determined for 69 fish prior to dissecting stomachs by counting the number of Diphyllobothrium cysts attached to the stomach, gut and internal walls of the body cavity. Diphyllobothrium cysts are easily identifiable as opaque white nodules usually attached to the gut and swim bladder as well as other organs.
The stomachs of 69 Arctic charr were dissected of which only 34 contained prey items. These were preserved in 70% ethanol and the contents later identified to family and where possible, species level. Stomach contents were then dried at 48°C for 48 h in a drying oven to calculate relative and total prey dry weight.
Approximately 1 cm2 of white muscle tissue was removed by dissection from the lateral muscle below the posterior edge of the dorsal fin and above the lateral line for stable isotope analysis for 69 fish. Tissue samples were initially frozen at −20°C then later thawed and the epidermal layer was removed. White muscle tissue was then dried at 48°C for 96 h and ground to a fine powder using a pestle and mortar. 0.7 mg (± 0.1 mg) subsamples were loaded into 5 × 5 mm tin capsules, ready for stable isotope analysis. Samples were analysed for δ15N and δ13C, at the Natural Environment Research Council Life Sciences Mass Spectrometry Facility, East Kilbride, via continuous flow isotope ratio mass spectrometry (CF-IRMS). This system employs an Elementar Pyrocube elemental analyser interfaced with a Delta XP IRMS. The standard deviation of multiple analyses of the internal gelatine standard in each experiment was ~0.1‰ for both δ 15N and δ13C.
The first gill arch from the left side of 40 fish was removed by dissection and the total number of gill rakers counted using a Brunel MONEX series AR Microscope illuminated with a EUROMEX LE 5210 external cold light source.
Morphological data (represented by the shape change associated with PC1 scores from the geometric morphometric analysis (hereafter, PC-morphology)) were combined with parasite and stable isotope data in a Principal Component Analysis (PCA) to look for putative discrete groupings of Arctic charr from Loch Dughaill. PC-morphology scores were positive and large for fish with a long, more pointed snout and a fusiform body. This shape is one indicative of charr specialising in plankton feeding that inhabit the pelagic zone (Skúlason et al., 1989; Adams et al., 1998). The intermediate host of the trophically transmitted Diphyllobothrium parasite is a planktonic copepod (Knudsen et al., 1996); thus, a high parasite loading is indicative of planktivorous fish in the pelagic zone. δ13C provides an indication of the ultimate carbon sources contributing to tissue formation. A high δ13C (relatively low δ13C content) in white muscle tissue is characteristic of fish that feed on organisms of a higher trophic position such zooplankton (Vander Zanden & Rasmussen, 1999). Principal Component 1 of the PC-morphology weighted, parasite loading and delta δ13C in the same direction (but negatively); thus, fish with a highly negative score indicated fish with a strong affinity to planktonic feeding.
A second PCA was used to combine morphological (PC-morphology) and capture depth data for fish that were returned alive and thus for which there are no parasite or stable isotope data. As before, the scores for morphology PC1 (taken from the same PC-morphology used above) were positive and large for planktonic feeding fish. Capture depth [measured as negative deviations from the surface (which = 0)] was also more positive (less negative) for fish that inhabit the surface of the water column (pelagic zone), typical of plankton feeding specialists. Both variables loaded in the same direction for this PC1 (but negatively) with individuals yielding highly negative scores indicative of fish with a plankton feeding-like morphology and inhabiting the pelagic zone.
PC scores from each of these two PCA’s (the full PCA and the PCA constrained to only non-destructive data) were used to define putative ecomorph groups which were then used as a factor, with body length as a covariate, in a linear model to explore a number of between group differences. For lipid content, mass was used as a covariate. Each comparison initially included a two-way interaction between factors and covariates. Comparisons that included an interaction between factors and covariates were subject to model simplification with the removal of non-significant interactions (P = < 0.05) (Crawley, 2007). Covariates were dropped in all models due to non-significance. Model diagnostics were assessed graphically by examining the residuals for heterogeneity. All analyses were conducted using R (R Development Core Team, 2011).
Results
Principal Component Analysis (PCA) of body and head shape (represented by PC1-morphology from the geometric morphometric analysis), Diphyllobothrium infection rate and δ13C stable isotope signature were thus carried out on 69 fish. PC1 explained 56% of the variation in these variables. PC1 coefficients indicate strong negative loadings for body and head shape −0.645, Diphyllobothrium infestation rate −0.426 and δ13C stable isotope signature −0.635. On the basis of the distribution of these data, 38 individuals were given a working classification as belonging to planktonic feeding specialist group (negative PC1 score) and 31 as belonging to another feeding group (positive PC1 score) (Fig. 2a).
