Abstract
Background
Neuronal ceroid lipofuscinosis is a group of neurodegenerative disorders with varying visual dysfunction. CLN3 is a subtype which commonly presents with visual decline. Visual symptomatology can be indistinct making early diagnosis difficult. This study reports ocular biomarkers of CLN3 patients to assist clinicians in early diagnosis, disease monitoring, and future therapy.
Methods
Retrospective review of 5 confirmed CLN3 patients in our eye clinic. Best corrected visual acuity (BCVA), electroretinogram (ERG), ultra-widefield (UWF) fundus photography and fundus autofluorescence (FAF), and optical coherence tomography (OCT) studies were undertaken.
Results
Five unrelated children, 4 females and 1 male, with median age of 6.2 years (4.6–11.7) at first assessment were investigated at the clinic from 2016 to 2021. Four homozygous and one heterozygous pathogenic CLN3 variants were found. Best corrected visual acuities (BCVAs) ranged from 0.18 to 0.88 logMAR at first presentation. Electronegative ERGs were identified in all patients. Bull’s eye maculopathies found in all patients. Hyper-autofluorescence ring surrounding hypo-autofluorescence fovea on FAF was found. Foveal ellipsoid zone (EZ) disruptions were found in all patients with additional inner and outer retinal microcystic changes in one patient. Neurological problems noted included autism, anxiety, motor dyspraxia, behavioural issue, and psychomotor regression.
Conclusions
CLN3 patients presented at median age 6.2 years with visual decline. Early onset maculopathy with an electronegative ERG and variable cognitive and motor decline should prompt further investigations including neuropaediatric evaluation and genetic assessment for CLN3 disease. The structural parameters such as EZ and FAF will facilitate ocular monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neuronal ceroid lipofuscinoses (NCLs) are a group of autosomal recessive lysosomal storage disorders (LSD) and together are one of the most frequent causes of neurodegenerative disease in children. The incidence of NCL ranges from 0.1 to 8 per 100,000 live births [1,2,3,4,5,6,7]. Isolated retinal CLN3 disease accounted for 1% of all inherited retinal disease (IRD) in a French cohort [8]. There are a number of recent publications reporting isolated retinal findings in patients with CLN3 mutations [9,10,11]. Analysis of these reports suggests that it is more likely that the 1 kb homozygous deletion is associated with the syndromic CLN3 phenotype, while compound heterozygous mutations are more likely to be found in the isolated retinal degeneration phenotype. NCL patients experience myoclonic seizures, progressive visual deterioration, cognitive dysfunction, motor decline, and premature death [11,12,13]. These clinical features often present asynchronously, making diagnosis difficult and often delayed. Classically, NCL was classified based on age at onset (congenital, infantile, late infantile, juvenile, and adult). To date, 13 causative genes have been identified (CLN1 to 8 and CLN10 to 14) with CLN3 being the most prevalent cause [11, 12, 14].
CLN3 disease was formerly known as ‘juvenile neuronal ceroid lipofuscinosis’ (JNCL) and can initially present as with isolated visual symptoms or with progressive neurological dysfunction. Wang et al. reported that the CLN3 associated visual symptoms can exhibit rod-cone or cone-rod dystrophy (RCD or CRD) phenotype [15]. In that study, 5 patients from a total of 123 retinal degeneration patients had a CLN3 mutation with 4 RCD and 1 CRD phenotype [15]. Data from our previous study showed that all CLN3 patients in our study centre had an electronegative ERG, suggesting its importance in this particular diagnosis [16].
CLN3 is a lysosomal membrane protein involved with glycosylation and phosphorylation at several sites, with localization to synaptic compartments in neuronal cells. This localization might suggest a distinctive role of the CLN3 protein within neurons that makes the central nervous system (CNS) susceptible in this disease [17].
Understanding of the ophthalmological findings is crucial to early diagnosis of CLN3-related disease, as these commonly precede the development of neurological signs, with retinal examination using multimodal imaging frequently identifying bull’s eye maculopathy, optic disc pallor, and/or bone spicule formation. These structural findings overlap with Stargardt disease or retinitis pigmentosa (RP) [18, 19]. Where CLN3 disease is a differential diagnosis, it is critical that a full-field electroretinogram (ffERG) is performed, as this may demonstrate an electronegative ERG (b:a ratio ≤ 1 in dark adapted 3.0 or 12.0 ERG) [16, 18, 20,21,22]. Other classical ocular features of CLN3 disease may then be elucidated on ophthalmic examination, alerting the clinician to the possibility of this disorder and the need for neurogenetic review.
Novel therapies for CLN3-related disease are currently emerging into clinical trials. Early diagnosis is therefore vital to increase the possibility of administering a novel CLN3 disease therapy at a time when maximal benefit might be achieved. Ocular biomarkers become challenging to obtain as neurological deterioration progress. The purpose of this study is to report ocular findings of CLN3 disease patients to aid early diagnosis, enable disease monitoring, and assist further trials of novel CLN3 therapies.
