Abstract
Purpose
To determine the full-field electroretinogram (ffERG) parameters, including the light-adapted (LA) 3 ERG and the photopic negative response (PhNR), in 6- to 12-year-old children.
Methods
ffERG data were obtained from 214 eyes of 214 healthy subjects. The amplitudes and peak time of the ffERG responses were obtained from children divided into 6- to 8-year-old and 9- to 12-year-old groups. Using a skin electrode, electrical signals were measured in response to white stimulating light and white background light (LA 3 ERG). A blue background light and red flashes were then used to elicit the PhNR.
Results
The a-wave amplitude ranged from 0.40 to 9.20 μV, the b-wave ranged from 4.70 to 30.80 μV, and the PhNR ranged from 1.30 to 39.90 μV. The b-wave peak time (33.20 ms) of 6- to 8-year-old groups was slightly shorter than that of the 9- to 12-year-old groups (33.60 ms, P = 0.01), but no differences in amplitudes or in peak time of other components. There were significant correlations between the amplitudes (a-wave and b-wave: r = 0.43, p < 0.001; a-wave and PhNR: r = 0.25, p < 0.001; b-wave and PhNR: r = 0.45, p < 0.001). There was a moderate correlation between the a-wave and b-wave peak time (r = 0.31, P < 0.001).
Conclusions
We determined the largest dataset of the LA 3 ERG and PhNR parameters in a population of healthy children, aged 6–12 years, which may provide a useful reference value when evaluating children with potential retinal defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because light stimulation can induce retinal electrical activity, electroretinograms (ERGs) are used extensively for clinical identification of retinal diseases and evaluation of their severity [1]. Recently, the International Society of Electrophysiology of Vision (ISCEV) standard specified six recording conditions for the ffERG and an extended protocol for the photopic negative response (PhNR) [2]. These conditions were established to reflect the function of the main physiological generators located in the different retinal layers [3,4,5].
Currently, the negative a-wave and the positive b-wave are the main ERG components used in clinical practice. Under photopic conditions, the negative a-wave is thought to predominantly reflect activity from cone cells and OFF bipolar cells [6]. The b-wave is mainly generated by ON bipolar cells [7], with some contributions of other post-receptoral sources [8]. The PhNR is predominantly generated by retinal ganglion cells [9].
Abnormal photopic ERG components were previously reported in diseases such as retinitis pigmentosa [10], optic nerve hypoplasia [11], age-related macular degeneration [12], diabetic retinopathy [13], and aniridia [14]. A reduction in the PhNR amplitude was also described in pathological diseases such as glaucoma [15,16,, 16], multiple sclerosis [17], childhood optic gliomas [18], and optic neuropathy [19]. Although standard ffERG values from the normal retina are required for assessment of pathological retina, these values have not been reported in a large cohort of healthy children. It also remains unclear whether the a-wave, b-wave, and PhNR amplitudes change with age especially in 6- to 12-year-old children. Furthermore, children have a preference for skin electrodes, although these typically produce lower amplitude responses than those obtained with a contact lens [20, 21]. Previous studies using skin electrodes also had relatively small sample sizes. For example, Soekamto et al. [22] recorded scotopic (rod) and photopic (cone) responses from only 20 healthy patients. Additionally, although there are some other reports of normal electrophysiological values [23,24,25,26], they are typically recorded from older subjects or do not assess the PhNR.
Therefore, the aims of this study were to record the LA 3 ERG and PhNR with a skin electrode in a large population of healthy children and to determine the relationship between these electrophysiological parameters and age.
Subjects and methods
Subjects
The present study included 214 eyes of 214 children with emmetropia or ametropia who volunteered to receive a full-field ERG examination at the Eye Hospital of Wenzhou Medical University at Hangzhou between September 2019 and September 2020. The inclusion criteria included: (1) age < 12 years old, (2) best-corrected visual acuity reaching the current age standard, (3) refractive error < ± 6.00 D mean sphere, (4) a pupil diameter of ≥ 6 mm with dilation, and (5) a normal intraocular pressure. Exclusion criteria included the presence of tropia, nystagmus, fundus disease, or any physical or mental disability that could affect cooperation of the subject. We divided children into 6- to 8-year-old and 9- to 12-year-old groups for comparisons of the electrophysiological parameters.