Distribution of individual Arctic charr assigned to either plankton feeding (pelagic) or non-plankton feeding (profundal) working classes using principal component analysis of ecological variables. Fish were assigned to bins of 0.3 intervals for lethal (a) and 0.4 for non-lethal (b) variables to aid visualisation. Individuals assigned to a plankton feeding working class are shown in white and non-plankton feeding are shown in grey. Grey dashed line indicates 0
In the PCA of non-lethal data, body and head shape (as described above) and net capture depth on 30 fish, PC1 explained 91% of the variation. PC1 coefficients indicate negative loadings for body and head shape −0.701 and capture depth −0.426. Again based on these variables, the results of the PCA found negative PC1 scores to be indicative of a planktonic feeding fish and positive PC scores indicative of fish feeding on an alternative food source. On the basis of this, an additional 15 individuals were given a working classification as planktonic feeding specialists (now referred to as pelagic fish) (negative PC1 score) and 15 as belonging to another feeding group (positive PC1 score) (Fig. 2b).
During June, the mean capture depth for the pelagic fish was significantly shallower (−2 m ± 0 SE) than the other group (−45.9 m ± 1.76 SE). This group was clearly occupying the profundal zone (now referred to as profundal fish) (t = 25.1841, 41; P = < 0.0001) (Fig. 3). However, both pelagic fish (−5.4 m ± 0.68 SE) and profundal fish (−7.6 m ± 0.9 SE) occupied shallow water during the sampling period in October (Fig. 3), although capture depth was still statistically different (t = 2.6561, 56; P = 0.0103).
Profundal fish were on average slightly larger but this was not statistically significant for length (t = 0.7321, 98; P = 0.491) or weight (t = 0.6911, 98; P = 0.557). Profundal fish length ranged from 157 to 277 mm (213.45 ± 4.02 SE) and 44–247 g (114.76 ± 5.85 SE) in weight, and pelagic fish length ranged from 125 to 287 mm (203.06 ± 5.22 SE) and 19–251 g (101.7 ± 7.01 SE) in weight. There was also no statistical difference in the relationship between length and weight for profundal fish compared with pelagic fish (t = 0.1362, 97; P = < 0.892).
Whole-body lipid content as a percentage of body mass (fat content) of pelagic fish (6.10% ± 0.43 SE) was significantly higher than that of the profundal fish (3.03% ± 0.12 SE) (t = 6.8551, 29; P = < 0.0001).
Average morphology was highly significantly different in a Discriminant Function analysis between profundal fish (N = 46) and pelagic fish (N = 53) (Procrustes distance 0.0296, P = < 0.0001, Mahalanobis distance 3.6411, P = < 0.0001) (Fig. 4). Landmarks that showed the most variation between groups was associated with pectoral fin length, which was longer and body depth, which was deeper, in profundal fish. Pelagic fish were more fusiform and their head were more delicate and snouts more pointed. Profundal fish in contrast were more thick set in their body and their head shape more rounded and robust (Fig. 4). Geometric morphometric analysis of pectoral fin shape of 10 individuals from each group also showed significant differences between them (Procrustes distance 0.0981, P = < 0.0001, Mahalanobis distance 2.2123, P = < 0.0010) with fins of profundal fish being wider relative to fin length than the pelagic fish.
Distribution of pelagic (white) and profundal (grey) Arctic charr from the discriminant function analysis on body shape. Wireframes represent the shape at the outer most point of each distribution (both profundal and pelagic scaled at −15 and +15, respectively). Below are images of pelagic (left) and profundal (right) Arctic charr from Loch Dughaill
Mean Diphyllobothrium cyst count was significantly lower in profundal fish (0.22 ± 0.1SE) than the pelagic fish (43.84 ± 5.93 SE) (t = −5.0731, 68; P = <0.0001).
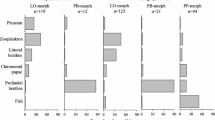
Prey items found in the stomachs of pelagic fish comprised only of Leptodora kindtii, a pelagic cladoceran. Stomachs of profundal fish comprised Pisidium sp. and Chironomid sp., both of which are known to be deep-water benthic organisms. There was no overlap in stomach contents between the two groups (Fig. 5).
