Abstract
SYHA1813 is a potent multikinase inhibitor that targets vascular endothelial growth factor receptors (VEGFRs)/colony-stimulating factor 1 receptor (CSF1R). This study aimed to evaluate the safety, pharmacokinetics (PK), and antitumor activity of escalating doses of SYHA1813 in patients with recurrent high-grade gliomas (HGGs) or advanced solid tumors. This study adopted a combination of accelerated titration and a 3 + 3 design for dose escalation, with a starting dose of 5 mg once daily. The dose escalation continued at successive dose levels until the maximum tolerated dose (MTD) was determined. A total of 14 patients were enrolled and treated, including 13 with WHO grade III or IV gliomas and 1 with colorectal cancer. Two patients experienced dose-limiting toxicities (grade 4 hypertension and grade 3 mucositis oral) at 30 mg SYHA1813. The MTD was defined as 15 mg once daily. Hypertension (n = 6, 42.9%) was the most frequent treatment-related adverse event. Among evaluable patients (n = 10), 2 (20%) patients achieved partial response, and 7 (70%) had stable disease. The exposure increased with increasing doses within the studied dose range of 5 to 30 mg. Biomarker assessments demonstrated significant reductions in the levels of soluble VEGFR2 (P = .0023) and increases in the levels of VEGFA (P = .0092) and placental growth factor (P = .0484). The toxicities of SYHA1813 were manageable, and encouraging antitumor efficacy was observed in patients with recurrent malignant glioma. This study is registered with the Chinese Clinical Trial Registry (www.chictr.org.cn/index.aspx; identifier ChiCTR2100045380).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in treating recurrent high-grade glioma (HGG, WHO grade III and IV gliomas), the prognosis remains poor, and much work still needs to be done for improvement. Malignant tumors require neovascularization for growth, invasion, and metastasis[1]. Vascular endothelial growth factor (VEGF) and its receptors (VEGFR-1 to -3) play major roles in tumor angiogenesis[2]. As VEGFR is attractive target, various VEGFR tyrosine kinase inhibitors (TKIs) have been successfully approved for multiple types of cancer. However, none of these TKIs have been approved for patients with HGG, who may rapidly develop resistance to anti-VEGFR therapy with activation of compensatory angiogenesis, growth, and survival pathways[3, 4].
Tumor-associated macrophages (TAMs), defined as macrophages infiltrating the tumor microenvironment (TME), comprise 30–40% of the tumor mass in HGG and play a major role in promoting tumor angiogenesis and progression[5, 6]. Macrophage colony-stimulating factor 1 (CSF1) is a classical protumor cytokine that binds to the CSF1 receptor (CSF1R) to polarize macrophages in the TME from an antitumor M1-like phenotype toward the protumor M2 phenotype and promotes tumor angiogenesis, proliferation, metastasis, and immune escape[7, 8]. Inhibitors of CSF1R can inhibit the infiltration of macrophages in the TME, reshape their polarity, promote CD8+ T-cell infiltration, and prevent tumor progression[9]. A previous study showed that the TAM infiltration level was inversely correlated with survival in HGG patients treated with anti-VEGF therapy[10, 11]. Furthermore, M2 macrophage polarization has proven to be related to resistance to antiangiogenic treatments[12, 13]. These findings suggest that repolarizing the M1-like phenotype of macrophages is a potential therapeutic mechanism to reverse resistance to anti-VEGF therapy.
SYHA1813 is a selective TKI that inhibits VEGFR-1 (half maximal inhibitory concentration [IC50] = 2.8 nmol/L), VEGFR-2 (IC50 = 0.3 nmol/L), VEGFR-3 (IC50 = 4.3 nmol/L), and CSF1R (IC50 = 19.3 nmol/L) in kinase enzyme assays[14, 15]. The antitumor activity of SYHA1813 has been demonstrated in diverse xenograft models, and this activity was consistent with potent inhibition of angiogenesis and CSF1R signaling in a preclinical study (Shanghai Institute of Materia Medica, unpublished data, 2022).
The present study is a dose-escalation phase I clinical trial to evaluate the safety, pharmacokinetics (PK), maximum tolerated dose (MTD), efficacy, and potential biomarkers of SYHA1813 in patients with recurrent HGG or advanced solid tumors.
