Abstract
Feeding habits of common dolphinfish (Coryphaena hippurus) were investigated in the Southeast Pacific Ocean using stomach content analysis. Stomachs were collected from 1506 individuals ranging between 18 and 187 cm in fork length. The fish were caught by longliner boats in 2009–2017. Based on percentage of wet weight (%Wi), percentage of number (%Ni), and frequency of occurrence (%Oi), flyingfishes were the predominant prey, followed by the cephalopods jumbo squid (Dosidicus gigas) and argonaut (Argonauta spp.). Permutational multivariate analysis of variance (PERMANOVA) detected significant differences between zones (p < 0.001). PERMANOVA also revealed two size-related shifts in feeding habits to occur at FL ~ 80 cm and FL ~ 110 cm (p < 0.001). Cumulative prey curves did not reach an asymptote in any of the zones and size classes, which may be attributed to the fine taxonomic resolution used. Spatial variations in daily meal ranged between 296.3 and 438.9 g day−1, corresponding to daily ration of 6.1–8.2% body mass BM day−1. Otherwise, size-related variations in both DM and DR were observed. Thus, while daily food intake increased with size from 177.3 for the smallest fish (LF < 80 cm) to 496.7 g day−1 for the largest ones (LF ≥ 110 cm), DR decreased from 13.0 ± 22.1 to 3.7 ± 0.7 BM day−1. The present results contribute to improve the understanding of dolphinfish feeding habits in the Southeast Pacific Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common dolphinfish (Coryphaena hippurus, Linnaeus 1758) is an epipelagic predator occurring in tropical and subtropical regions of the Pacific, Indian, and Atlantic oceans (Palko et al. 1982). This species is caught in open and coastal waters (Briggs 1960), and spends most of its time at the upper 30 m of the water column (Palko et al. 1982; Tripp-Valdez et al. 2015). With a high socio-economic importance, the dolphinfish supports commercial and recreational fisheries in all seas worldwide. Furthermore, it plays an important ecological role delineating pelagic food-webs by “top down” control mechanisms (Olson and Galván-Magaña 2002; Varela et al. 2017a).
Like other large pelagic fishes inhabiting the southeastern Pacific Ocean, the dolphinfish shows exceptionally high standard metabolic rates (Benetti et al. 1995; Crossley et al. 2021), which allow fast somatic and gonadal growth (Palko et al. 1982). This, therefore, requires a high consumption of prey, which may significantly impact on the abundance, diversity, or distribution of other species within the food web. In this context, stomach content analysis (SCA) is a powerful tool to understand prey-predator relationships.
Although previous studies carried out in the Southeast Pacific Ocean provide useful data on the feeding habits of dolphinfish, they do not consider spatial-related variations (Lasso and Zapata 1999; Varela et al. 2017a). Thus, while Lasso and Zapata (1999) reported that this species feeds on large number of fishes, mollusks, and crustaceans in the Pacific coast of Colombia and Panama, Varela et al. (2017a) found that the family Exocoetidae was the predominant prey species off Ecuador. The only research conducted to assess spatial-related shifts in the feeding patterns of this top predator was carried out two decades ago by Olson and Galván-Magaña (2002). Therefore, new data on this issue may be useful to better understand the trophic role of this predator in the Southeast Pacific Ocean ecosystem.
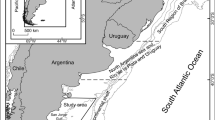
The Southeast Pacific coast is characterized by a marked spatial variation in the oceanographic conditions. Thus, the northernmost area is affected by the Panama current (warm and nutrient-poor water), whereas the southernmost area is influenced by the Chile-Peru current (cold and nutrient-rich water) (Sonnenholzner et al. 2013; Martínez-Ortiz et al. 2015). These singular oceanographic features may cause geographic variation in the abundance and distribution of prey between both areas. Moreover, differences in the dietary composition between inshore and offshore waters have been reported for dolphinfish and other large pelagic fishes from the region (Alverson 1963; Olson and Galván-Magaña 2002; Olson et al. 2014). The present study was conducted to assess spatial- and size-related shifts in the dietary composition of the dolphinfish in the southeastern Pacific Ocean considering four regions: North, South, inshore, and offshore (see Fig. 1).