Stable isotope analysis of white muscle showed profundal fish to have a significantly lower δ13C value (δ13C −29.38 ± 0.11 SE) than pelagic fish (δ15C −28.76 ± 0.09 SE) (t = −6.3881, 68; P = < 0.0001). No difference was found between the δ15N values of profundal fish (δ15N 7.49 ± 0.07 SE) and pelagic fish (δ15N 7.47 ± 0.11 SE) (t = 0.8821, 68; P = 0.381).
The number of gill rakers of profundal fish (17.9 ± 0.28 SE) and pelagic fish (18.2 ± 0.6 SE) was not significantly different (t = −0.4551, 39; P = 0.652).
Discussion
This is the first description of sympatric profundal and pelagic habitat Arctic charr specialists in Scotland. This differential habitat use in sympatry has been documented relatively infrequently (we can find only 13 records previously) in the accessible literature, of which only eight populations still persist (Table 2). The comparisons of morphology, ecology and behaviour between profundal and pelagic fish reported here support the hypothesis that the two populations of Arctic charr in Loch Dughaill have become isolated through the utilisation of two contrasting trophic niches. Differences in morphology, size and colouration (Hesthagen et al., 1995; Alekseyev & Pichugin, 1998; Knudsen et al., 2006; Soreide et al., 2006) and often temporal and spatial isolation in spawning behaviour (Klemetsen, 2010) are known to maintain genetic isolation in sympatric populations. However, direct interactions between morphs are less likely compared to other littoral-zoobenthos—pelagic sympatric populations as their habitats are more separated.
Differences in morphology can solely arise through the effect of plasticity; however, the differences seen in Loch Dughaill would appear too extreme (Fig. 4) to be explained by plasticity alone and thus we speculate in the absence of any specific data, that at least some of the morphological characteristics are genetic in origin. The profundal and pelagic Arctic charr in Loch Dughaill show many of the parallelisms shared with other polymorphic lakes systems that support pelagic and littoral-benthic foraging specialists of Arctic charr (Alekseyev & Pichugin, 1998; Adams et al., 1998, 2003; Klemetsen et al., 2002) as well as brown trout (Ferguson & Mason, 1981), whitefish (Amundsen et al., 2004; Harrod et al., 2010; Siwertsson et al., 2013) and sticklebacks (McPhail, 1984). The profundal fish had much shorter, round and robust heads and large sub-terminal mouths (Fig. 4) which are suited to foraging from the substrate (Fugi et al. 2001), a feeding behaviour characteristic of feeding on Pisidium, the main component of their diet (Fig. 5). The bodies of profundal fish were much deeper and cryptic in colour (Fig. 4), often seen in fish that inhabit deeper water (Jonsson & Jonsson, 2001; Klemetsen, 2010). Pelagic fish had more pointed, delicate heads in comparison (Fig. 4), again which is suited to catching smaller pelagic prey items (Adams & Huntingford, 2002) such as Leptodora kindtii, the only prey item found in the stomachs of pelagic fish (Fig. 5). Pelagic fish bodies were more streamlined in appearance (Fig. 4) (and it was noted during dissection that pelagic fish had noticeably thicker, tougher more muscular body walls than that of profundal fish which were in contrast extremely thin and required little force to make an incision) supporting the need for increased swimming activity associated with foraging in open water. The ventral side of pelagic fish had pale to dark red pigmentation in contrast to the profundal fish which had pale skin with some grey shading. Pectoral fins were much larger in profundal fish compared to the pelagic fish. Large fan like pectoral fins are characteristics of many benthic feeding fish that have to manoeuvre and orientate with accuracy in order to find, capture and manipulate prey. The narrower pectoral fins of the pelagic fish are more suitable in structure for greater swimming efficiency (Walker & Westneat, 2002). These are repeatedly reported features of benthic and pelagic feeding specialists in Arctic charr (Klemetsen, 2010).