Methods
Patient eligibility
Eligible patients were ≥ 18 years of age with recurrent or advanced solid tumors confirmed by histology or cytology refractory to standard therapy or for which no effective therapy was available. Patients had at least one measurable lesion during the baseline period (primary central nervous system tumors were assessed as per the Response Assessment in Neuro-Oncology [RANO] criteria; other solid tumors were assessed as per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1). The time interval between the end of the last antitumor treatment and the first administration of SYHA1813 was ≥ four weeks for cytotoxic drugs, immunotherapy, macromolecular targeted drugs, and biological therapy; ≥ two weeks or five half-lives (whichever is longer) for oral small-molecule targeted drug therapy, anticancer traditional Chinese medicines or proprietary Chinese medicines; and ≥ four weeks for radiotherapy (≥ two weeks for palliative local radiotherapy for symptom relief). Other inclusion criteria included a Karnofsky Performance Status (KPS) of 60 or more for patients with central nervous system tumors, an Eastern Cooperative Oncology Group (ECOG) physical performance status of 0 to 2 for patients with other solid tumors, and a life expectancy of 12 weeks.
Exclusion criteria included participation in other interventional clinical studies within four weeks, major surgery within four weeks, use of glucocorticoids at a dose equivalent to more than 5 mg dexamethasone within five days for glioma or brain metastases, and use of bevacizumab, ramucirumab, and other anti-VEGF/VEGFR antibodies within three months for glioma or brain metastases. Patients were also excluded if they had impaired cardiac function or clinically significant cardiovascular and cerebrovascular diseases, including but not limited to a history of myocardial infarction, congestive heart failure, and unstable angina pectoris within six months; cerebrovascular accident within six months (patients with transient ischemic attack or lacunar infarction with no clinical significance could be enrolled); hypertension uncontrollable after medication (repeated blood pressure measurement at least 1 h apart, and blood pressure 150/90 mmHg at two consecutive tests); uncontrolled arrhythmia requiring medical treatment; QTc interval > 470 ms on electrocardiogram (ECG) examination; or left ventricular ejection fraction < 50%.
Study design and drug administration
This was a phase Ia, multicenter, open-label, dose-escalation study of SYHA1813. The overall study design is presented in Supplementary Fig. S1. The primary endpoints were the safety and tolerability of SYHA1813, including the occurrence of dose-limiting toxicities (DLTs) and the establishment of the maximum tolerated dose (MTD). Secondary endpoints included determination of the PK profile and preliminary antitumor activity based on the objective response rate (ORR), and disease control rate (DCR), as well as exploration of potential biomarkers related to SYHA1813 therapy. The study was approved by the National Medical Products Administration (NMPA) for clinical trials. We conducted the study according to the ethical principles of the Declaration of Helsinki and Good Clinical Practices guidelines. The study was approved by an institutional review board at each participating site. All patients provided written informed consent. The study was registered at chictr.org.cn (trial registration ID: ChiCTR2100045380).
The maximum recommended starting dose (based body weight 60 kg) of 11 mg was estimated based on 1/6 of the highest non-severely toxic dose in the beagle dog study. Based on further safety considerations, 5 mg was selected as the starting dose in human subjects. In the dose-escalation study, patients were enrolled sequentially to receive a single oral dose of SYHA1813 (5, 15, 30, 60, 100, 150, and 200 mg) followed by a 3-day observation period with safety and PK assessments, subsequent once-daily treatment at the same dose level during 21-day multiple-dose period followed by a 4-day observation period with safety, PK and efficacy assessments. From the second cycle onwards, the study drug was given every day (3 weeks/cycle) if the treatment was well tolerated and beneficial.
Safety assessments and definition of DLT
DLT was defined as the occurrence of adverse events (AEs) that the investigator judged to be related to SYHA1813 within 28 days of the first dose during the dose-escalation period (Supplementary Table S1). The MTD was defined as the maximum dose for which the probability of a DLT was ≤ 33%.