Material and methods
Sampling and stomach content analysis
Stomachs of dolphinfish (n = 1506) were collected aboard surface longline boats fishing in the Southeast Pacific Ocean in 2009–2017. The main species employed as bait during the fishing operations were jumbo squid (Dosidicus gigas) and frigate tuna (Auxis thazard).
Most fish were measured to the nearest centimeters (fork length, FL) and the stomachs were stored at − 20 °C until analysis. In the laboratory, they were thawed and opened and prey species were weighed, counted, and identified to the lowest possible taxonomic level using a stereoscopic microscope. Food items considered as bait were not taken into consideration for analysis, and the stomachs containing only these species were treated as empty. Fish and cephalopod species were identified from otoliths and lower beaks, respectively, using available guides (Clarke 1986; Harvey et al. 2000; García-Godos Naveda 2001).
Data analysis
The contribution of each prey to the diet was evaluated by three indices: (1) percentage of wet weight (%Wi), (2) percentage of number (%Ni), and (3) frequency of occurrence (%Oi).
Spatial- and size-related shifts in dietary composition were tested by a permutational multivariate analysis of variance (PERMANOVA) (Anderson 2001; McArdle and Anderson 2001; Anderson et al. 2008). The analytical design considered “zone” as fixed factor with four levels. As in Varela et al. (2017a), we also considered the factor “size class” with 3 levels (< 80 cm in FL, 80–110 cm in FL, ≥ 110 cm in FL). The analysis was based on a Bray–Curtis similarity matrix calculated from the prey weight values, after performing a fourth-root transformation (Van Ginderdeuren et al 2014; Kvaavik et al. 2019; Valls et al. 2022). Significant terms were investigated using a posteriori pair-wise comparisons with PERMANOVA test. Multivariate dispersion among zones was tested by PERMDISP (Anderson 2006). Both PERMANOVA and PERMDISP were performed using the software PRIMER v6.1.13 and the PERMANOVA + v1.0.3 statistical package (PRIMER-E Ltd., Plymouth, UK).
Cumulative prey curves (CPCs) were used to evaluate if the sample size was sufficient to describe the food habits of dolphinfish in each zone, size class, and in the whole area (Ferry and Cailliet 1996). CPCs were built using the vegan package (Oksanen et al., 2010) in R (R Core Team, 2020). To determine whether the curve reached an asymptote, the slope of the linear regression estimated from the last four stomachs was compared with a horizontal asymptote by t test.
The food consumption rate was estimated from the formula \(\widehat{\mathrm r}=\sum_{i=0}^I{\overline{\mathrm W}}_i/{\mathrm A}_i\) (Olson and Mullen 1986), where r̂ is the feeding rate measured in grams per hour, \({\overline{\mathrm{W}} }_{i}\) is the weight of prey i divided by the total number of stomachs, and \({\mathrm{A}}_{i}\) represents the average time required to evacuate the average proportion of prey i. As in Olson and Galván-Magaña (2002), we applied \({\mathrm{A}}_{i}\) values estimated for yellowfin tuna (Thunnus albacares) (Olson and Boggs 1986). Considering that the dolphinfish feeds during both day and night hours (Olson and Galván-Magaña 2002), daily meal (DM) was estimated by multiplying r̂ by 24 h. Daily ration (DR) is equivalent to the daily meal expressed as percent of the dolphinfish body mass (BM). BM was estimated from the length using the equation proposed by Lasso and Zapata (1999).
Stomach content data from unmeasured fish were not considered in the analysis involving FL.
Results
FL of the sampled dolphinfish ranged between 18 and 187 (mean ± SD = 96.7 ± 20.1 cm). A total of 1506 stomachs were analyzed, of which 1270 (84.3%) contained at least one prey (Table 1). The diet was made up of 57 prey items, including 35 fishes, 19 cephalopods, and 3 crustaceans. Fishes were the most important prey group (%W = 60.9, %N = 54.5, and %O = 67.9), followed by cephalopods (%W = 39.1, %N = 54.5, and %O = 50.9) and crustaceans (%W < 0.1, %N = 0.1, and %O = 0.2). Among all the prey species, flyingfishes were the most abundant (%W = 30.7, %N = 34.8, and %O = 39.8), followed by the cephalopods jumbo squid (Dosidicus gigas) (%W = 34.9, %N = 13.0, and %O = 21.0) and argonaut (Argonauta spp.) (%W = 1.2%, N = 24.9, and %O = 24.4) (Table 2).
