Abstract
Small molecule Bruton’s tyrosine kinase (BTK) inhibitors have been developed for the treatment of various haemato-oncological diseases, and ibrutinib was approved as the first BTK inhibitor for anticancer therapy in 2013. Previous reports proved the receptor kinase human epidermal growth factor receptor 2 (HER2) to be a valid off-target kinase of ibrutinib and potentially other irreversible BTK inhibitors, as it possesses a druggable cysteine residue in the active site of the enzyme. These findings suggest ibrutinib as a candidate drug for repositioning in HER2-positive breast cancer (BCa). This subtype of breast cancer belongs to one of the most common classes of breast tumours, and its prognosis is characterized by a high rate of recurrence and tumour invasiveness. Based on their similar kinase selectivity profiles, we investigated the anticancer effect of zanubrutinib, evobrutinib, tirabrutinib and acalabrutinib in different BCa cell lines and sought to determine whether it is linked with targeting the epidermal growth factor receptor family (ERBB) pathway. We found that zanubrutinib is a potential inhibitor of the HER2 signalling pathway, displaying an antiproliferative effect in HER2-positive BCa cell lines. Zanubrutinib effectively inhibits the phosphorylation of proteins in the ERBB signalling cascade, including the downstream kinases Akt and ERK, which mediate key signals ensuring the survival and proliferation of cancer cells. We thus propose zanubrutinib as another suitable candidate for repurposing in HER2-amplified solid tumours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
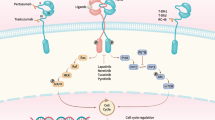
HER2-positive breast accounts for nearly 20–30% of breast cancer cases. The amplification or overexpression of the HER2 gene as well as its amenability to pharmacological modulation makes it an attractive target for drug discovery. The HER2 kinase is a member of the ERBB tyrosine kinase receptor family, together with EGFR, HER3 and HER4. Despite their molecular homology, ERBB kinases affect different signalling pathways depending on their homo- and heterodimerization, which is crucial for their enzymatic activity [1]. The HER2 pathway contributes to the regulation of cell growth, proliferation and survival via various signalling cascades, including the Ras/Raf/MAPK, mTOR and PI3K/Akt pathways [2]. Oncogenic activation of HER2 contributes to deregulated proliferation of breast tissue cells, leading to tumorigenesis. Among the molecular subtypes, HER2-positive breast cancer has a poor prognosis, with a higher rate of recurrence and tumour invasiveness [3].
However, HER2-positive carcinomas benefit from standard therapy combined with targeted therapy using anti-HER2 antibodies and small molecule inhibitors. The first approved monoclonal antibody, trastuzumab, acts through multiple mechanisms. Upon binding of the drug to the receptor, the RAS/MAPK and PI3K/AKT signalling pathways downstream of HER2 are inhibited, and ubiquitin-mediated degradation of HER2 is induced [4]. Another possible mechanism of action is through the attraction of innate immune cells [5]. Another example of an anti-HER2 agent is pertuzumab, which is frequently used in targeted therapy together with trastuzumab. The combination of these two antibodies appears to show high efficacy, as the inhibitory mechanism of pertuzumab complements that of trastuzumab by binding to a different epitope in the extracellular domain of the HER2 receptor [6]. Although acquired resistance to targeted therapies is a common obstacle in setting up a treatment regimen, insensitivity can be overcome by using small molecule inhibitors such as lapatinib, neratinib or tucatinib that target the catalytic function of the kinase [7, 8]. In addition, new drugs are still being sought. Apart from traditional drug discovery approaches, alternative ways include drug repurposing, i.e., searching for drugs among already approved pharmaceuticals.
Bruton’s tyrosine kinase (BTK) inhibitors are a group of low-molecular-weight kinase inhibitors that are effective in certain B-cell malignancies. Their target, the nonreceptor kinase BTK, is a key effector in the B-cell receptor pathway. Ibrutinib is a covalent BTK inhibitor that reacts with Cys481 in the active site of the kinase. As a first-in-class it that has been approved for the treatment of chronic lymphocytic leukaemia, the non-Hodgkin’s lymphomas, follicular lymphoma, mantle cell lymphoma, and Waldenström’s macroglobulinemia [9]. A variety of second-generation BTK inhibitors with improved pharmacological properties have been developed, including acalabrutinib, zanubrutinib, tirabrutinib and evobrutinib [9]. Their biochemical properties were compared in a review article by Estupiñán et al., 2021 [10].