An accelerated titration design with one patient enrolled was utilized for the first two dose levels (5 and 15 mg). Then, a 3 + 3 dose-escalation design was employed starting from the third dose level (30 mg). If grade 3 or above adverse events (AEs, non-DLTs) occurred in one patient at the first two doses, two more patients were enrolled for further observation. If DLT was observed in one of the 3 patients, up to 3 more patients were enrolled at the same level. If 2 or more out of 3 to 6 patients experienced DLT at a dose level, the dose was decreased to the previous dose group. When decreasing to the previous dose group, if there were only 3 patients in the dose group, 3 more patients were added; if there were already 6 patients, the dose escalation ended, and the dose was defined as the MTD. Any patient who withdrew from the study before the completion of the DLT observation period due to a reason other than DLT was replaced. Dose de-escalation or reduction was permitted if safety re-evaluation was deemed necessary.
Safety analyses were conducted for all patients who received at least one dose of SYHA1813. AEs were assessed throughout the study using National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The health status assessment of the patients included a physical examination (vital signs, weight, PS score, etc.), hematologic and biochemical profiling, routine urine and stool tests, and electrocardiogram assessment performed at screening and throughout the study.
PK evaluation
Blood samples for the assessment of PK parameters were collected at predefined time points during the DLT observation period as follows: Cycle 0 Day 1 (predose; 0.5, 1, 2, 4, 8, and 12 h postdose), Day 2 (24 h postdose), and Day 3 (48 h postdose); Cycle 1 Day 1 (predose), Day 8 (predose), Day 15 (predose), Day 21 (predose; 0.5, 1, 2, 4, 8, and 12 h postdose), Day 22 (24 h postdose), Day 23 (48 h postdose), and Day 24 (72 h postdose). Plasma SYHA1813 levels were measured using a validated high-performance liquid chromatography‒mass spectrometry method.
Single- and multiple-dose PK parameters of SYHA1813 were estimated using noncompartmental analysis, including maximum observed plasma concentration (Cmax), Cmax/dose, time to Cmax (Tmax), area under curve extrapolated to infinity (AUCinf), AUCinf/dose, volume of distribution (Vz/F), terminal elimination half-life (t1/2), and oral clearance (CL/F) after the first dose. PK parameters at steady-state (ss), including Cmax, ss, Cmax, ss/dose, minimum observed plasma concentration (Cmin, ss), AUCinf, AUCinf/dose, Tmax, ss, Vss, t1/2, CLss/F after multiple doses, and accumulation index (Rac), were also estimated.
Efficacy assessment
Radiographic assessments were conducted at screening and at the end of the DLT observation period and repeated every six weeks during the extended treatment period until disease progression, intolerable toxicity, or withdrawal of consent (whichever came first). The patients with central nervous system tumors were evaluated for responses according to the RANO criteria by enhanced magnetic resonance imaging (MRI) scan. Patients with other solid tumors were assessed by computed tomography (CT) or MRI scan according to RECIST version 1.1. The categories used for the evaluation of the response to treatment included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR and DCR were defined as CR + PR and CR + PR + SD, respectively.
Exploratory biomarker analysis
Samples for biomarker analyses were collected on Cycle 1 Day 1 (predose), Cycle 1 Day 24 (72 h post-dose), and 14 days after the end of treatment. Soluble VEGFR2, VEGF, and CSF1 levels were measured using multiplex enzyme-link immunosorbent assay (ELISA) plates from R&D Systems (Minneapolis, MN), and placental growth factor (PlGF) levels were measured using an electrochemiluminescence immunoassay (ECLIA) kit from Roche Diagnostics (Mannheim, Germany).
Statistical analysis
All statistical analyses were performed using SAS® 9.4 (SAS Institute, Inc., Cary, NC, USA) except for the calculation of PK parameters using Phoenix® WinNonlin 8.1 (Pharsight Corp., Certara, Princeton, NJ, USA). Exploratory biomarker analyses were performed by a two-tailed paired t test using GraphPad Prism 9.3 (GraphPad Software, Inc., CA, USA).
Results
Patients and treatments
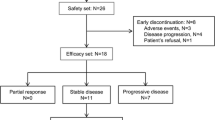
This dose-escalation study was conducted at 4 study centers in China starting on May 26, 2021. As of the data cutoff date of February 15, 2022, 14 patients had received SYHA1813, including 1 patient in the 5-mg group, 8 patients in the 15-mg group, and 5 patients in the 30-mg group. Ten patients remained in the study for the entire DLT observation period (Supplementary Fig. S2). Reasons for discontinuing treatment were AEs (n = 3), withdrawal of consent (n = 2), DLT (n = 2), and investigator discretion (n = 1). The baseline characteristics of the patients are summarized in Table 1. The median age was 46 years, and 64.3% of the patients were male. Among the 14 enrolled patients, 4 patients were diagnosed with WHO grade IV glioblastoma, 4 with WHO grade III astrocytoma, 2 with WHO grade III oligodendroglioma, 2 with WHO grade II-III astrocytoma, 1 with WHO grade III to IV glioma, and 1 with colorectal cancer.