The dolphinfish caught in zone A ranged from 36.0 to 187.0 cm in FL (mean SD: 94.0 ± 20.6 cm), whereas the size rage of the individuals sampled in zone B was 36–187 in FL (85.8 ± 28.7 cm). The dolphinfish from the zone C ranged between 26 and 154 cm in FL (98.9 ± 16.2), and the fish caught in zone D sized from 37 to 187 in FL (96.7 ± 20.1). Although fishes were the most representative prey group in all zones, spatial variations in dietary composition were found. In zone A, flyingfishes were the most abundant prey species (%W = 50.1, %N = 40.6, and %O = 8.1%), followed by jumbo squid (%W = 26.3, %N = 15.7, and %O = 7.5) and frigate tuna (Auxis spp.) (%W = 15.6, %N = 6.5, and %O = 5.9). Dolphinfish caught in zone B mainly consumed flyingfishes (%W = 40.6, %N = 47.4, and %O = 19.2), frigate tuna (%W = 11.4, %N = 5.6, and %O = 10.5), and argonauts (%W = 0.8, %N = 13.7, and %O = 11.0). In zone C, flyingfishes (%W = 27.7, %N = 32.8, and %O = 38.6), jumbo squid (%W = 33.6, %N = 16.5, %O = 49.8), and frigate tuna (%W = 26.4, %N = 12.1, %O = 53.4%) were the most representative prey food. The diet of the specimens from zone D was mainly composed of flyingfishes (%W = 27.5, %N = 29.8, and %O = 34.0), argonauts (%W = 1.4, % = 35.6, and %O = 53.2), and jumbo squid (%W = 42.1, %N = 11.0, and %O = 33.0) (Table 2). Fishes were also the predominant prey group in the three size classes, but dietary composition changed with body size. Class I fish (< 80 cm) fed mainly on flyingfishes (%W = 43.1, %N = 29.4, and %O = 30.9), argonauts (%W = 2.9, %N = 30.4, and %O = 19.1), and jumbo squid (%W = 19.7, %N = 9.5, and %O = 14.9) (Table 3). The medium-sized fish (80–110 cm, FL) mainly consumed flyingfishes (%W = 29.8, %N = 37.0, and %O = 31.0), frigate tuna (%W = 23.6, %N = 11.4, and %O = 20.9), and argonauts (%W = 0.9, %N = 19.6, and %O = 24.8). The largest fish (≥ 110 cm) preyed fed mainly on flyingfishes (%W = 25.2, %N = 32.3, and %O = 27.7), jumbo squid (%W = 35.9, %N = 14.4, and %O = 28.5), and frigate tuna (%W = 14.2, %N = 8.1, and %O = 16.4) (Table 2). Despite the large number of stomachs analyzed (n = 1506), CPCs did not reach an asymptote in any of the zones, size classes, or in the whole stomach sample (t test, p < 0.05).
The PERMANOVA analysis detected significant spatial- and size-related differences in the dietary composition (p < 0.05) (Table 4). The interactions between both factors were also significantly different, indicating that the differences in “zone” were not homogeneous across the levels of the “size classes” factor (PERMANOVA, p < 0.05) (Table 4). Pair-wise PERMANOVA comparisons indicated significant differences between the four zones or between the three size classes (p < 0.05). Overall, significant dietary differences between size classes were found by zone (p < 0.05), except in zone C (p > 0.05) (Table 5). The PERMDISP analysis showed no significant differences (p > 0.05), suggesting that the differences detected by PERMANOVA were not due to multivariate dispersion.
DM showed similar values in zones A, B, and C, whereas highest values were found in zone C. DR estimates were comparable in all zones, except in zone B where they were highest (Table 2). Otherwise, size-related variations in both DM and DR were observed. Thus, while daily food intake increased with size from 177.3 for the smallest fish (LF < 80 cm) to 496.7 g day−1 for the largest ones (LF ≥ 110 cm), DR decreased from 13.0 ± 22.1 to 3.7 ± 0.7 BM day−1 (Table 3).