In addition to their primary use in B-cell malignancies, repurposing of BTK inhibitors is being extensively investigated [11,12,13]. The drugs could be used for therapy of other cancer types expressing BTK, and some of them are currently being investigated in clinical trials (e.g., in acute myeloid leukaemia [14], colorectal carcinoma [15], prostate cancer [16]). In addition, the off-target kinases of BTK inhibitors provide a possibility to expand the use of these inhibitors in other cancers. Some of these sensitive kinases include ITK, TEC, BMX and TXK (members of the TEC kinase family, which also includes BTK) and the less related EGFR, HER2 and HER4 (members of the ERBB family) [17]. These kinases share a suitably positioned cysteine residue (analogous to Cys481 in BTK) in the active site. Importantly, ibrutinib and acalabrutinib have been identified to potently block ERBB and TEC kinases expressed in solid tumours [18,19,20]. Currently, there are over 20 ongoing clinical trials to verify the therapeutic efficacy of these two drugs beyond haemato-oncological malignancies, either in combination therapies or, more interestingly, as monotherapies.
The BTK inhibitor zanubrutinib has been approved for the treatment of mantle cell lymphoma and Waldenström’s macroglobulinemia [21]. Regarding its use in haemato-oncological malignancies, zanubrutinib has similar or slightly improved selectivity towards kinases in the TEC and ERBB families compared to ibrutinib, yet it is less potent than acalabrutinib [10]. However, its kinase selectivity profile has revealed promising potency towards ERBB kinases, namely, EGFR and HER4 [22, 23] (Supplementary Table 1). In fact, its biochemical properties are similar to those of ibrutinib [9, 10, 22], indicating that it could be another suitable candidate for repurposing in HER2-amplified solid tumours. Among the next-generation BTK inhibitors, zanubrutinib possesses the lowest IC50 values for the HER2 receptor [10]. We therefore investigated the anticancer effect of zanubrutinib in breast cancer cell lines. We describe zanubrutinib as a potential inhibitor of the HER2 signalling pathway, displaying antiproliferative effects in HER2-positive breast cancer cell lines, and we propose zanubrutinib as a candidate drug to be further investigated as a therapeutic agent in HER2-amplified breast cancer.
Methods
Cell lines and compounds
Human cancer cell lines (obtained from ATCC, USA, or DSMZ, Germany) were cultured according to the distributors’ instructions. In brief, MCF7, SKBR3, BT20, JIMT1 and BT474 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10–15% FBS, and T47D, HCC1806 and EFM192A cells were maintained in RPMI 1640 medium supplemented with 10–20% FBS. All media were supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM glutamine. For treatment, cells were seeded at densities of 1.5-2 million cells per dish in 60 mm dishes and allowed to adhere overnight. The BTK inhibitors ibrutinib, evobrutinib, tirabrutinib, acalabrutinib and zanubrutinib were purchased from MedChemExpress, and the EGFR/HER2 inhibitor lapatinib was purchased from LC Laboratories.
Cytotoxicity assay
For the cytotoxicity assays, cells were seeded into 96-well plates. After overnight preincubation, cells were treated in triplicate with six different concentrations of each compound for 72 h. After treatment, resazurin solution (Sigma Aldrich) was added for 4 h, and the fluorescence of resorufin, corresponding to living cells, was measured at 544 nm/590 nm (excitation/emission) using a Fluoroskan Ascent microplate reader (Labsystems). The results of the assays were used to construct sigmoidal dose‒response curves, and to determine GI50 values (the drug concentration lethal to 50% of the cells) using Origin 6.0 software. The experiments were performed in technical triplicates in at least three independent biological replicates, and mean values ± standard deviations were calculated.