Written informed consent was obtained from all the parents or guardians of each subject after a thorough explanation of the study. This study was approved by the Institutional Ethics Committee of Wenzhou Medical University. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was registered at www.clinicaltrials.gov (NCT04427748).
Examination
Axial length (AL) was measured using an IOL-Master 700 (Carl Zeiss Meditec AG, Jena, Germany). The manifest refraction was also assessed in all subjects.
ERG recordings
The ffERG was performed using the Metrovision vision monitor (Metrovision, Pérenchies, France) and Ag–AgCl electrodes (EEGWO2, Brain Science Electronic&Technology Co, Qindao, China). ISCEV standard protocols for recording the LA 3 ERG and PhNR were followed as strictly as possible. The ffERG was performed binocularly on the eyes with pupils dilated using 1% tropicamide and active skin electrodes. The active electrode was taped on the skin at 2.5 mm below the margin of the lower eyelid, the earth electrode was taped at the mid-frontal position, and the reference electrode was taped at the temporal canthus position. Good electrical conduction was ensured by using an abrasive conductive gel before electrode taping. The impedances of skin electrodes were accepted be 5 kΩ or less in our study.
Video monitoring with a near-infrared sensor was used to record the eye image to ensure fixation. The LA 3 ERG condition was elicited by white flashes (3.1 cd s/m2) on a steady white background (31 cd/m2) after 10 min of light adaptation with a steady background light (31 cd/m2). PhNR was then elicited by red flashes (wavelength, 619 nm; 1.2 cd/m2) presented on a steady blue background (wavelength, 465 nm; 8 cd/m2). Signals were then filtered (1–35 Hz for LA 3 ERG; 1–288 Hz for PhNR), amplified (50 K), and digitized at 2 kHz. Fifty test flashes for LA 3 ERG and 200 tests for PhNR were averaged with automatic artifact rejection. The PhNR was excluded if continuous, large, or small transients were present in the waveform.
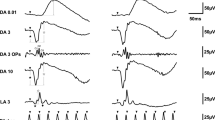
The a-wave and b-wave amplitudes and peak time in LA 3 ERG and the PhNR amplitude were measured. The a-wave amplitude of the LA 3 ERG was measured from baseline to its trough. The b-wave amplitude of the LA 3 ERG was measured between its first negative trough and the first positive peak. The amplitude of the PhNR was measured between the baseline and the most negative trough prior to 100 ms time window. The peak time was measured from the presentation of the stimulus to the trough or peak of the relevant wave (Fig. 1).
Statistical analysis
Statistical analysis was performed using statistical software (SPSS software v. 21.0). The normality of data was checked by Kolmogorov–Smirnov test. Analysis of variance was used to compare electrophysiological parameters between the two groups of subjects. Pearson’s coefficient was used to correlate between parameter amplitudes and between parameter peak time. Partial correlation analysis was used to examine the association between electrophysiological parameters and AL and between the electrophysiological parameters and ages. A p-value < 0.05 was considered statistically significant.
Results
General characteristics
A total of 214 eyes of 214 children were enrolled in this study (Fig. 2). The mean age was 8.81 ± 1.70 years old (range, 6–12 years old). The gender distribution was 43.6% boys and 56.4% girls. The median logarithm of the minimal angle resolution best-corrected visual acuity in all patients was 0.00°. Demographics and ocular parameters are shown in Table 1.
Reference values of LA 3 ERG and PhNR
The reference values for the a-wave and b-wave of the LA 3 ERG and the PhNR amplitudes and peak time are shown in Table 2. 214 eyes for LA 3 ERG and 206 eyes for PhNR, because 8 eyes failed to obtain the true PhNR.