The depth at which individuals were captured can be used to make inferences about habitat use. There was no overlap in the depth use for either morphs during summer (June). Only benthic set nets (set between 35 and 60 m) caught profundal fish and pelagic nets set at the surface (0–6 m) caught only pelagic fish, indicating very strong spatial depth segregation (Fig. 3). The overlap in depth use in October is probably a change in behaviour and habitat use associated with spawning. These data presented here indicate that at spawning time, spatial depth segregation between these forms is eroded and it is probable that there is a temporal overlap in spawning of both Arctic charr ecomorphs in Loch Dughaill. Locational data from the nets that caught fertile male and female fish of both morphs would suggest that spawning in both morphs is likely to take place in waters approximately 15–20 m deep towards the north of the lake and at a similar time as both fecund male and female fish of both morphs were caught in the same nets. However, this is purely speculative. If genetic isolation between the morphs persists, it would most likely be maintained by two factors. Either spatially, with each morph spawning in a different location, or through positive assortative mating, with each morph showing a preference to spawn with conspecifics. Genetic data will confirm if the morphs are genetically isolated and positional information from an ongoing telemetry study will give more insight as to what degree their spawning habits overlaps both temporally, as well as spatially. This will allow a more precise explanation of the possible processes that are maintaining two sympatric morphs/populations in such a small lake.
Stomach content analysis suggests that profundal and pelagic fish have stable and precise foraging niches. There was no change or overlap in the prey items being consumed by profundal and pelagic fish during either sampling period. The diet of profundal fish consisted of items that were exclusively deep-water benthic in their ecology by consuming predominantly Pisidium which contributed 95% of benthic prey items found in stomachs and larval chironomids the other 5%. Pelagic fish consumed exclusively Leptodora kindtii. More interestingly, there was no stomach contents overlap in fish sampled during October where there is evidence of a temporary overlap in habitat with profundal fish inhabiting much shallower water. It would appear that the change in behaviour during the October sampling that causes a shift in habitat use does not influence the trophic ecology of profundal fish supporting a strong dietary segregation that still persists during the speculated spawning period.
Stable isotope ratios (δ15N and δ13C) of an animal allow its trophic position and carbon source to be quantified (Kelly, 2000). Due to the difficulty in sampling the zoobenthos at >50 m depth, it was not possible to collect samples of dominant prey items for stable isotope analysis. In freshwater aquatic systems, the δ13C signature of planktonic food items is more depleted than that of benthic invertebrates (Harrod et al., 2010). This was supported in the stable isotope analysis of δ13C which was significantly more depleted in the white muscle tissue of the profundal fish. This is characteristic of animals that forage in the benthic zone (Harrod et al., 2010), providing evidence of long-term and temporally stable differences in trophic ecology between the two ecomorphs. Of the 15 profundal stomachs that contained identifiable prey items, six fish had consumed chironomid larvae and three individuals had fed exclusively on chironomids indicating they contribute a significant proportion of the profundal diet. Some species of chironomid (larvae) harbour methanotrophic bacteria in areas of O2 depletion. Since biogenic CH4 has exceptionally low δ13C, this can result in very low δ13C for the chironomids (−20 to −70‰) and anything that consumes them (Jones et al., 2008). Nitrogen stable isotope ratios of a consumer may become enriched by 3–4‰ (Vander Zanden & Rasmussen, 1999; Kelly, 2000) of their prey and thus are a good indicator of the trophic level at which at animal feeds. Profundal organisms tend to have enriched δ15N as the profundal environment is dominated by detritus derived from species higher in the food chain (Vander Zanden & Rasmussen, 1999). However, stable isotope analysis did not find differences in δ15N indicating that although the two morphs in Loch Dughaill feed on different prey items, the similar δ15N signatures of the prey are maintained through different routes.
The first intermediate host of the trophically transmitted Diphyllobothrium parasite is a planktonic copepod (Knudsen et al., 1996); thus, a high parasite loading is indicative of fish that feed on plankton in the pelagic zone. In profundal fish, the mean number of Diphyllobothrium cysts was significantly lower than in pelagic fish. Diphyllobothrium cysts were only present in six of the 31 profundal fish sampled (19%) compared 37 of the 38 pelagic fish (97%). This adds support to the stomach contents and stable isotope data that the specific niches both morphs exploit are stable over space and time, and the different diets are not ontogenetic shifts. The only parasite data recorded were on Diphyllobothrium cysts as they can be can be easily identified in the body cavity. These cysts persist long after the prey that has resulted in the infection has been digested. Therefore, their presence/absence can be used to make inference about prey choices of individuals with empty stomachs making it a good identifier of long-term niche exploitation. Due to the high specificity of some parasites with respect to their life cycle, information on parasite diversity and abundance can also provide information on niche width (Knudsen et al., 1996). In some sympatric polymorphic populations, it has been suggested that parasitism may help maintain trophic segregation as the level of infection positively correlated with the degree of genetic segregation (Karvonen et al., 2013).