Immunoblotting
The cells were lysed using RIPA lysis buffer supplemented with NaF (1 mM), Na3VO4 (1 mM), DTT (1 mM), PMSF (1 mM), aprotinin (0.5 µg/ml) and leupeptin (2 µg/ml). After cell lysis, proteins were separated using SDS‒PAGE and electroblotted onto a nitrocellulose membrane. After 1 h of blocking with bovine serum albumin, the membrane was incubated overnight with specific primary antibodies and then for 1 h with peroxidase-conjugated secondary antibodies. Peroxidase activity was then detected with SuperSignal West Pico reagents, and band intensities were measured using a LAS-4000 CCD camera. The following specific antibodies were used and purchased from Cell Signaling: anti-EGFR (D38B1), anti-HER2/ErbB2 (D8F12), anti-HER3/ErbB3 (D22C5), anti-HER4/ErbB4 (111B2), anti-phospho-EGFR Y1068 (D7A5), anti-phospho-HER2/ErbB2 Y1221/1222 (6B12), anti-phospho-HER3/ErbB3 Y1289 (D1B5), anti-phospho-HER4/ErbB4 Y1284/EGFR Y1173 (21A9), anti-Akt (pan) (C67E7), anti-phospho-Akt S473 (D9E), anti-p44/42 MAPK (Erk1/2), anti-phospho-p44/42 MAPK (phospho-Erk1/2) T202/Y204), and anti-PARP (46D11). Anti-β-actin (C4) was purchased from Santa Cruz Biotechnology. Each experiment (treatment and immunoblotting analysis) was performed at least twice, and the representative figures are shown.
Cell cycle analysis
Analysis of the cell cycle distribution was performed in 96-well plates. Asynchronous cells were seeded and treated with different concentrations of the compounds for 24 h. After incubation, cells were trypsinised and then stained with 5× staining solution (17 mM trisodium citrate dihydrate, 0.5% IGEPAL CA-630, 7.5 mM spermine tetrahydrochloride, and 2.5 mM Tris; pH 7.6) supplemented with propidium idodide (50 µg/mL). The DNA content of the cells was measured by flow cytometry using a 488 nm laser (BD FACSVerse with BD FACSuite software, version 1.0.6.). The cell cycle distribution was analysed with ModFit LT (Verity Software House, version 4.1.7). The experiments were performed in three independent biological replicates, and mean values ± standard deviations were calculated.
Colony formation
Cells were seeded at a density of 5000 cells/ml in 6-well plates and allowed to adhere overnight. Cells were then treated with compounds and incubated for 10 days. After the treatment, colonies were fixed with 70% ethanol, washed with PBS and stained with crystal violet. After 1 h of incubation at RT, excess stain was removed by washing with PBS and distilled water, and the stained cell colonies were imaged by scanning. Colony formation was quantified by measuring the absorbance (570 nm, Infinite 200 Pro microplate reader, Tecan, Life Sciences) of crystal violet after solubilization with 1 ml 1% SDS. The experiments were performed in at least two independent biological replicates, the mean values ± standard deviations were calculated, and the representative images are shown.
Results
Anticancer effect of BTK inhibitors in breast cancer cell lines in vitro
The anticancer effect of the BTK inhibitors ibrutinib, acalabrutinib, tirabrutinib, evobrutinib and zanubrutinib was evaluated in a panel of ten breast cancer cell lines in vitro. The cell lines were categorized into two groups according to the reported expression status of the receptor: HER2-positive and HER2-negative [24, 25]. HER2 amplification and protein expression in the panel of cell lines were confirmed by FISH and western blot analysis, respectively (Supplementary Fig. 1).
The effect of the BTK inhibitors in the cell line panel was investigated, and GI50 values were determined after 72 h of treatment using a resazurin assay to assess viability (Table 1). Lapatinib was used as a positive control. The HER2-negative cell lines (MCF7, T47D, BT20, HCC1806) were insensitive to treatment with all compounds. In contrast, four HER2-positive cell lines were found to be sensitive to ibrutinib, zanubrutinib and acalabrutinib, with submicromolar GI50 values ranging from 0.09 µM to 0.88 µM for ibrutinib and zanubrutinib and single-digit micromolar GI50 values for acalabrutinib. The viability of the HER2-positive cell line JIMT-1 was not affected by the tested compounds, but these cells are known to be trastuzumab resistant [25]. Interestingly, the novel BTK inhibitor zanubrutinib was active at concentrations comparable to lapatinib. The activity of ibrutinib was approximately 1.5-8 times higher than that of lapatinib, which is in agreement with previously published data [19].