ERG characteristics according to age
The group-averaged amplitude and peak time data according to age are shown in Table 3 and Fig. 2. There was a significant difference in the peak time of the b-wave (p = 0.01) between the two groups, but no differences in any other parameters. We investigated correlations between each ERG parameter and age using age as a continuous variable. Unfortunately, there were no correlations between each ERG parameter and age (a-wave: amplitude r = 0.01, P = 0.90, peak time r = 0.09, P = 0.21; b-wave: amplitude r = − 0.06, P = 0.40, peak time r = 0.16, P = 0.02; PhNR: amplitude r = − 0.06, P = 0.40).
Correlations between the a-wave, b-wave, and PhNR parameters
The correlations between the electrophysiological parameters in normal children are shown in Figs. 3 and 4. There was a moderate correlation between the a-wave and b-wave amplitudes (r = 0.43, p < 0.001). There was also a mild correlation between the amplitudes of the a-wave and the PhNR (r = 0.25, p < 0.001) and a moderate correlation between the b-wave and the PhNR (r = 0.45, p < 0.001). There was a moderate correlation between the a-wave and b-wave peak time (r = 0.31,P < 0.001).
Correlations between electrophysiological parameters and AL
There were no correlations between the electrophysiological parameters and AL (amplitudes a-wave: r = 0.06, p = 0.42; b-wave: r = 0.03, p = 0.71; PhNR: r = 0.13, p = 0.07; peak time a-wave: r = 0.05, P = 0.47; b-wave: r = 0.09, P = 0.20).
Discussion
Changes in the ffERG have been reported in children with various retinal disorders, including Stargardt disease [27] and retinopathy of prematurity [28]. However, the normal ffERG values for children in those studies were derived from healthy control groups with a small sample size and a wide age range. ERG is also affected by the type of electrophysiological recording system, subject age, light stimulation parameters, and electrode type [18, 29,30,31,32,33]. Therefore, in the present study, we determined the normal values of LA 3 ERG and PhNR in a large cohort of children and also examined whether the a-wave, b-wave, and PhNR changed with age especially in 6- to 12-year-old children. Furthermore, we used skin electrodes, which were reported to provide accurate ffERG recording in clinical practice [7, 34, 35] to improve cooperation in children.
Using the Metrovision electrophysiological system, we recorded LA 3 ERG from 214 healthy subjects (mean age, 8.81 years; range, 6–12 years). The mean peak time in the a-wave (16.20 ms) was similar to that reported by Schwitzer et al. (18.6 ms) [29], Esposito et al. (15.73 ms) [31], and Bhatti et al. (14.3 ms) [36]. Furthermore, the mean peak time in the b-wave (33.41 ms) was similar to that reported by Schwitzer et al. (35.80 ms) [29], Esposito et al. (32.16 ms) [31], Abed et al. (30.29 ms) [18], and Bhatti et al. (29.3 ms) [36]. By contrast, the wave amplitudes in our study were similar with previous studies using skin electrodes and were lower than those previously reported using other electrodes. For example, the mean a-wave amplitude (4.25 μV) was similar with Esposito et al. (6.09 μV) [31] and was lower than that reported by Schwitzer et al. (10.8 μV) [29], Lin et al. (91.4 μV) [30], and Bhatti et al. (22.1 μV) [36]. Similarly, the mean b-wave amplitude (14.78 μV) was similar with Esposito et al. (17.37 μV) [31] and Abed et al. (22.35 μV) [18] and was lower than that reported by Schwitzer et al. (48.0 μV) [29], and Bhatti et al. (95.0 μV) [36]. The amplitude in our study which is lower than that in previous studies reported by Lin et al. and Bhatti et al. significantly, because amplitudes are lower with skin electrodes [34]. We can see that the values of different literatures differ greatly, and their sample size is relatively small except for Bhatti et al. study, and age range is relatively large. The data of our large sample may be able to somehow correct bias caused by small samples.