The significantly higher lipid levels in pelagic fish suggest that the rate of accumulation of surplus energy is higher in this morph. Although benthic food items have been shown to contribute significantly to food webs in lakes (Jones et al., 2008), differences in lipid levels could be reflecting a relatively more productive feeding resource at the time of sampling due to the seasonal abundance of pelagic prey (Persson et al., 1996). It is uncertain if the difference in lipid deposition rate between morphs remains stable throughout the year as lipid deposition can drop during less productive periods. Alternatively, it could be a reflection of the lipid levels in the prey items being consumed, rather than prey abundance itself (Eloranta et al., 2013a).
There have been numerous accounts of differing numbers of gill rakers between benthic/profundal and pelagic ecomorphs, most notably in whitefish (Lindsey, 1981; Amundsen et al., 2004; Kahilainen et al., 2011) and to some lesser extent sticklebacks (Schluter, 1993) and Arctic charr (Sandlund et al., 1992). Surprisingly, we found no difference in the number of gill rakers between profundal and pelagic fish in Loch Dughaill. It would be reasonable to expect the profoundal specialist, described here as feeding predominantly on Pisidium which live buried in the deep-water substrate, to have a lower gill raker count as this would benefit the feeding behaviour characteristic of fish that forage by sifting through sediment.
Greater lake surface area and depth are often seen as a driver behind sympatric divergence as it provides habitat heterogeneity (Nosil & Reimchen, 2005). The size of Loch Dughaill (1.15 km2) is very small compared with other systems that support polymorphic populations; however, it is very deep by comparison (62 m). Given this example of such an extreme difference in habitat use in what is a comparably small polymorphic system (Table 2), it is surprising the level of habitat heterogeneity is great enough to support such a divergence. This shows that habitat structuring, even in small ecosystems, can promote and maintain divergence. The combination of a narrow niche and high-intraspecific competition of the two forms described here means there is likely to be strong selection to evolve morphological and behavioural traits related to these foraging specialisms. It is likely that the profundal morph evolved to be an effective soft bottom feeder in sympatry with the pelagic morph by diverging from an ancestral form that is closer to the plankton feeding form described here [as the ancestral niche of Arctic charr does not include this type of soft bottom feeding (Knudsen et al., 2006)]. Competition for available resources is an important driver behind ecological speciation (Rundle & Nosil, 2005). Thus, we speculate that high-intraspecific competition in the pelagic zone of Loch Dughaill may have forced individuals to utilise an alternative niche. This alternative niche in a majority of polymorphic charr populations is the littoral-zoobenthos zone. A switch to the profundal zone is a more extreme foraging niche change and arguably requiring a more significant divergence from an ancestral foraging form and thus less common (Klemetsen, 2010). For such a divergence to occur, a possible hypothesis could suggest either an unsuitable littoral-zoobenthos foraging zone at Loch Dughaill which is already dominated by a more aggressive conspecific, such as brown trout which are known to displace Arctic charr from shallow benthic habitats (Jansen et al., 2002; Forseth et al., 2003; Eloranta et al., 2013b).
Conclusions
The results from the various comparisons in morphology, physiology, ecology and behaviour that have been presented and supported by parallelisms in the literature suggest the proximate driver behind the sympatric divergence was the successful exploitation of the benthic profundal zone, a previously untapped niche. It is essential in ecological speciation that a population both expand its current range and exploit a new stable resource successfully. This relies on a combination of morphological, physiological and behavioural adaptations that can arise through natural selection, disruptive selection, plasticity or a combination of selection and plasticity. Selection pressure would include changes in resource availability and the ability to forage at low temperatures. The pelagic resource (Leptodora sp.) is abundant during the summer but this decreases during winter; therefore, it is likely that difference in foraging strategies between forms may not persist as clearly in winter as one food source declines in abundance. This transition to permanent profundal feeding >50 m, in an almost lightless habitat, on food items with a hard shell and buried in the benthos would require the evolution of morphological and behavioural traits associated with this type of foraging. The consequences of this has driven functional adaptations in morphology and changes in behaviour to allow this divergence to become stable over time. This supports the theory of sympatric divergence through utilisation of profundal resources.
References
Adams, C. E. & F. A. Huntingford, 2002. The functional significance of inherited differences in feeding morphology in a sympatric polymorphic population of Arctic charr. Evolutionary Ecology 16: 15–25.
Adams, C. E., D. Fraser, F. A. Huntingford, R. B. Greer, C. M. Askew & A. F. Walker, 1998. Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. Journal of Fish Biology 52: 1259–1271.