Zanubrutinib and acalabrutinib inhibit ERBB signalling in HER2-positive breast cancer cell lines
The effect of the tested BTK inhibitors on ERBB signalling was analysed in cancer cell lines stimulated by heregulin; a similar experimental setup was used in a recently published study with ibrutinib [19]. Heregulin is the most broadly active ERBB ligand in HER2-positive breast cancer cells [26]. Heregulin is a potent activator, especially of HER3 and HER4, and possesses mitogenic activity in breast cancer cells [27, 28].
Specifically, the activating autophosphorylation of ERBB2 and subsequent phosphorylation of the downstream kinases Akt and ERK1/2 were analysed in cells treated with BTK inhibitors at a single concentration of 10 µM for 16 h (Fig. 1). According to previous reports, ibrutinib impaired the phosphorylation of ERBB receptors and the downstream kinases Akt and ERK1/2 [19, 29, 30]. Among the other compounds, zanubrutinib was similarly effective. Acalabrutinib and tirabrutinib showed slight inhibitory effects against EGFR and HER2 in some cell lines. Treatment with evobrutinib did not cause any changes in the phosphorylation of the evaluated proteins, probably due to its selectivity profile with minimum effects towards ERBB receptor kinases [31]. In the HER2-negative cell line MCF7, no changes in the phosphorylation of the ERBB downstream target (pERK1/2 T202/Y204) were detected (Supplementary Fig. 2).
Effects of BTK inhibitors on signalling pathways in HER2-positive breast cancer cell lines. Compounds were used at a 10 µM concentration for 16 h of treatment. Cells were stimulated by heregulin (HRG, 0.1 µg/mL) 30 min prior to harvesting. β-Actin served as a control for equal loading. The representative results from at least two biological replicates are shown
Next, the dose-dependent effect of zanubrutinib was examined in BT474 and SKBR3 cells treated for 16 h. Activation of ERBB receptors was impaired starting at a 1 µM concentration of the compound in BT474 cells, and the effect was dose related (Fig. 2).
Dose-dependent effect of zanubrutinib in HER2-positive BT474 and SKBR3 cell lines. Cells were treated with increasing concentrations of zanubrutinib for 16 h. Cells were stimulated by heregulin (HRG, 0.1 µg/mL) 30 min prior to harvesting. β-Actin served as a control for equal loading. The representative results from at least two biological replicates are shown
BTK inhibitors cause G1 arrest in cell lines overexpressing HER2
Cell cycle arrest at the G1-S transition induced by ibrutinib has been previously reported [19, 29]. As expected, zanubrutinib was found to have a similar effect on cell cycle progression in the HER2-positive cell lines BT474 and SKBR3. The compound increased the number of cells in G1 phase at higher concentrations than did ibrutinib (Fig. 3). The potent and specific EGFR/HER2 inhibitor lapatinib, used as a positive control, also resulted in accumulation of cells in G1 phase, although at much lower concentrations. In control experiments, no changes in the cell cycle were observed in HER2-negative MCF7 cells upon exposure to these three tested compounds (Supplementary Fig. 3).
Heregulin impairs the effect of BTK inhibitors on the cell cycle distribution
We hypothesized that the addition of heregulin to cells exposed to BTK inhibitors would decrease the potency of these inhibitors and thus help to confirm that their mechanism of action is related to ERBB inhibition. We first analysed the effect of heregulin on the growth and viability of HER2-positive cells treated with ibrutinib and zanubrutinib for 72 h. The results showed that cell growth and viability were restored when the treated cells were exposed simultaneously to heregulin (Table 2).
Next, we tested the effect of different concentrations of ibrutinib, zanubrutinib, and lapatinib on the cell cycle in HER2-positive cells stimulated with heregulin. Cotreatment with heregulin clearly rescued BT474 cells from G1 phase arrest at lower concentrations of the compounds (Fig. 3). The effect of the inhibitors was delayed under cotreatment with heregulin in comparison with treatment with each compound alone. The ability of heregulin to rescue HER2-positive breast cancer cells from the growth inhibition induced by ibrutinib and lapatinib is consistent with other findings [29].