Discussion
SCA data showed that the diet of dolphinfish from the Southeast Pacific Ocean is mainly composed of epipelagic fishes and cephalopods (flyingfishes, frigate tuna, jumbo squid, and argonauts). This finding is consistent with earlier studies carried out in the region (Lasso and Zapata 1999; Olson and Galván-Magaña 2002; Varela et al. 2017a). Lasso and Zapata (1999) and Varela et al. (2017a, b) reported that family Exocoetidae was the most important prey in the diet of dolphinfish caught in the Pacific coast of Colombia, Panama, and Ecuador, whereas Olson and Galván-Magaña (2002) found that flyingfishes and epipelagic cephalopods were the predominant prey species in the coast of Ecuador and Peru. Similarly, Campos et al. (1993) reported that the dolphinfish preyed mainly on exocoetids and squids off Costa Rica. In the southeastern Gulf of California, Tripp-Valdez et al. (2010) and Bergés-Tiznado et al. (2019), however, observed that the dolphinfish fed primarily on the fishes longfin halfbeak (Hemiramphus saltator) and finescale triggerfish (Balistes polylepis), respectively. Torres-Rojas et al. (2014) and Tripp-Valdez et al. (2015) found that crustaceans (Pleuroncodes planipes and Hemisquilla californiensis) were the dominant prey group in the southern Gulf of California. These marked spatial variations indicate that dolphinfish show high feeding plasticity, adjusting their diet to local prey availability.
Comparing dolphinfish diet with that of other large pelagic fishes co-occurring in the region may be useful to infer trophic relationships. Assuming that interspecific food competition occurs when the presence of a food item is more than 25% in two or more predators (Johnson 1977), we found that niche partitioning occurs between dolphinfish and yellowfin tuna, swordfish (Xiphias gladius), and striped marlin (Kajikia audax) in the southeastern Pacific Ocean (see previous studies for yellowfin tuna, striped marlin and swordfish; Alverson 1963; Baque-Menoscal et al. 2012; Rosas-Luis et al. 2016; Varela et al. 2017b; Loor-Andrade et al. 2017; Zambrano-Zambrano et al., 2019). Off eastern Australia, Young et al. (2010) reported that these predators feed on different prey sizes, depths, and/or times, which would allow them to reduce their trophic competition. These authors, however, stated that this pattern may be not consistent across all oceans. Moteki et al. (2001), in fact, reported that dolphinfish and yellowfin tuna may compete for exocoetids in the eastern tropical Pacific Ocean, whereas Oxenford and Hunte (1999) suggested that these two predators may compete for flying gurnards (Dactylopterus volitans) and for filefish (Monacanthidae) in Barbados waters (see Lewis and Axelsen 1967). Conversely, Kojadinovic et al. (2008), based on stable isotope analysis, observed trophic segregation between common dolphinfish and tuna species in Reunion Islands waters.
Although flyingfishes were the dominant prey in all zones, interspecific variations in their abundance were observed. Thus, while smallwing flyingfish (Oxyporhamphus micropterus) was the main prey species in the North region, barbel flyingfish (Exocoetus monocirrhus) was the most abundant prey species in the South region (see Table 2). These differences may be related to variations in the oceanographic features. Lewallen et al. (2018), in fact, reported that surface temperature and salinity are key parameters defining flyingfish habitats. Otherwise, spatial variations between inshore and offshore waters were also found, epipelagic cephalopods such as argonauts being more prevalent in inshore caught fish. Similarly, Olson and Galván-Magaña (2002) found that epipelagic cephalopods were more abundant in fish caught nearshore. Multivariate analysis also suggested two size-related shifts in feeding habits. The first shift in the feeding habits was detected between the small- and the medium-sized fish, and was caused by a decrease in the consumption of barbel flyingfish along with an increased proportion of frigate tuna. The second shift was found around 110 cm, where the cephalopods jumbo squid and argonauts showed a higher contribution to the diet than flyingfishes. This finding is consistent with previous studies carried out in Brazilian waters and EPO area (Zavala-Camin 1986; Olson and Galván-Magaña, 2002), where large-sized dolphinfish also show greater predation on cephalopods than on flyingfishes.