The amplitude of the PhNR (13.90 μV) in the present study was higher than that reported by Mortlock et al. (11.43 μV) [32], Esposito et al. (9.40 μV) [31] and was lower than Abed et al. (19.17 μV) [18] using the same types of electrodes. These differences may relate to differences in the light stimulation parameters and subject age. The amplitudes in all of these studies, including ours, were lower than those reported by Banerjee et al. [33] and Bhatti et al. [36], which likely relates to the different electrodes used in that study.
In the present study, there was only a small difference in the peak time of the b-wave between the 6- to 8-year-old and the 9- to 12-year-old groups, which may be of limited clinical significance, while there were no differences in any other parameters. This indicates that the electrophysiology of retinal cells in school-age children does not change with age. It is also possible that because the amplitude obtained by the skin electrode is small, the correlation is not easily detected.
The significant correlation between the amplitudes of the a-wave and b-wave in LA 3 ERG was consistent with the findings of Esposito et al.[31], which confirms the reliability and repeatability of our study. Skin electrodes placed farther from the eyelid margin can reduce amplitude responses, though less effect on peak time [37]. In order to be more reflective of retinal neuronal processing times, we also analyzed correlation between the peak time of the a-wave and b-wave. The significant correlation between the a-wave and b-wave peak time also existed. These findings suggest the existence of a strict functional relationship between cone cell pathway components in the eye [31]. We also found a positive correlation between PhNR amplitude and the a-wave and b-wave amplitudes, as reported by Esposito et al. [31], suggesting that ganglion cell function is affected by more distal retinal elements in the eye. In addition, the farther the active electrode is from the eyelid margin, the lower the recorded signal amplitude [37, 38]. So, we made sure that our electrode positioning is consistent with Esposito et al. However, the extent to the amplitude of each wave reduction remains unclear. So, the influence of electrode position on correlation needs to be further explored.
By contrast, we found no correlation between the electrophysiological parameters and AL. Previous studies have reported a reduction in electrophysiological amplitudes in high myopia and pathological myopia patients [39, 40]. In adults, axial elongation of the myopic eye can stretch the retina across the interior of the globe, thereby reducing the sampling density of retinal neurons and altering retinal physiology [41]. Previous study has reported peak time showed minimal delay with increase in axial length in adults. However, this has not yet been reported in children. Additionally, the present study only included children with a refractive error < ± 6.00 D mean sphere, which resulted in a small AL range (21.37–26.32 mm). Thus, we did not find the same relationship as observed in adults. Further studies are required in children with a larger AL range.
There are some limitations to our study. First, the electrophysiological examination was not repeated for every child, although only stable electrophysiological results from cooperative children were selected for analysis. Second, because we used skin electrodes, the ffERG values are not comparable to studies using corneal electrodes. However, skin ERG electrodes can facilitate better testing cooperation in children. Thirdly, we recorded only LA 3 ERG and PhNR. However, in order to record the ERG responses for the younger children, who are not able to cooperate with the longer examination, we could not record other 5 standard ffERG responses. Due to the difficulty of examining children, and to ensure the reliability of the data, most of the published papers did not report records including 6 standard full-field ERG responses. Although establishing laboratory-specific reference values is the most optimal process, now lacking large sample electrophysiological data in children as a basic reference, we recorded a large number of electrophysiological results in 6- to 12-year-old children, especially including PhNR, which can at least provide a reference value for public. Fourthly, our study filtered to a much narrower range of frequencies than is conventional, but the ffERG results obtained in the 1–35 Hz range were more stable and reproducible in our laboratory with reference to previous research [42]. In addition, we detected ffERG following ISCEV standard protocols as strictly as possible.
To our knowledge, this study provides the largest dataset of LA 3 ERG and PhNR parameters in a population of healthy children. These electrophysiological parameters may be useful reference values when evaluating children with potential retinal defects. However, these results should be interpreted with caution because of different skin electrodes and narrower range of frequencies.