Heregulin rescues HER2-positive cells from G1 arrest induced by BTK inhibitors. Cells were treated for 24 h with increasing concentrations of the selected compounds, with or without activation by heregulin (HRG, 0.1 µg/ml). Results are averages of biological triplicates, the error bars represent standard deviation
Ibrutinib and zanubrutinib suppress colony formation and induce apoptosis in HER2-positive breast cancer cells
To further confirm the anticancer effect of zanubrutinib in the HER2-positive cell lines SKBR3 and BT474, we performed a long-term treatment experiment. SKBR3 cells showed a reduced ability to form colonies in the presence of zanubrutinib in a dose-dependent manner (Fig. 4A), although the effect was weaker than that of ibrutinib [29]. In agreement with the insensitivity of HER2-negative breast cancer cells to the studied BTK inhibitors (Table 1), the control cell line MCF7 also showed no decrease in colony formation after treatment with any of the compounds.
BTK inhibitors not only block the proliferation of HER2-positive cells but also directly induce their death. The level of cleaved PARP-1 was investigated in SKBR3, BT474 and MCF7 cells. Treatment with zanubrutinib and ibrutinib resulted in an increase in the cleaved PARP-1 fraction after 24 h in HER2-positive cell lines (Fig. 4B). In contrast, no change in the PARP-1 level was observed in HER2-negative MCF7 cells.
(A) Ibrutinib and zanubrutinib inhibit colony formation in HER2 + SKBR3 cells, while the proliferation rate of HER2- MCF7 cells remains unaffected. Colonies were stained with crystal violet after 10 days of treatment. The numbers indicate the percentages of colonies formed (calculated from the absorbance values, numbers are averages of biological duplicates ± SD). (B) Immunoblot analysis of lysates of cells treated with 1 µM zanubrutinib and ibrutinib. β-Actin served as a control for equal loading. The representative images are shown
Discussion
A current trend and attractive subject of research in drug development is the reuse of already known and approved drugs for new diseases, a strategy known as drug repurposing. Although modern approaches in novel drug development allow the design and dynamic development of potential pharmaceuticals, the search for suitable candidate compounds and their subsequent translation into therapies remains a relatively high hurdle. In the case of drug repurposing strategies, the application of established drugs for other indications brings with it some advantages. These include the already known safety profiles of the drugs, the acceleration of the approval process, and the consequent reduction in costs.
Ibrutinib and its follow-up BTK inhibitors are being investigated in a variety of clinical trials beyond their primary indications approved by the FDA. The inclusion of the drugs in preclinical tests utilizes both the primary target, BTK, and off-target kinases possessing a homologous Cys481 residue in the active site of the enzyme (e.g., EGFR, HER2, ITK) [17]. For ibrutinib and acalabrutinib, several clinical trials in solid tumours are ongoing. Zanubrutinib, the most recently approved BTK inhibitor, has not yet been evaluated for repurposing for solid tumours, although its biochemical and pharmacokinetic properties suggest possible activity towards HER2-overexpressing cancers. In our study, we therefore aimed to examine the potential repurposing of zanubrutinib and other selected BTK inhibitors in breast cancer cell line models.
Among the drugs tested in this study, ibrutinib and zanubrutinib demonstrated clear effects on cell models of HER2-positive breast carcinomas, as expected. The efficiency of ibrutinib in these cancers has already been proposed in previous publications [19, 29, 30], and we indeed found ibrutinib to be the most potent BTK inhibitor of ERBB signalling in breast cancer cell lines, once again confirming its anticancer effects. The potency of other tested BTK inhibitors against HER2-positive cell lines reflects the previously reported inhibition of individual ERBB receptors. Most interestingly among these other BTK inhibitors, zanubrutinib has been proven to inhibit the activity of EGFR and HER4 (86 and 96%, respectively) and of HER2 by 40% at 1 µM [22]. Our findings are in agreement with previously stated inhibitory potencies and reveal zanubrutinib as an effective inhibitor of proliferation and signalling in HER2-positive breast cancers.
We demonstrated that zanubrutinib effectively reduced the levels of phosphorylated forms of ERBB receptors in the HER2-overexpressing cell lines BT474, SKBR3, AU565 and EFM192A. Subsequently, downstream signalling of the Akt and ERK pathways was impaired, leading to reduced proliferation of the cells and proapoptotic effects. The effect of zanubrutinib was detectable when cells were treated with 3 µM zanubrutinib overnight (Fig. 2). The importance of the HER2 receptor in signalling and survival in HER2-positive breast cancer cell lines was supported by the strong antiproliferative effect of ibrutinib and zanubrutinib at submicromolar concentrations in these cell lines compared to cells with low expression of HER2, which were insensitive to BTK inhibitors.