Similar to what was observed by Varela et al. (2017a) in dolphinfish caught in Ecuadorian waters, DR decrease with body size. Benetti et al. (1995) stated that the dolphinfish mass-specific standard metabolic rate (SMR) diminishes with increasing body weight. This fact, therefore, suggests that smaller fish may need to consume proportionally larger amount of food to meet their SMR, and that DR decreases with increasing size has also been reported in others top predators. For instance, Ménard et al. (2000) found that DR of yellowfin tuna in the equatorial Atlantic Ocean decreased from 16.0% BM day−1 for > 90 cm fish to 2.6% BM day−1 for > 90 cm fish. Similarly, Griffiths et al. (2007) found that DR values of longtail tuna (Thunnus tonggol) in Australian waters decreased from 2.2% BM day−1 for < 60 cm fish to 1% BM day−1 for > 130 cm fish. The highest DR values (17.6%BM day−1) in zone B may be attributed to the smaller fish size in this region (FL = 85.8 ± 28.7 cm). This result is consistent with the findings of Olson and Galván-Magaña (2002) who reported DR values ~ 20% BM day−1 for individuals ranging between 65 and 80 cm in FL.
In comparison with other large pelagic fishes inhabiting the study area, our DR estimates were greater than those reported for yellowfin tuna (3.9% BM day−1: Olson and Boggs 1986) and swordfish (1.6–8.2% BM day−1; Zambrano-Zambrano et al. 2019). Such differences may be attributed to species-specific factors that may affect prey evacuation rates, such as metabolic rates or gut morphology (Griffiths et al., 2007; Perelman et al., 2017). Another factor explaining DR differences among these species may be related to the consumed prey type (Buckel and Conover 1997). Thus, Olson and Boggs (1986) reported that scombrids were the main prey species in the diet of yellowfin tuna, whereas Zambrano-Zambrano et al. (2019) found that the diet of swordfish was mainly composed of squids, which are more easily digested and evacuated (Varghese and Somvanshi, 2016).
The presence of conspecifics in the stomachs was not unexpected, since dolphinfish cannibalism has been widely reported in all seas worldwide (Oxenford and Hunte 1999; Olson and Galván-Magaña 2002; Castriota et al. 2007; Rudershausen et al. 2010; Brewton et al. 2016; Luckhurst 2017). The cannibalism rate found in the present study (0.6 based on %O) was similar to that previously reported in the eastern Pacific Ocean. Thus, Olson and Galván-Magaña (2002) reported %O = 0.4, whereas Torres-Rojas et al. (2014) found O% values of 2.2. However, Moteki et al. (2001) based on a low sampled size (n = 38) reported higher cannibalism rates (%O = 10.5). According to Torres-Rojas et al. (2014), dolphinfish cannibalism may be sporadic as this species shows spatial stratification by size.
The present study provides new information on the feeding habits and consumption rate of common dolphinfish in the Southeast Pacific Ocean. Our findings indicate that this species is an epipelagic carnivorous predator that shows two size-related dietary changes to occur at a FL ∼ 80 cm and FL ∼110 cm. Although flyingfishes seem to be the main prey species in the whole sampling area, interspecific variations among zones were found. Considering that surface temperature and salinity are factors that define flyingfish habitats, this spatial variation may be related the oceanographic features of each zone. For instance, the northern area is affected by the Panama current (warm water), whereas the southern area is influenced by the Chile-Peru current (cold water). Otherwise, our estimates of daily ration suggest that this species play an important trophic role structuring pelagic ecosystems. However, the lack of information on specific gastric evacuation rates for dolphinfish may bias our estimates. Therefore, further investigations should be addressed to determine accurate rates of gastric evacuation for this predator.
Data availability
Datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
No applicable.
Change history
07 March 2022
The following Latin binomials in the reference list were incorrectly written as a single word: Thunnus albacares, Coryphaena hippurus, Acanthocybium solandri, Stizostedion vitreum vitreum, Pomatomus saltatrix, Thunnus tonggol, Thunnus alalunga, Perca flavescens, Catostomus commersoni, Scombrus scomber, and Coryphaena equiselis.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2006) Distance-based tests for homogeneityof multivariatedispersions. Biometrics 62:245–253
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E: Plymouth, UK.