Adams, C. E., D. Fraser, I. McCarthy, S. Shields, S. Waldron & G. Alexander, 2003. Stable isotope analysis reveals ecological segregation in a bimodal size polymorphism in Arctic charr from Loch Tay, Scotland. Journal of Fish Biology 62: 474–481.
Alekseyev, S. S. & M. Y. Pichugin, 1998. A new form of charr Salvelinus alpinus from lake Davatchan in Transbaikalia and its morphological differences from sympatric forms. Journal of Ichthyology 38: 328–337.
Amundsen, P. A., R. Knudsen & A. Klemetsen, 2008. Seasonal and ontogenetic variations in resource use by two sympatric Arctic charr morphs. Environmental Biology of Fishes 83: 45–55.
Amundsen, P. A., R. Knudsen, A. Klemetsen & R. Kristoffersen, 2004. Resource competition and interactive segregation between sympatric whitefish morphs. Annales Zoologici Fennici 41: 301–307.
Bolnick, D. I. & B. M. Fitzpatrick, 2007. Sympatric speciation: models and empirical evidence. Annual Review of Ecology and Systematics 38: 459–487.
Brenner, T., 1980. The Arctic charr, Salvelinus alpinus salvelinus, in the prealpine Attersee, Austria. In Balon, E. K. (ed.), Charrs: Salmonid Fishes of the Genus Salvelinus. Dr. W. Junk, The Hague: 765–772.
Corrigan, L. J., M. C. Lucas, I. J. Winfield & A. R. Hoelzel, 2011. Environmental factors associtated with genetic and phenotypic divergence among sympatric populations of Arctic charr (Salvelinus alpinus). Journal of Evolutionary Biology 24: 1906–1917.
Crawley, M. J., 2007. Statistical modelling. In The R Book. Wiley, Chichester: 323–386.
Dorfel, H. J., 1974. Untersuchungen zur Probiematik der Saiblingspopulationen (Salvelinus alpinus L.) im Ukrlinger See (Bodensee). Archiv fur Hydrobiologie Supplement 47: 80–105.
Eloranta, A. P., H. L. Mariash, M. Rautio & M. Power, 2013a. Lipid-rich zooplankton subsidise the winter diet of benthivorous Arctic charr (Salvelinus alpinus) in a subarctic lake. Freshwater Biology 58: 2541–2554.
Eloranta, A. P., R. Knudsen & P. A. Amundsen, 2013b. Niche segregation of coexisting Arctic charr (Salvelinus alpinus) and brown trout (Salmo trutta) constrains food web coupling in subarctic lakes. Freshwater Biology 58: 207–221.
Ferguson, A. & F. M. Mason, 1981. Allozyme evidence for reproductively isolated sympatric populations of brown trout Salmo trutta L. in Lough Melvin, Ireland. Journal of Fish Biology 18: 629–642.
Forseth, T., O. Ugedal, B. Jonsson & I. A. Fleming, 2003. Selection on Arctic charr generated by competition from brown trout. Oikos 101: 467–478.
Fugi, R., A. A. Agostinho & N. S. Hahn, 2001. Trophic morphology of five benthic-feeding fish species of a tropical floodplain. Revista Brasileira de Biologia 61: 27–33.
Garduño-Paz, M. V., C. E. Adams, E. Verspoor, D. Knox & C. Harrod, 2012. Convergent evolutionary processes driven by foraging opportunity in two sympatric morph pairs of Arctic charr with contrasting post-glacial origins. Biological Journal of the Linnean Society 106: 794–806.
Garduño-Paz, M. V., M. Demetriou & C. E. Adams, 2010. Variation in scale shape among alternative sympatric phenotypes of Arctic charr Salvelinus alpinus from two lakes in Scotland. Journal of Fish Biology 76: 1491–1497.
Harrod, C., J. Mallela & K. K. Kahilainen, 2010. Phenotype–environment correlations in a putative whitefish adaptive radiation. Journal of Animal Ecology 79: 1057–1068.
Hesthagen, T., K. Hindar, B. Jonsson, J. Ousdal & H. Holthe, 1995. Effects of acidification on normal and dwarf Arctic charr Salvelinus alpinus (L.). Biological Conservation 74: 115–123.
Hindar, K. & B. Jonsson, 1982. Habitat and food segregation of dwarf and normal Arctic charr (Salvelinus alpinus) from Vangsvatnet Lake, Western Norway. Canadian Journal of Fisheries and Aquatic Sciences 39: 1030–1045.