References
Robson AG, Frishman LJ, Grigg J et al (2022) ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol Adv Ophthalmol 144(3):165–177. https://doi.org/10.1007/s10633-022-09872-0. [published Online First: Epub Date]
Frishman L, Sustar M, Kremers J et al (2018) ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol Adv Ophthalmol 136(3):207–211. https://doi.org/10.1007/s10633-018-9638-x. [published Online First: Epub Date]
Hood DC, Birch DG (1990) A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci 5(4):379–387. https://doi.org/10.1017/s0952523800000468. [published Online First: Epub Date]
Bui BV, Armitage JA, Vingrys AJ (2002) Extraction and modelling of oscillatory potentials. Doc Ophthalmol 104(1):17–36. https://doi.org/10.1023/a:1014401502
Vinberg F, Kolesnikov AV, Kefalov VJ (2014) Ex vivo ERG analysis of photoreceptors using an in vivo ERG system. Vision Res 101:108–117. https://doi.org/10.1016/j.visres.2014.06.003
Robson JG, Saszik SM, Ahmed J, Frishman LJ (2003) Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 547(Pt 2):509–530. https://doi.org/10.1113/jphysiol.2002.030304. [published Online First: Epub Date]
Stockton RA, Slaughter MM (1989) B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J General Physiol 93(1):101–22. https://doi.org/10.1085/jgp.93.1.101. [published Online First: Epub Date]
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11(3):519–532. https://doi.org/10.1017/s0952523800002431. [published Online First: Epub Date]
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136.
Iijima H, Yamaguchi S, Hosaka O (1993) Photopic electroretinogram implicit time in retinitis pigmentosa. Jpn J Ophthalmol 37(2):130–135
Dunn FA, Wong RO (2012) Diverse strategies engaged in establishing stereotypic wiring patterns among neurons sharing a common input at the visual system’s first synapse. J Neurosci Official J Soc Neurosci 32(30):10306–10317. https://doi.org/10.1523/jneurosci.1581-12.2012. [published Online First: Epub Date]
Forshaw TRJ, Subhi Y, Andréasson S, Sørensen TL (2022) Full-field electroretinography changes associated with age-related macular degeneration: a systematic review with meta-analyses. Ophthalmologica. Journal international d'ophtalmologie. Int J Ophthalmol. Zeitschrift fur Augenheilkunde 245(3):195–203. https://doi.org/10.1159/000521834. [published Online First: Epub Date]
Jansson RW, Raeder MB, Krohn J (2015) Photopic full-field electroretinography and optical coherence tomography in type 1 diabetic retinopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 253(7):989–997. https://doi.org/10.1007/s00417-015-3034-y. [published Online First: Epub Date]
Dangremond T, Wang K, Helms M, Bhattarai S, Pfeifer W, Drack AV (2021) Correlation between electroretinography, foveal anatomy and visual acuity in aniridia due to PAX6 mutations. Doc Ophthalmol Adv Ophthalmol 143(3):283–295. https://doi.org/10.1007/s10633-021-09844-w. [published Online First: Epub Date]
North RV, Jones AL, Drasdo N, Wild JM, Morgan JE (2010) Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 51(2):1216–1222. https://doi.org/10.1167/iovs.09-3409. [published Online First: Epub Date]
Kirkiewicz M, Lubiński W, Penkala K (2016) Photopic negative response of full-field electroretinography in patients with different stages of glaucomatous optic neuropathy. Doc Ophthalmol Adv Ophthalmol 132(1):57–65. https://doi.org/10.1007/s10633-016-9528-z. [published Online First: Epub Date]
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53(3):1315–1323. https://doi.org/10.1167/iovs.11-8461. [published Online First: Epub Date]
Abed E, Piccardi M, Rizzo D et al (2015) Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci 56(4):2469–2474. https://doi.org/10.1167/iovs.14-16235. [published Online First: Epub Date]