Alverson F (1963) The food of yellowfin and skipjack tunas in the eastern tropical Pacific Ocean. Inter Amer Trop Tuna Comm Bull 7:295–396
Baque-Menoscal J, Páez-Rosas D, Wolff M (2012) Hábitos alimentarios de dos peces pelágicos Thunnus albacares y Acanthocybium solandri de la Reserva Marina de Galápagos. Rev Biol Mar Oceanogr 47:1–11
Benetti DD, Brill RW, Kraul SA (1995) The standard metabolic rate of dolphin fish. J Fish Biol 46:987–996
Bergés-Tiznado ME, Márquez-Farías JF, Osuna-Martínez CC, Torres-Rojas Y, Galván-Magaña F, Páez-Osuna F (2019) Patterns of mercury and selenium in tissues and stomach contents of the dolphinfish Coryphaena hippurus from the SE Gulf of California, Mexico: concentrations, biomagnification and dietary intake. Mar Pollut Bull 138:84–92
Brewton RA, Ajemian MJ, Young PC, Stunz GW (2016) Feeding ecology of dolphinfish in western Gulf of Mexico. Trans Am Fish Soc 145:839–853
Briggs JC (1960) Fishes of worldwide (circumtropical) distribution. Copeia 1960:171–180
Buckel JA, Conover DO (1997) Movements, feeding periods, and daily ration of piscivorous young-of-the-year bluefish, Pomatomus saltatrix, in the Hudson River estuary. Fish Bull 95:665–679
Campos JA, Segura A, Lizano O, Madrigal E (1993) Ecología básica de Coryphaena hippurus (Pisces: Coryphaenidae) y abundancia de otros grandes pelágicos en el Pacífico de Costa Rica. Rev Biol Trop 41:783–790
Castriota L, Pipitone C, Campagnuolo S, Romanelli M, Potoschi A, Andaloro F (2007) Diet of Coryphaena hippurus (Coryphaenidae) associated with FADs in the Ionian and Southern Tyrrhenian Seas. Cybium 31:435–441
Clarke MR (1986) A Handbook for the Identification of Cephalopod Beaks. Clarendon Press, Oxford
Crossley DA II, Stieglitz JD, Benetti DD, Grosell M (2021) The effects of acute temperature change and digestive status on in situ cardiac function in mahi-mahi (Coryphaena hippurus). Comp Biochem Physiol Part A 255:110915
Ferry LA, Cailliet GM (1996) Sample size and data: are we characterizing and comparing diet properly? In: MacKinlay D, Shearer K (eds) Feeding ecology and nutrition in fish; proceedings of the symposium on feeding ecology and nutrition in fish. American Fisheries Society, San Francisco, pp 71–80
García-Godos Naveda I (2001) Patrones morfológicos del otolito sagitta de algunos peces óseos del mar Peruano. Bol Inst Mar Perú 20:1–83
Griffiths SP, Fry GC, Manson FJ, Pillans RD (2007) Feeding dynamics, consumption rates and daily ration of longtail tuna (Thunnus tonggol) in Australian waters, with emphasis on the consumption of commercially important prawns. Mar Freshw Res 58:376–397
Harvey JT, Loughlin TR, Perez MA, Oxman DS (2000) Relationship between fish size and otolith length for 63 species of fishes from the eastern North Pacific Ocean. NOAA Tech Rep NMFS 150:1–35
Johnson FH (1977) Responses of walleye (Stizostedion vitreum vitreum) and yellow perch (Perca flavescens) population to the removal of white sucker Catostomus commersoni) from a Minnesota Lake in 1966. J Fish Res Board Can 34:1633–1642
Kojadinovic J, Ménard F, Bustamante P, Cosson RP, Le Corre M (2008) Trophic ecology of marine birds and pelagic fishes from Reunion Island as determined by stable isotope analysis. Mar Ecol Prog Ser 36:239–251
Kvaavik C, Óskarsson GJ, Daníelsdóttir AK, Marteinsdóttir G (2019) Diet and feeding strategy of Northeast Atlantic mackerel (Scombrus scomber) in Icelandic waters. Plos One 14:e022555
Lasso J, Zapata L (1999) Fisheries and biology of Coryphaena hippurus (Pisces: Coryphaenidae) in the Pacific coast of Colombia and Panama. Sci Mar 63:387–399