DLTs and MTD
According to the accelerated titration design, two patients successively completed the DLT observation period (28 days) of treatment at 5 and 15 mg. However, the patient treated with 15 mg experienced grade 3 hypertension (non-DLT), and another two patients were enrolled at 15 mg based on the study design. Of those patients, one patient discontinued the treatment due to grade 2 bradycardia, which the investigator considered not related to the treatment. As a result, one more patient was enrolled at 15 mg for safety evaluation, and no more than grade 3 AEs were reported. In the 30 mg group, two out of five patients experienced DLTs (grade 3 mucositis oral and grade 4 hypertension), and the drug administration to the sixth patient in the 30 mg group was stopped according to the investigator’s decision to protect the safety and welfare of patients. Hence, for safety re-evaluation, four additional patients were enrolled in the lower dose level of 15 mg (one patient withdrew consent and was replaced), and no DLTs occurred. The dose-escalation process is shown in Supplementary Fig. S3.
Two patients in the dose escalation had DLTs. The first patient with WHO grade III anaplastic astrocytoma in the 30-mg group had a DLT (grade 3 mucositis oral) on Cycle 1 Day 18, which lasted 5 days. The second patient with WHO grade IV glioblastoma in the 30-mg group had a DLT (grade 4 hypertension) on Cycle 1 Day 15, which lasted 8 days. No DLT was observed at doses of 15 mg and lower. Based on protocol-defined criteria, the MTD for SYHA1813 was determined to be 15 mg once daily.
Safety and tolerability
All 14 patients were evaluated for safety. Overall, 13 (92.9%) patients had treatment-emergent AEs (TEAEs) during the study (Table 2). The most common TEAEs were hypertension (42.9%), sinus bradycardia (42.9%), platelet count decreased (35.7%), blood triglyceride increased (35.7%), alanine aminotransferase increased (28.6%), aspartate aminotransferase increased (28.6%), and urinary tract infection (28.6%). The majority of TEAEs were ≤ grade 2 and resolved spontaneously after drug discontinuation. Grade ≥ 3 TEAEs, including hypertension (35.7%), platelet count decreased (14.3%), and mucositis oral (7.1%), were all resolved after treatment withdrawal. There were 11 (78.6%) patients who experienced treatment-related AEs (TRAEs) (Supplementary Table S2).
Four patients experienced treatment interruptions and dose reductions due to SYHA1813-related toxicities, which were grade 2 sinus bradycardia, grade 2 platelet count decreased, grade 3 mucositis oral, and grade 4 hypertension. Serious adverse events considered to be related to SYHA1813 treatment were reported for one (7.1%) patient with grade 4 hypertension in the 30-mg group. No grade 5 AEs occurred.
PK
The plasma PK parameters are summarized in Supplementary Table 3. The mean plasma concentration-time curves of SYHA1813 were plotted after a single dose (Fig. 1a, b) and multiple doses (Fig. 1c, d) at 5, 15, and 30 mg. After single-dose administration ranging from 5 mg to 30 mg, SYHA1813 exhibited rapid absorption with median Tmax values of 2.0 h, independent of dose. Subsequently, the concentration declined slowly; the mean t1/2 was approximately 26.5 to 36.8 h. The mean apparent volume of distribution (Vz/F) and apparent clearance (CL/F) were 188.8 ~ 231.0 L and 4.3 ~ 5.6 L/h, respectively. The Cmax and AUCinf increased with increasing doses and generally demonstrated linear PK properties. At steady state, the Cmax, ss was reached after approximately 1.0 ~ 8.0 h, and the mean t1/2 was 27.6 ~ 35.7 h. In addition, the accumulation ratio Rac (Cmax) and Rac (AUC) of SYHA1813 ranged from 1.1 to 2.6 and 1.8 to 3.3, respectively, suggesting weak to moderate accumulation[16].