In the presence of zanubrutinib, HER2-positive breast cancer cells exhibited G1 arrest. The deregulation of cell cycle progression in HER2-amplified cells reflects the detected inhibitory effect of BTK inhibitors on the phosphorylation of proteins in ERBB receptor-controlled pathways. G1 arrest was also observed in cells treated with ibrutinib and lapatinib, which were used as controls, suggesting that zanubrutinib acts via a similar mechanism [29].
Importantly, the HER2-positive breast cancer cell lines tested in this study (BT474, SKBR3, AU565, EFM192A) are sensitive to submicromolar concentrations of zanubrutinib (Table 1 ; Fig. 4), which are lower than the maximum plasma concentration (cmax) in humans (1.4 µM for a dose of 320 mg per os) [32]. The ratio of the measured GI50 values to the known clinically achievable plasma concentration of zanubrutinib indicates its potential efficacy in HER2-positive breast cancer therapy. Ibrutinib, which is already being investigated in clinical trials for HER2-positive breast cancer, and acalabrutinib are also active in HER2-positive cells at concentrations similar to cmax [33, 34].
In previous studies, the BTK-C transcript was detected in HER2-positive breast cancer cells [35]. Based on that finding, it was proposed that BTK-C signalling could be involved in the appearance of ligand-dependent lapatinib resistance in HER2-positive breast cancer cells and thus may be a potential therapeutic target in combination with HER2 in this subtype of breast carcinoma [29]. The molecular weight of BTK-C is 79.9 kDa [35], and its expression is detectable using a commercial antibody against BTK-A; however, in our study, we were not able to detect the expression of BTK in any of the tested breast cancer cell lines (Supplementary Fig. 1). Moreover, the study by Eifert et al. revealed BTK expression in a HER2-negative cell line and showed increased levels of apoptosis in BTK knockdown cells, whether HER2-positive or HER2-negative [35]. Thus, the presence of BTK in breast cancer cells may contribute to the effect of BTK inhibitors in HER2-positive cells. However, we assume that the effect of ibrutinib and zanubrutinib in this breast cancer subtype presumably stems from inhibition of ERBB signalling, as we did not observe any antiproliferative effects of the compounds in HER2-negative cells. In particular, inhibition of EGFR, HER3 and HER4 may contribute to the effects of zanubrutinib, as we have detected their dephosphorylation and previous reports have demonstrated their inhibition by zanubrutinib [22]. However, a report by Stanley et al. showed no significant differences in EGFR, HER3 and HER4 expression between the HER2-positive cell lines BT474, SKBR3 and HER2-negative MCF7 cells [36].
In conclusion, our results support the potential indication of the BTK inhibitor zanubrutinib for use in HER2-amplified breast carcinomas. The anticancer effects of zanubrutinib have been described in vitro and we suggest that the compound is an attractive drug to be tested in further in vivo models and trials for potential repositioning outside of haemato-oncological diseases.