Lewallen EA, van Wijnen AJ, Bonin CA, Lovejoy NR (2018) Flyingfish (Exocoetidae) species diversity and habitats in the eastern tropical Pacific Ocean. Mar Biodiver 48:1755–1765
Lewis JB, Axelsen F (1967) Food of the dolphin, Coryphaena hippurus (Linnaeus) and of the yellowfin tuna, Thunnus albacares (Lowe), from Barbados, West Indies. J Fish Res Board Can 24:683–686
Loor-Andrade P, Pincay-Espinoza J, Carrera-Fernández M, Rosas-Luis R (2017) Feeding habits of billfishes (Carangaria: Istiophoriformes) in the Ecuadorian Pacific Ocean. Neotrop Ichthyol 15:e160162
Luckhurst BE (2017) Aspects of the migration, seasonality and habitat use of two mid-trophic level predators, dolphinfish (Coryphaena hippurus) and wahoo (Acanthocybium solandri), in the pelagic ecosystem of the western Atlantic including the Sargasso Sea. Collect Vol Sci Papers ICCAT 73:3163–3174
Martínez-Ortiz J, Aires-Da-Silva AM, Lennert-Cody CE, Maunder MN (2015) The Ecuadorian artisanal fishery for large pelagics: species composition and spatio-temporal dynamics. PLoS One 10:e0135136
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Ménard F, Stéquert B, Rubin A, Herrera M, Marchal E (2000) Food consumption of tuna in the Equatorial Atlantic ocean: FAD-associated versus unassociated schools. Aquat Living Resour 13:233–240
Moteki M, Arai M, Tsuchiya K, Okamoto H (2001) Composition of piscine prey in the diet of large pelagic fish in the eastern tropical Pacific Ocean. Fish Sci 67:1063–1074
Oksanen J, Blanchet F, Kindt R, Legendre P, O’Hara RG, Simpson G, Solymos P, Stevens MHH, Szoecs E, Wagner H (2010) Vegan: community ecology package. R package version 2.5–5. Available at http://CRAN.R-project.org/package=vegan
Olson RJ, Mullen CH (1986) Recent developments for making gastric evacuation and daily ration determinations. Environ Biol Fish 16:183–191
Olson RJ, Boggs CH (1986) Apex predation by yellowfin tuna (Thunnus albacares): independent estimates from gastric evacuation and stomach contents, bioenergetics, and cesium concentrations. Can J Fish Aquat Sci 43:1760–1775
Olson RJ, Galván-Magaña F (2002) Food habits and consumption rates of common dolphinfish (Coryphaena hippurus) in the eastern Pacific Ocean. Fish Bull 100:279–298
Olson RJ, Duffy LM, Kuhnert PM, Galván-Magaña F, Bocanegra-Castillo N, Alatorre-Ramirez V (2014) Decadal diet shift in yellowfin tuna (Thunnus albacares) suggests broad-scale food web changes in the eastern tropical Pacific Ocean. Mar Ecol Prog Ser 497:157–178
Oxenford HA, Hunte W (1999) Feeding habits of the dolphinfish (Coryphaena hippurus) in the eastern Caribbean. Sci Mar 63:303–315
Palko BJ, Beardsley GL, Richards WJ (1982) Synopsis of the biologicaldata on dolphin-fishes, Coryphaena hippurus Linnaeus and Coryphaena equiselis Linnaeus. NOAA Tech Rep NMFS Circ 443:1–28
Perelman JN, Schmidt KN, Haro I, Tibbetts IR, Zischke MT (2017) Feeding dynamics, consumption rates and daily ration of wahoo Acanthocybium solandri in Indo-Pacific waters. J Fish Biol 90:1842–1860
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing. www.r-project.org, Vienna
Rosas-Luis R, Loor-Andrade P, Carrera-Fernández M, Pincay-Espinoza JE, Vinces-Ortega C, Chompoy-Salazar L (2016) Cephalopod species in the diet of large pelagic fish (sharks and billfishes) in Ecuadorian waters. Fish Res 173:159–168
Rudershausen PJ, Buckel JA, Damon JE, Gannon P, Butler CM, Averett TW (2010) Feeding ecology of blue narlins, dolphinfish, yellowfin tuna, and wahoos from the North Atlantic Ocean and comparisons with other oceans. Trans Am Fish Soc 139:1335–1359