Efficacy
Ten patients were assessable for tumor response per investigator assessment, 9 with glioma (RANO criteria) and 1 with colorectal cancer (RECIST version 1.1); the other four enrolled patients withdrew from the study before the first tumor assessment. In the efficacy-evaluable population, the ORR was 20% (2/10), with all responses being PRs (Supplementary Table S3).
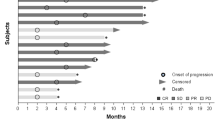
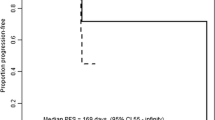
For glioma, the best percent change in tumor size from baseline and the best response and time on therapy for patients with HGG are shown in Fig. 2a and b. Two patients achieved PR: one with WHO grade III astrocytoma in the 15-mg group and one with WHO grade IV glioblastoma in the 30-mg group. Seven (70%) patients achieved SD. One patient with anaplastic astrocytoma (WHO grade III) achieved prolonged SD longer than six months in the 5-mg group. One (10%) patient with WHO grade III astrocytoma developed PD. The overall response and disease control rates were 20% and 90%, respectively. An example of objective response in one patient with WHO grade III astrocytoma treated at 15 mg is shown in Supplementary Fig. S4. The tumor volume score decreased by 88.4% in this patient after four months of treatment, indicating a substantial tumor burden reduction. Alleviation of cerebral edema persisted for at least three months, as shown in axial FLAIR MRI images (Supplementary Fig. S5).
The other advanced solid tumor patient, a 49-year-old male patient with stage IV colorectal cancer, received standard regimens and developed PD. He was then enrolled in the 15-mg SYHA1813 group and achieved SD assessed on Cycle 1 Day 25, which had lasted 2 weeks by the study cutoff date.
Analysis of biomarkers
Five patients were excluded from the biomarker analysis set due to a lack of postdose measurements. After multiple-dose administration of SYHA1813, there were significant increases in the serum levels of PlGF (P = .0484, Supplementary Fig. S6a) and VEGFA (P = .0092, Supplementary Fig. S6b). Compared with those at baseline, the levels of sVEGFR2 were significantly decreased by Day 24 (P = .0023, Supplementary Fig. S6c). The level of serum CSF1 increased in 7 of 9 (77.8%) patients (Supplementary Fig. S6d).
Discussion
This trial was the first to investigate the safety, PK profile, and preliminary antitumor activity of single and multiple doses of SYHA1813. The MTD of SYHA1813 was defined as 15 mg once daily.
While most patients experienced at least one TRAE, most treatment-related toxicities were mild and included hypertension, platelet count decreased, and sinus bradycardia. Doses up to 15 mg per day were considered tolerable. Two patients in the 30-mg group experienced DLTs. Hypertension and platelet count decreased were the most frequently reported grade 3–4 AEs. The occurrence of hypertension was likely the result of effective inhibition of the VEGF signaling pathway, consistent with the observations of increased incidence of hypertension in other clinical studies with small-molecule VEGF TKIs[17]. This effect is considered to be mediated by the disruption of nitric oxide-activated VEGFR2 signaling, which can result in physiological vasodilation[18]. Close monitoring and timely adjustment of blood pressure according to standard blood pressure treatment guidelines are recommended in future studies. Platelets as carriers of VEGF have been suggested to play a role as regulators of endothelial cell function, possibly affected during treatment with SYHA1813 [19, 20]. In general, the on-target toxicities caused by SYHA1813 were hypertension, mucositis oral, and platelet count decreased, similar to those caused by other small molecular VEGFR TKIs [20,21,22,23].
When administered as a single dose, the exposure (Cmax and AUCinf) of SYHA1813 increased approximately linearly over the studied dose range. The results of the current study established the MTD of SYHA1813 as 15 mg once daily. After multiple doses of 5 mg SYHA1813, the unbound trough concentration at steady state was much higher than the in vitro IC50 of VEGFRs and CSF1R, supporting that a daily oral dose of 5 mg and above is expected to be efficacious. Thus, 15 mg was selected as the recommended dose for the dose-expansion cohort to further determine the therapeutic activity of SYHA1813.