Hindar, K., N. Ryman & G. Ståhl, 1986. Genetic differentia tion among local populations and morphotypes of Arctic charr, Salvelinus alpinus. Biological Journal of the Linnean Society 27: 269–285.
Jansen, P. A., H. Slettvold, A. G. Finstad & A. Langeland, 2002. Niche segregation between Arctic charr (Salvelinus alpinus) and brown trout (Salmo trutta): an experimental study of mechanisms. Canadian Journal of Fisheries and Aquatic Sciences 59: 6–11.
Jensen, J. W. & T. Hesthagen, 1996. Direct estimates of the selectivity of a multimesh and a series of single gillnets for brown trout. Journal of Fish Biology 49: 33–40.
Jones, R. I., C. E. Carter, A. Kelly, S. E. Ward, D. Kelly & J. Grey, 2008. Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 89: 857–864.
Jonsson, B. & K. Hindar, 1982. Reproductive strategy of dwarf and normal Arctic charr (Salvelinus alpinus) from Vansvatnet Lake, western Norway. Canadian Journal of Fisheries and Aquatic Sciences 39: 1404–1413.
Jonsson, B. & N. Jonsson, 2001. Polymorphism and speciation in Arctic charr. Journal of Fish Biology 58: 605–638.
Jonsson, B., S. Skúlason, S. S. Snorrason, O. T. Sandlund, H. J. Malmquist, P. Jónasson, R. Cydemo & T. Lindem, 1988. Life history variation of Polymorphic Arctic charr (Salvelinus alpinus) in Thingvallavatn, Iceland. Canadian Journal of Fisheries and Aquatic Sciences 14: 1537–1547.
Kahilainen, K. K., A. Siwertsson, K. Ø. Gjelland, R. Knudesn, T. Bøhn & P. A. Amundsen, 2011. Sympatric diversification as influenced by ecological opportunity and historical contingency in a young species lineage of whitefish. Evolutionary Ecology 25: 575–588.
Karvonen, A., B. Lundsgaard-Hansen, J. Jokela & O. Seehausen, 2013. Differentiation in parasitism among ecotypes of whitefish segregating along depth gradients. Oikos 122: 122–128.
Kelly, J. F., 2000. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Canadian Journal of Zoology 78: 1–27.
Klemetsen, A., 2010. The charr problem revisited: exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshwater Reviews 3: 49–74.
Klemetsen, A., J. M. Elliott, R. Knudsen & P. Sørensen, 2002. Evidence for genetic differences in the offspring of two sympatric morphs of Arctic charr. Journal of Fish Biology 60: 933–950.
Klemetsen, A., P. A. Amundsen, B. Dempson, B. Jonsson, N. Jonsson, M. F. O’Connell & E. Mortensen, 2003. Atlantic salmon Salmo salar (L.), brown trout Salmo trutta (L.) and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecology of Freshwater Fish 12: 1–59.
Klingenberg, C. P., 1998. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Cambridge Philosophical Society 73: 79–123.
Klingenberg, C. P., 2011. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources 11: 353–357.
Knudsen, R., A. Klemetsen & F. Staldvik, 1996. Parasites as indicators of individual feeding specialization in Arctic charr during winter in northern Norway. Journal of Fish Biology 48: 1256–1265.
Knudsen, R., A. Klemetsen, P. A. Amundsen & B. Hermansen, 2006. Incipient speciation through niche expansion: an example from the Arctic charr in a subarctic lake. Proceedings of the Royal Society Biological Sciences 273: 2291–2298.
Lindsey, C. C., 1981. Stocks are chameleons: plasticity in gill rakers of coregonid fishes. Canadian Journal of Fisheries and Aquatic Sciences 38: 1497–1506.
Malmquist, H. J., S. S. Snorrason, S. Skúlason, B. Jonsson, O. T. Sandlund & P. M. Jonasson, 1992. Diet differentiation in polymorphic Arctic charr in Thingvallavatn, Iceland. Journal of Animal Ecology 61: 21–25.
McPhail, J. D., 1984. Ecology and evolution of sympatric sticklebacks (Gasterosteus): morphological and genetic evidence for a species pair in Enos Lake, British Columbia. Canadian Journal of Zoology 62: 1402–1408.
Mitteroecker, P. & P. Gunz, 2009. Advances in geometric morphometrics. Evolutionary Biology 36: 235–247.