References
Jones RB, Gordus A, Krall JA, MacBeath G (2006) A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature 439:168–174. https://doi.org/10.1038/nature04177
Yarden Y, Pines G (2012) The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 12:553–563. https://doi.org/10.1038/nrc3309
Schlam I, Swain SM (2021) HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. npj Breast Cancer 7:56. https://doi.org/10.1038/s41523-021-00265-1
Vu T, Claret FX (2012) Trastuzumab: updated mechanisms of action and resistance in breast Cancer. Front Oncol 2:62. https://doi.org/10.3389/fonc.2012.00062
Kreutzfeldt J, Rozeboom B, Dey N, De P (2020) The trastuzumab era: current and upcoming targeted HER2 + breast cancer therapies. Am J Cancer Res 10:1045–1067
Scheuer W, Friess T, Burtscher H et al (2009) Strongly enhanced Antitumor Activity of Trastuzumab and Pertuzumab Combination Treatment on HER2-Positive human xenograft Tumor Models. Cancer Res 69:9330–9336. https://doi.org/10.1158/0008-5472.CAN-08-4597
Hicks M, Macrae ER, Abdel-Rasoul M et al (2015) Neoadjuvant Dual HER2-Targeted Therapy with Lapatinib and Trastuzumab improves pathologic complete response in patients with early stage HER2-Positive breast Cancer: a Meta-analysis of randomized prospective clinical trials. Oncologist 20:337–343. https://doi.org/10.1634/theoncologist.2014-0334
Wahdan-Alaswad R, Liu B, Thor AD (2020) Targeted lapatinib anti-HER2/ErbB2 therapy resistance in breast cancer: opportunities to overcome a difficult problem. Cancer Drug Resist 3:179–198. https://doi.org/10.20517/cdr.2019.92
Kaptein A, de Bruin G, Emmelot-van Hoek M et al (2018) Potency and selectivity of BTK inhibitors in Clinical Development for B-Cell Malignancies. Blood 132:1871–1871. https://doi.org/10.1182/blood-2018-99-109973
Estupiñán HY, Berglöf A, Zain R, Smith CIE (2021) Comparative analysis of BTK inhibitors and mechanisms underlying adverse Effects. Front Cell Dev Biol 9:630942. https://doi.org/10.3389/fcell.2021.630942
Shi Y, Guryanova OA, Zhou W et al (2018) Ibrutinib inactivates BMX-STAT3 in glioma stem cells to impair malignant growth and radioresistance. Sci Transl Med 10:eaah6816. https://doi.org/10.1126/scitranslmed.aah6816
Zhu S, Gokhale S, Jung J et al (2021) Multifaceted Immunomodulatory Effects of the BTK inhibitors Ibrutinib and Acalabrutinib on different Immune Cell Subsets – Beyond B Lymphocytes. Front Cell Dev Biol 9:727531. https://doi.org/10.3389/fcell.2021.727531
Gao W, Wang M, Wang L et al (2014) Selective Antitumor activity of Ibrutinib in EGFR-Mutant non–small cell Lung Cancer cells. JNCI J Natl Cancer Inst 106:dju204. https://doi.org/10.1093/jnci/dju204
Cortes JE, Jonas BA, Graef T et al (2019) Clinical experience with Ibrutinib alone or in combination with either cytarabine or azacitidine in patients with Acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 19:509–515e1. https://doi.org/10.1016/j.clml.2019.05.008
Kim DW, Tan E, Zhou J-M et al (2021) A phase 1/2 trial of ibrutinib in combination with pembrolizumab in patients with mismatch repair proficient metastatic colorectal cancer. Br J Cancer 124:1803–1808. https://doi.org/10.1038/s41416-021-01368-z
Pachynski R, Reimers M, Weimholt C et al (2021) 423 a phase 2 study of ibrutinib as neoadjuvant therapy in patients with localized prostate cancer. J Immunother Cancer 9:A453–A453. https://doi.org/10.1136/jitc-2021-SITC2021.423
Singh J, Petter RC, Kluge AF (2010) Targeted covalent drugs of the kinase family. Curr Opin Chem Biol 14:475–480. https://doi.org/10.1016/j.cbpa.2010.06.168
Rauf F, Festa F, Park JG et al (2018) Ibrutinib inhibition of ERBB4 reduces cell growth in a WNT5A-dependent manner. Oncogene 37:2237–2250. https://doi.org/10.1038/s41388-017-0079-x
Chen J, Kinoshita T, Sukbuntherng J et al (2016) Ibrutinib inhibits ERBB receptor tyrosine kinases and HER2-amplified breast cancer cell growth. Mol Cancer Ther 15:2835–2844. https://doi.org/10.1158/1535-7163.MCT-15-0923
Wang A, Yan X-E, Wu H et al (2016) Ibrutinib targets mutant-EGFR kinase with a distinct binding conformation. Oncotarget 7:69760–69769. https://doi.org/10.18632/oncotarget.11951