SYHA1813 administration resulted in a significant decrease in the level of sVEGFR2 from baseline, consistent with previous clinical findings with other VEGFR inhibitors, such as cediranib and pazopanib. Previous preclinical studies suggested that changes in the level of sVEGFR2 could be a pharmacodynamic marker of systemic exposure to drug, not a predictive marker of tumor response or clinical benefit. Similar to a previous study of the CSF1R inhibitor pexidartinib, we also observed an increased plasma concentration of CSF1 following multiple-dose administration [24]. A major implication of such an increase in CSF1 level is that there was strong on-target inhibition of CSF1R [25].
SYHA1813 has promising antitumor activity in recurrent HGG. A sustained tumor response was observed in HGG. Similar to the results of the phase II studies of cediranib (ORR of 56.7%) and pazopanib (ORR of 5.9%) in patients with recurrent malignant gliomas, SYHA1813 monotherapy showed encouraging antitumor activity (ORR of 20%) [4, 22]. Our findings are in line with the therapeutic mechanisms SYHA1813 was designed to have. Neoangiogenesis is a critical feature of glioblastoma, and several VEGFR-targeting small molecules have been extensively investigated for the treatment of glioblastoma [26]. However, exploration of multitarget TKIs such as cediranib and pazopanib as monotherapy have failed to improve the survival of recurrent glioblastoma patients [4, 21, 22]. CSF1R is an interesting target because it is overexpressed in HGG; and its levels are associated with glioma grade, and high CSF1R levels are associated with poor clinical outcomes [27,28,29]. Although pexidartinib (a TKI of CSR1R) is approved by the FDA based on its significant improvement of ORR, it did not show meaningful improvement in progression-free survival compared with previous data in recurrent glioblastoma [9]. The inhibition of multiple VEGFRs and CSF1R by SYHA1813 may lead to synergistic effects through the immunoregulation of macrophages to potentially overcome resistance to anti-VEGFR treatment and enhance antitumor activity.
In conclusion, oral administration of SYHA1813 at 15 mg daily demonstrated acceptable tolerability and preliminary antitumor activity in recurrent HGG. The subsequent dose-expansion cohort and phase Ib clinical trials of SYHA1813 should include HGG as a focus.
References
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353–364. https://doi.org/10.1016/s0092-8674(00)80108-7
Kim KJ, Li B, Winer J et al (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844. https://doi.org/10.1038/362841a0
Winkler F, Kozin SV, Tong RT et al (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–563. https://doi.org/10.1016/j.ccr.2004.10.011
Batchelor TT, Duda DG, di Tomaso E et al (2010) Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 28:2817–2823. https://doi.org/10.1200/jco.2009.26.3988
Hambardzumyan D, Gutmann DH, Kettenmann H (2016) The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 19:20–27. https://doi.org/10.1038/nn.4185
Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H (2012) The brain tumor microenvironment. Glia 60:502–514. https://doi.org/10.1002/glia.21264
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41:49–61. https://doi.org/10.1016/j.immuni.2014.06.010
Tan Y, Wang M, Zhang Y et al (2021) Tumor-Associated Macrophages: a potential target for Cancer Therapy. Front Oncol 11:693517. https://doi.org/10.3389/fonc.2021.693517
Butowski N, Colman H, De Groot JF et al (2016) Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase clinical trials Consortium phase II study. Neuro Oncol 18:557–564. https://doi.org/10.1093/neuonc/nov245
Lu-Emerson C, Snuderl M, Kirkpatrick ND et al (2013) Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol 15:1079–1087. https://doi.org/10.1093/neuonc/not082
Pombo Antunes AR, Scheyltjens I, Lodi F et al (2021) Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci 24:595–610. https://doi.org/10.1038/s41593-020-00789-y
Castro BA, Flanigan P, Jahangiri A et al (2017) Macrophage migration inhibitory factor downregulation: a novel mechanism of resistance to anti-angiogenic therapy. Oncogene 36:3749–3759. https://doi.org/10.1038/onc.2017.1
Dalton HJ, Pradeep S, McGuire M et al (2017) Macrophages facilitate resistance to Anti-VEGF therapy by altered VEGFR expression. Clin Cancer Res 23:7034–7046. https://doi.org/10.1158/1078-0432.Ccr-17-0647