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol (Chicago, Ill:1960) 122(3):341–346. https://doi.org/10.1001/archopht.122.3.341.. [published Online First: Epub Date]
Esakowitz L, Kriss A, Shawkat F (1993) A comparison of flash electroretinograms recorded from Burian Allen, JET, C-glide, gold foil, DTL and skin electrodes. Eye (Lond) 7(Pt 1):169–171. https://doi.org/10.1038/eye.1993.36. [published Online First: Epub Date]
Kriss A (1994) Skin ERGs: their effectiveness in paediatric visual assessment, confounding factors, and comparison with ERGs recorded using various types of corneal electrode. Int J Psychophysiol Official J Int Organiz Psychophysiol 16(2–3):137–146. https://doi.org/10.1016/0167-8760(89)90040-8. [published Online First: Epub Date]
Soekamto CD, Gupta R, Keck KM (2021) Using the RETeval device in healthy children to establish normative electroretinogram values. J Pediatr Ophthalmol Strabismus 58(1):17–22. https://doi.org/10.3928/01913913-20200910-03. [published Online First: Epub Date]
Asakawa K, Amino K, Iwase M et al (2017) New Mydriasis-free electroretinogram recorded with skin electrodes in healthy subjects. Biomed Res Int 2017:8539747. https://doi.org/10.1155/2017/8539747. [published Online First: Epub Date]
Ortiz G, Drucker D, Hyde C, Staffetti J, Kremers J, Tzekov R (2020) The photopic negative response of the Light-adapted 3.0 ERG in clinical settings. Doc Ophthalmol 140(2):115–128
Kergoat H, Kergoat MJ, Justino L (2001) Age-related changes in the flash electroretinogram and oscillatory potentials in individuals age 75 and older. J Am Geriatr Soc 49(9):1212–1217. https://doi.org/10.1046/j.1532-5415.2001.49239.x. [published Online First: Epub Date]
Azad R, Ghatak U, Sharma YR, Chandra P (2012) Multifocal electroretinogram in normal emmetropic subjects: correlation with optical coherence tomography. Indian J Ophthalmol 60(1):49–52. https://doi.org/10.4103/0301-4738.91345. [published Online First: Epub Date]
Bax NM, Lambertus S, Cremers FPM, Klevering BJ, Hoyng CB (2019)The absence of fundus abnormalities in Stargardt disease. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 257(6):1147–1157. https://doi.org/10.1007/s00417-019-04280-8. [published Online First: Epub Date]
Molnar AEC, Andréasson SO, Larsson EKB, Åkerblom HM, Holmström GE (2017) Reduction of rod and cone function in 6.5-year-old children born extremely preterm. JAMA Ophthalmol 135(8):854–861. https://doi.org/10.1001/jamaophthalmol.2017.2069. [published Online First: Epub Date]
Schwitzer T, Schwan R, Angioi-Duprez K et al (2018) Delayed bipolar and ganglion cells neuroretinal processing in regular cannabis users: The retina as a relevant site to investigate brain synaptic transmission dysfunctions. J Psychiatr Res 103:75–82. https://doi.org/10.1016/j.jpsychires.2018.04.021. [published Online First: Epub Date]
Lin YB, Liu JH, Chang Y (2012) Hypoxia reduces the effect of photoreceptor bleaching. J Physiol Sci JPSS 62(4):309–315. https://doi.org/10.1007/s12576-012-0201-3. [published Online First: Epub Date]
Esposito Veneruso P, Ziccardi L, Magli G, Parisi V, Falsini B, Magli A (2017) Early light deprivation effects on human cone-driven retinal function. Acta Ophthalmol 95(2):133–139. https://doi.org/10.1111/aos.13191. [published Online First: Epub Date]
Mortlock KE, Binns AM, Aldebasi YH, North RV (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol Adv Ophthalmol 121(2):123–134. https://doi.org/10.1007/s10633-010-9239-9. [published Online First: Epub Date]
Banerjee A, Khurana M, Sachidanandam R, Sen P (2019) Comparison between broadband and monochromatic photopic negative response in full-field electroretinogram in controls and subjects with primary open-angle glaucoma. Doc Ophthalmol Adv Ophthalmol 138(1):21–33. https://doi.org/10.1007/s10633-018-09668-1. [published Online First: Epub Date]