Nosil, P. & T. E. Reimchen, 2005. Ecological opportunity and levels of morphological variance within freshwater stickleback populations. Biological Journal of the Linnean Society 86: 297–308.
O’Connell, M. F. & B. J. Dempson, 2002. The biology of Arctic charr, Salvelinus alpinus, of Gander Lake, a large, deep, oligotrophic lake in Newfoundland, Canada. Environmental Biology of Fishes 64: 115–126.
Østbye, K., P. A. Amundsen, L. Bernatchez, A. Klemetsen, R. Knudsen, R. Kristoffersen, T. F. Næsje & K. Hindar, 2006. Parallel evolution of ecomorphological traits in European whitefish Coregonus lavaratus (L.) species complex during postglacial times. Molecular Ecology 15: 3983–4001.
Persson, L., J. Andersson, E. Wahlström & P. Eklöv, 1996. Size-specific interactions in lake systems: predator gape limitation and prey growth rate and mortality. Ecology 77: 900–911.
Quartier, A. A., 1951. Morphologie et biologie de Salvelinus alpinus dans le lac Neuchâtel. Revue Suisse de Zoologie 58: 631–637.
R Development Core Team, 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rohlf, F. J., 2006a. tpsDig version 2.10 New York: Department of ecology and evolution, State University, Stony Brook.
Rohlf, F. J., 2006b. tpsUtil version 2.10 New York: Department of ecology and evolution, State University, Stony Brook.
Rohlf, F. J. & D. Slice, 1989. Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology 39: 40–59.
Rundle, N. & P. Nosil, 2005. Ecological speciation. Ecology Letters 8: 336–352.
Sandlund, O. T., K. Gunnarsson, P. M. Jónasson, B. Jonsson, T. Lindem, K. P. Magnusson, H. J. Malmquist, H. Sigurjonsdottir, S. Skúlason & S. S. Snorrason, 1992. The arctic charr Salvelinus alpinus in Thingvallavatn. Oikos 64: 305–351.
Schluter, D., 1993. Adaptive radiation in sticklebacks: size, shape, and habitat use efficiency. Ecology 74: 699–709.
Siwertsson, A., R. Knudsen, C. E. Adams, K. Præbel & P. A. Amundsen, 2013. Parallel and non-parallel morphological divergence among foraging specialists in European whitefish (Coregonus lavaretus). Ecology and Evolution 3: 1590–1602.
Skoglund, S., A. Siwertsson, P. A. Amundsen & R. Knudsen, 2015. Morphological divergence between three Arctic charr morphs - the significance of the deep-water environment. Ecology and Evolution 5: 3114–3129.
Skúlason, S. & T. Smith, 1995. Resource polymorphisms in vertebrates. Trends in Ecology and Evolution 10: 366–370.
Skúlason, S., D. L. G. Noakes & S. S. Snorrason, 1989. Ontogeny of trophic morphology in four sympatric morphs of Arctic charr Salvelinus alpinus in Thingvallavatn, Iceland. Biological Journal of the Linnean Society 38: 281–301.
Smith, T. & S. Skúlason, 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics 27: 111–133.
Soreide, F., D. Dolmen & K. Hindar, 2006. Den mystiske dypvannsfisken i Tinnsjoen. Fauna 59: 122–129 (In Norwegian with an English summary).
Taylor, E. B. & J. D. McPhail, 1999. Evolutionary history of an adaptive radiation in species of threespine sticklebacks (Gasterosteus): insights from mitochondrial DNA. The Biological Journal of the Linnean Society 66: 271–291.
Vander Zanden, M. J. & J. B. Rasmussen, 1999. Primary Consumer d 15N and d 13C and the trophic position of aquatic consumers. Ecology 80: 1395–1404.
Walker, J. A. & M. W. Westneat, 2002. Performance limits of labriform propulsion and correlates with fin shape and motion. Journal of Experimental Biology 205: 177–187.
West-Eberhard, M. J., 1989. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology 20: 249–278.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Power, R. Knudsen, C. Adams, M. J. Hansen, J. B. Dempson, M. Jobling & M. Ferguson / Advances in Charr Ecology and Evolution
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hooker, O.E., Barry, J., Van Leeuwen, T.E. et al. Morphological, ecological and behavioural differentiation of sympatric profundal and pelagic Arctic charr (Salvelinus alpinus) in Loch Dughaill Scotland. Hydrobiologia 783, 209–221 (2016). https://doi.org/10.1007/s10750-015-2599-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2599-0