Muñoz J, Paludo J, Sarosiek S, Castillo JJ (2022) Coming of age for BTK inhibitor therapy: a review of Zanubrutinib in Waldenström Macroglobulinemia. Cells 11:3287. https://doi.org/10.3390/cells11203287
Guo Y, Liu Y, Hu N et al (2019) Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 62:7923–7940. https://doi.org/10.1021/acs.jmedchem.9b00687
Crawford JJ, Johnson AR, Misner DL et al (2018) Discovery of GDC-0853: a potent, selective, and Noncovalent Bruton’s tyrosine kinase inhibitor in early clinical development. J Med Chem 61:2227–2245. https://doi.org/10.1021/acs.jmedchem.7b01712
Dai X, Cheng H, Bai Z, Li J (2017) Breast Cancer cell line classification and its relevance with breast tumor subtyping. J Cancer 8:3131–3141. https://doi.org/10.7150/jca.18457
Tanner M, Kapanen AI, Junttila T et al (2004) Characterization of a novel cell line established from a patient with herceptin-resistant breast cancer. Mol Cancer Ther 3:1585–1592. https://doi.org/10.1158/1535-7163.1585.3.12
Grant S, Qiao L, Dent P (2002) Roles of Erbb family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front Biosci 7:d376–d389. https://doi.org/10.2741/grant
Lim S-J, Lopez-Berestein G, Hung M-C et al (2000) Grb2 downregulation leads to akt inactivation in heregulin-stimulated and ErbB2-overexpressing breast cancer cells. Oncogene 19:6271–6276. https://doi.org/10.1038/sj.onc.1204014
Falls D (2003) Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284:14–30. https://doi.org/10.1016/S0014-4827(02)00102-7
Wang X, Wong J, Sevinsky CJ et al (2016) Bruton’s tyrosine kinase inhibitors prevent therapeutic escape in breast cancer cells. Mol Cancer Ther 15:2198–2208. https://doi.org/10.1158/1535-7163.MCT-15-0813
Grabinski N, Ewald F (2014) Ibrutinib (Imbruvica™) potently inhibits ErbB receptor phosphorylation and cell viability of ErbB2-positive breast cancer cells. Invest New Drugs 32:1096–1104. https://doi.org/10.1007/s10637-014-0141-2
Caldwell RD, Qiu H, Askew BC et al (2019) Discovery of Evobrutinib: an oral, potent, and highly selective, covalent Bruton’s tyrosine kinase (BTK) inhibitor for the treatment of Immunological Diseases. J Med Chem 62:7643–7655. https://doi.org/10.1021/acs.jmedchem.9b00794
Tam CS, Trotman J, Opat S et al (2019) Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 134:851–859. https://doi.org/10.1182/blood.2019001160
Liston DR, Davis M (2017) Clinically relevant concentrations of Anticancer Drugs: a Guide for Nonclinical Studies. Clin Cancer Res 23:3489–3498. https://doi.org/10.1158/1078-0432.CCR-16-3083
Patel VK, Lamothe B, Ayres ML et al (2018) Pharmacodynamics and proteomic analysis of acalabrutinib therapy: similarity of on-target effects to ibrutinib and rationale for combination therapy. Leukemia 32:920–930. https://doi.org/10.1038/leu.2017.321
Eifert C, Wang X, Kokabee L et al (2013) A novel isoform of the B cell tyrosine kinase BTK protects breast cancer cells from apoptosis. Genes Chromosom Cancer 52:961–975. https://doi.org/10.1002/gcc.22091
Stanley A, Ashrafi GH, Seddon AM, Modjtahedi H (2017) Synergistic effects of various her inhibitors in combination with IGF-1R, C-MET and src targeting agents in breast cancer cell lines. Sci Rep 7:3964. https://doi.org/10.1038/s41598-017-04301-8
Acknowledgements
We thank Vladimíra Koudeláková for performing FISH analysis.
Funding
The work was supported by the European Union—Next Generation EU (The project National Institute for Cancer Research, Programme EXCELES, ID No. LX22NPO5102), Czech Science Foundation (21-06553S) and Palacký University Olomouc (IGA_PrF_2022_007).
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data acquisition and analysis as well as preparing the first draft was performed by H.D., data analysis and writing of the manuscript was done by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dostálová, H., Jorda, R., Řezníčková, E. et al. Anticancer effect of zanubrutinib in HER2-positive breast cancer cell lines. Invest New Drugs 41, 210–219 (2023). https://doi.org/10.1007/s10637-023-01346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01346-7