Abstract
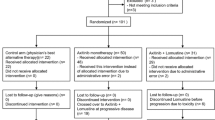
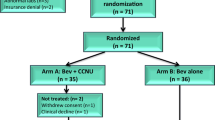
We conducted a randomized, non-comparative, multi center, phase II clinical trial in order to investigate the efficacy of axitinib, an oral small molecule tyrosine kinase inhibitor with high affinity and specificity for the vascular endothelial growth factor receptors, in patients with recurrent glioblastoma following prior treatment with radiation and temozolomide. Forty-four patients were randomly assigned to receive treatment with axitinib (5 mg BID starting dose; N = 22) or “physicians best alternative choice of therapy” that consisted of bevacizumab (N = 20) or lomustine (N = 2). Six-month progression-free survival served as the primary endpoint. The estimated 6-month progression-free survival rate was 34 % (95 % CI 14–54) for patients treated with axitinib and 28 % (95 % CI 8–48) with best alternative treatment; median overall survival was 29 and 17 weeks, respectively. Objective responses according to RANO criteria were documented in 28 % of patients treated with axitinib and 23 % of patients treated with best alternative therapy. A decrease in maximal uptake of 18F-fluoro-ethyl-tyrosine (18F-FET) by the glioblastoma on PET imaging was documented in 85 % of patients at the time of response on axitinib. Corticosteroid treatment could be stopped in four and tapered in seven out of the 15 patients who were treated with steroids at baseline in the axitinib cohort. Most frequent axitinib related grade ≥3 adverse events consisted of fatigue (9 %), diarrhea (9 %), and oral hyperesthesia (4.5 %). We conclude that axitinib has single-agent clinical activity and a manageable toxicity profile in patients with recurrent glioblastoma.


Similar content being viewed by others
References
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Lamborn KR, Yung WKA, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170
Puputti M, Tynninen O, Sihto H, Blom T, Mäenpää H, Isola J, Paetau A, Joensuu H, Nupponen NN (2006) Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res 4:927–934
Knizetova P, Darling JL, Bartek J (2008) Vascular endothelial growth factor in astroglioma stem cell biology and response to therapy. J Cell Mol Med 12:111–125
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66:7843–7848
Lu L, Saha D, Martuza RL, Rabkin SD, Wakimoto H (2014) Single agent efficacy of the VEGFR kinase inhibitor axitinib in preclinical models of glioblastoma. J Neurooncol. doi:10.1007/s11060-014-1612-1
Reardon DA, Turner S, Peters KB et al (2011) A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Cancer Netw 9:414–427
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218
Kratzsch T, Gruenwald V, Vajkoczy P, Kuhn S (2013) Use of axitinib, a new-generation tyrosine kinase inhibitor, to decrease glioblastoma growth despite primary resistance to the VEGF-antibody bevacizumab. ASCO Meet Abstr 2013(31):2077
Galldiks N, Rapp M, Stoffels G, Fink GR, Shah NJ, Coenen HH, Sabel M, Langen K-J (2013) Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging 40:22–33
Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, Danenberg PV, Laird PW (2000) MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res 28:E32
Widschwendter M, Siegmund KD, Müller HM, Fiegl H, Marth C, Müller-Holzner E, Jones PA, Laird PW (2004) Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res 64:3807–3813
Ogino S, Kawasaki T, Brahmandam M et al (2006) Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn 8:209–217
D’Haene N, Le Mercier M, De Nève N et al (2015) Clinical validation of targeted next generation sequencing for colon and lung cancers. PLoS One 10:e0138245
Le Mercier M, D’Haene N, De Nève N, Blanchard O, Degand C, Rorive S, Salmon I (2015) Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology 66:215–224
Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192
Hoogstraat M, Hinrichs JWJ, Besselink NJM et al (2015) Simultaneous detection of clinically relevant mutations and amplifications for routine cancer pathology. J Mol Diagn 17:10–18
Duerinck J, Clement PM, Bouttens F et al (2015) Patient outcome in the Belgian medical need program on bevacizumab for recurrent glioblastoma. J Neurol. doi:10.1007/s00415-014-7633-z
Fack F, Espedal H, Keunen O et al (2015) Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathol 129:115–131
Götz I, Grosu AL (2013) [(18)F]FET-PET imaging for treatment and response monitoring of radiation therapy in malignant glioma patients—a review. Front Oncol 3:104
Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, Loeffler JS, Batchelor TT, Jain RK (2015) Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol 33:1197–1213
Zhang X, Fang X, Gao Z et al (2014) Axitinib, a selective inhibitor of vascular endothelial growth factor receptor, exerts an anticancer effect in melanoma through promoting antitumor immunity. Anticancer Drugs 25:204–211
Stehle F, Schulz K, Fahldieck C, Kalich J, Lichtenfels R, Riemann D, Seliger B (2013) Reduced immunosuppressive properties of axitinib in comparison with other tyrosine kinase inhibitors. J Biol Chem 288:16334–16347
Bose A, Lowe DB, Rao A, Storkus WJ (2012) Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res 22:236–243
Du Four S, Maenhout SK, De Pierre K, Renmans D, Niclou SP, Thielemans K, Neyns B, Aerts JL (2015) Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology 4:e998107
Neyns B, Sadones J, Chaskis C et al (2011) Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol 103:491–501
Acknowledgments
We would like to acknowledge the patients who consented to participate in this study, their families, and Pfizer Belgium for the provision of axitinib study medication and a research grant. We would also like to thank the data manager Katrien Van den Bossche and Kathleen Mooren (UZ Brussel) for their help with the data collection and analysis, and Ludwig Van den Hove, PhD, Pfizer Belgium, for his support and critical review of the manuscript.
Funding
Supported by a research grant from Pfizer to the UZ Brussel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Additional information
ClinicalTrials.gov Identifier: NCT01562197.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2016_2092_MOESM1_ESM.jpg
Supplementary material 1 (JPEG 120 kb) Baseline and post-treatment magnetic resonance (MR) and FET-PET/T2 MRI fusion images of a 54 year old female glioblastoma patient treated with axitinib at the time of first recurrence, illustrating an unconfirmed complete response followed by non-enhancing progression of disease. Post-contrast axial T1-weighted and non-enhanced T2/FET-PET fusion images at baseline (A, B), after 6 weeks (C, D), and 18 weeks (E, F) of axitinib treatment; coronal FLAIR MR scans and non-enhanced T2/FET-PET fusion images at baseline (G, H), after 6 weeks (I,J), and 18 weeks (K,L) of axitinib treatment
Rights and permissions
About this article
Cite this article
Duerinck, J., Du Four, S., Vandervorst, F. et al. Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J Neurooncol 128, 147–155 (2016). https://doi.org/10.1007/s11060-016-2092-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2092-2