Bradshaw K, Hansen R, Fulton A (2004) Comparison of ERGs recorded with skin and corneal-contact electrodes in normal children and adults. Doc Ophthalmol Adv Ophthalmol 109(1):43–55. https://doi.org/10.1007/s10633-004-1751-3. [published Online First: Epub Date]
Wu Z, Hadoux X, Fan Gaskin JC, Sarossy MG, Crowston JG (2016) Measuring the photopic negative response: viability of skin electrodes and variability across disease severities in glaucoma. Trans Vis Sci Technol 5(2):13. https://doi.org/10.1167/tvst.5.2.13. [published Online First: Epub Date]
Bhatti T, Tariq A, Shen T, Williams KM, Hammond CJ, Mahroo OA (2017) Relative genetic and environmental contributions to variations in human retinal electrical responses quantified in a twin study. Ophthalmology 124(8):1175–1185. https://doi.org/10.1016/j.ophtha.2017.03.017. [published Online First: Epub Date]
Hobby AE, Kozareva D, Yonova-Doing E et al (2018) Effect of varying skin surface electrode position on electroretinogram responses recorded using a handheld stimulating and recording system. Doc Ophthalmol Adv Ophthalmol 137(2):79–86. https://doi.org/10.1007/s10633-018-9652-z. [published Online First: Epub Date]
Kurtenbach A, Kramer S, Strasser T, Zrenner E, Langrová H (2017) The importance of electrode position in visual electrophysiology. Doc Ophthalmol Adv Ophthalmol 134(2):129–134. https://doi.org/10.1007/s10633-017-9579-9. [published Online First: Epub Date]
Sachidanandam R, Ravi P, Sen P (2017) Effect of axial length on full-field and multifocal electroretinograms. Clin Exp Optom 100(6):668–675. https://doi.org/10.1111/cxo.12529. [published Online First: Epub Date]
Uchida A (1977) Studies of electrical activities of the eye in high myopia (author’s transl). Nippon Ganka Gakkai Zasshi 81(9):1328–1350. [published Online First: Epub Date]
Chui TY, Yap MK, Chan HH, Thibos LN (2005) Retinal stretching limits peripheral visual acuity in myopia. Vis Res 45(5):593–605. https://doi.org/10.1016/j.visres.2004.09.016
Porciatti V, Ventura LM (2004) Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology 111(1):161–168. https://doi.org/10.1016/j.ophtha.2003.04.007. [published Online First: Epub Date]
Funding
This study was supported by the research grant approved by the National Natural Science Foundation of China (No. 81870680), the Provincial Construction Project of Zhejiang (No. WKJ-ZJ-2135), and the Zhejiang Provincial Program of China for the Cultivation of health leading talents (Y.E.Z.). The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial in the subject matter or materials discussed in this manuscript.
Ethical approval
This study was approved by the Institutional Ethics Committee of Wenzhou Medical University.
Statement of human rights
Written informed consent was obtained from all the parents or guardians of each subject after a thorough explanation of the study. This study was approved by the Institutional Ethics Committee of Wenzhou Medical University. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was registered at www.clinicaltrials.gov (NCT04427748).
Statement on the welfare of animals
No animals were used in this research.
Informed consent
Informed consent was obtained from all the parents or guardians of each subject after a thorough explanation of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Wang, Y., Guan, W. et al. Full-field electroretinogram recorded with skin electrodes in 6- to 12-year-old children. Doc Ophthalmol 147, 179–188 (2023). https://doi.org/10.1007/s10633-023-09944-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09944-9