Sonnenholzner J, Brandt M, Francisco V, Hearn A, Luzuriaga M, Guarderas P, Navarro JC (2013) Echinoderms of Ecuador. In: Alvarado JJ, Solís-Marín F (eds) Echinoderm Research and Diversity in Latin America. Springer-Verlag, Berlin, pp 183–233
Torres-Rojas YE, Hernández-Herrera A, Ortega-García S, Soto-Jiménez MF (2014) Feeding habits variability and trophic position of dolphinfish in waters south of the Baja California Peninsula, Mexico. Trans Am Fish Soc 143:528–542
Tripp-Valdez A, Galván-Magaña F, Ortega-García S (2010) Feeding habits of dolphinfish Coryphaena hippurus in the southeastern Gulf of California, Mexico. J Appl Ichthyol 26:578–582
Tripp-Valdez A, Galván-Magaña F, Ortega-García S (2015) Food sources of common dolphinfish (Coryphaena hippurus) based on stomach content and stable isotopes analyses. J Mar Biol Assoc UK 95:579–591
Valls M, Saber S, Gómez MJ, Reglero P, Mele S (2022) Diet and feeding behaviour of albacore Thunnus alalunga in the western Mediterranean Sea during the spawning period. J Fish Biol 100:203–217
Van Ginderdeuren K, Vandendriessche S, Prössler Y, Matola H, Vincx M, Hostens K (2014) Selective feeding by pelagic fish in the Belgian part of the North Sea. ICES J Mar Sci 71:808–820
Varela JL, Lucas-Pilozo CR, González-Duarte MM (2017a) Diet of common dolphinfish (Coryphaena hippurus) in the Pacific coast of Ecuador. J Mar Biol Assoc UK 97:207–213
Varela JL, Intriago KM, Flores JC, Lucas-Pilozo CR (2017b) Feeding habits of juvenile yellowfin tuna (Thunnus albacares) in Ecuadorian waters assessed fromstomach content and stable isotope analysis. Fish Res 194:89–98
Varghese SJ, Somvanshi VS (2016) Feeding ecology and consumption rates of yellowfin tuna Thunnus albacares (Bonnaterre, 1788) in the eastern Arabian Sea. Indian J Fish 63:16–26
Young JW, Lansdell MJ, Campbell RA, Cooper SP, Juanes F, Guest MA (2010) Feeding ecology and niche segregation in oceanic top predators off eastern Australia. Mar Biol 157:2347–2368
Zambrano-Zambrano RW, Mendoza-Moreira PE, Gómez-Zamora W, Varela JL (2019) Feeding ecology and consumption rate of broadbill swordfish (Xiphias gladius) in Ecuadorian waters. Mar Biodiver 49:373–380
Zavala-Camin LA (1986) Conteúdo estomocal e distribuiçao do dourado Coryphaena hippurus e ocorrencia de C. equiselis no Brasil (24°S– 33°S). Bol Inst Pesca Brazil 13:5–14
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Andalusian Government (P12-RNM733, FEDER-UCA18-107069), CEI⋅MAR Foundation (CEIJ19-C02.1), and Spanish Ministry of Economy, Industry and Competitiveness (CTM2017-82808-R, AEI/FEDER, EU).
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception, design, laboratory work, data analysis, and manuscript writing.
Corresponding author
Ethics declarations
Ethics approval
The present research involved no animal experimentation or harm. Samples were collected from fish harvested for commercial purposes, and permits for animal collections were not required.
Consent to participate
Not applicable as this research did not involve human participants.
Consent for publication
The authors all agree with the content of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pincay-Espinoza, J.E., Varela, J.L. Spatial- and size-related shifts in feeding habits of the common dolphinfish (Coryphaena hippurus) in the Southeast Pacific Ocean. Environ Biol Fish 105, 313–326 (2022). https://doi.org/10.1007/s10641-022-01231-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01231-x