Wenhu D, Jian D, Yongcong L, Hua X (2015) inventors; Shanghai Institute of Materia Medica, Chinese Academy of Sciences, assignee. Naphthalamide compounds, preparation method and application thereof. China patent CN104860885A. https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=chagngui
Ding J, Duan W, Xie H et al (2022) inventors; Shanghai Institute of Materia Medica, Chinese Academy of Sciences, assignee. CSF1R kinase inhibitor and use thereof. China patent PCT/CN2021/119663. https://patentscope2.wipo.int/search/zh/detail.jsf?docId=WO2022063134&_cid=JP2-L9ZDVB-68911-1
Li L, Li X, Xu L, Sheng Y, Huang J, Zheng Q (2013) Systematic evaluation of dose accumulation studies in clinical pharmacokinetics. Curr Drug Metab 14:605–615. https://doi.org/10.2174/13892002113149990002
Dobbin SJH, Petrie MC, Myles RC, Touyz RM, Lang NN (2021) Cardiotoxic effects of angiogenesis inhibitors. Clin Sci (Lond) 135:71–100. https://doi.org/10.1042/cs20200305
Dy GK, Adjei AA (2013) Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin 63. https://doi.org/10.3322/caac.21184. :249 – 79
Selheim F, Holmsen H, Vassbotn FS (2002) Identification of functional VEGF receptors on human platelets. FEBS Lett. https://doi.org/10.1016/s0014-5793(02)02232-9. 512:107 – 10
Kuenen BC, Levi M, Meijers JC et al (2003) Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol 21:2192–2198. https://doi.org/10.1200/jco.2003.08.046
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218. https://doi.org/10.1200/jco.2012.47.2464
Iwamoto FM, Lamborn KR, Robins HI et al (2010) Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06 – 02). Neuro Oncol 12:855–861. https://doi.org/10.1093/neuonc/noq025
Duerinck J, Du Four S, Vandervorst F et al (2016) Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J Neurooncol 128:147–155. https://doi.org/10.1007/s11060-016-2092-2
Lee JH, Chen TW, Hsu CH et al (2020) A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in asian patients with advanced solid tumors. Invest New Drugs 38:99–110. https://doi.org/10.1007/s10637-019-00745-z
Benner B, Good L, Quiroga D et al (2020) Pexidartinib, a Novel Small Molecule CSF-1R inhibitor in Use for Tenosynovial Giant Cell Tumor: a systematic review of pre-clinical and clinical development. Drug Des Devel Ther 14:1693–1704. https://doi.org/10.2147/dddt.S253232
Kim G, Ko YT (2020) Small molecule tyrosine kinase inhibitors in glioblastoma. Arch Pharm Res 43:385–394. https://doi.org/10.1007/s12272-020-01232-3
Komohara Y, Horlad H, Ohnishi K et al (2012) Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci 103:2165–2172. https://doi.org/10.1111/cas.12015
Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216:15–24. https://doi.org/10.1002/path.2370
Pyonteck SM, Akkari L, Schuhmacher AJ et al (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19:1264–1272. https://doi.org/10.1038/nm.3337
Acknowledgements
We would like to thank all the patients, their families, and all the investigators involved in this study.
Funding
The study was funded by Shanghai Runshi Medical Technology Co., Ltd, China.
Author information
Authors and Affiliations
Contributions
ZK developed the study protocol, conducted the clinical study, and coordinated with the participating hospitals. SL, YL, YL, YM, JZ, and TL were the principal researchers in each hospital and helped to contribute to participant recruitment and provide feedback about the study procedure. HW, YS, YY, and JQ performed the statistical analysis, analyzed and interpreted the results, and provided comments. WL designed the research and supervised the report. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
HW, YS, YY, and JQ are employed by CSPC Pharmaceutical Group Limited. The other authors have no conflicts of interest.
Ethics approval
The studies involving human participants were reviewed and approved by an institutional review board at each participating site. This study is registered at chictr.org.cn (trial registration ID: ChiCTR2100045380).
Consent to participate
All patients provided written informed consent prior to the initiation of any study-related procedures.
Consent to publish
The authors affirm that human research participants provided informed consent for the publication of the images in Supplementary Figures S4 and S5.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, Z., Li, S., Lin, Y. et al. A phase I dose-escalation study of SYHA1813, a VEGFR and CSF1R inhibitor, in patients with recurrent High-Grade Gliomas or Advanced Solid Tumors. Invest New Drugs 41, 296–305 (2023). https://doi.org/10.1007/s10637-022-01325-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01325-4