Methods
Retrospective evaluation of 5 confirmed CLN3 disease patients in our tertiary referral clinic were included in this study. They were referred for ophthalmic review and subsequently underwent single genetic testing for CLN3 disease. The age when the patients were referred and the age of ocular and neurological onset were recorded. Age of ocular and neurological onset was determined by the earliest time point of reported ocular and neurological symptoms. Best corrected visual acuity (BCVA), retinal imaging, spectral domain-optical coherence tomography (SD-OCT), and full-field electroretinogram (ffERG) data were reviewed at baseline (BL) and follow-up (FU). BCVA was measured using a logarithm of minimum angle of resolution (logMAR). Patients with BCVA worse than 1.0 logMAR (6/60 on Snellen) were examined using Sheridan-Gardiner single letter and if failed this continued to finger counting, hand movement, and perception of light. BCVA values were then converted to logMAR equivalent values as described by Lange et al. [23, 24].
The study followed the tenets of the Declaration of Helsinki and was approved by the local ethical committee. Disease severity was calculated using the recently described Hamburg CLN3 Ophthalmic Rating Scale [25]. This scale consists of visual acuity, fundus, and OCT score with maximum points of 14. The scale then is translated into CLN3 grades of grade 0 (unaffected) = 14 points, grade 1 (affected) = 10–13 points, grade 2 (severely affected) = 5–9 points, and grade 3 (end stage) = 0–4 points.
Retinal imaging
Ultra-widefield (UWF) fundus pseudocolour imaging and UWF-fundus autofluorescence (UWF-FAF) were performed using the Optos system (Optos plc, Dunfermline, UK).
Spectral domain-optical coherence tomography (SD-OCT)
SD-OCT imaging was acquired using the Heidelberg Spectralis (Heidelberg Engineering, Germany) and Zeiss Cirrus (Carl Zeiss Meditec, Dublin, CA, USA). Retinal layers and central macular thickness were examined. Bruch membrane and internal limiting membrane markers were manually adjusted to ensure precision in measuring retinal thickness. Central subfield thickness (CST) and central macular thickness (CMT) are both commonly used terms in ophthalmology to describe the thickness of the central retina. Central subfield thickness (CST), also known as foveal thickness, was defined as the average thickness of the central 1 mm subfield centred at the fovea on ETDRS grid [26].
Electrophysiology
Testing strategies included pattern ERG (pERG) and full-field ERG (ffERG) using Espion (Diagnosys, Lowell, Massachusetts USA). Visual electrophysiology was performed according to International Society for Clinical Electrophysiology of Vision (ISCEV) standards [27,28,29]. Gold foil; Dawson, Trick, and Litzkow (DTL); or skin electrodes were used depending on the level of patient’s cooperation. Paediatric non-standard abbreviated ERG protocol was done using a modified Great Ormond Street Hospital (GOSH) protocol utilizing handheld Grass (Gr) strobe for the most uncooperative patient [28, 30]. Pulse period 2 (2/s) and flicker Gr intensity 1 (Gr1) were used instead of 3/s and Gr4, respectively. The b:a wave ratio was calculated from dark adapted (DA) ffERG 3.0 or 12.0 with a value of ≤ 1.0 defined as an electronegative ERG [16, 31].
Results
Five unrelated children with biallelic CLN3 pathogenic variants were included in the study, 4 females and 1 male with median age at referral of 6.2 (4.6–11.7) years (yrs). Median age at ocular onset was 5.1 (2.6–11.6) yrs with P1 who had the earliest ocular onset while P5 the latest. Two patients (P1 and P2) had FU data. Hamburg CLN3 ophthalmic rating scale at BL was ranging from 9 (affected) to 13 (severely affected) (Table 1). P1 progressed from affected to end stage while P2 from affected to severely affected.
Genetics and pathology investigations
The cohort in this study consisted of 4 patients with a previously reported homozygous pathogenic CLN3 variant (P1-P4) and one patient with compound heterozygous CLN3 variants (P5) (Table 1). The recurrent pathogenic variant CLN3: c.461-280_677 + 382del was identified in all 5 patients investigated. Only P3 underwent peripheral blood film microscopy and electron microscopy both with positive result of vacuolated lymphocytes and fingerprint inclusions, respectively.
BCVA
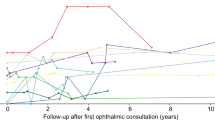
BCVAs ranged from 0.18 to 0.88 logMAR at BL with follow-up (FU) obtainable from 2 patients (P1 and P2) (Table 1). These 2 patients (4 eyes) had an average of 0.75 (0.41) logMAR loss per year during an average of 3.9 (2) years of FU and worst eventual FU BCVA (2.7 logMAR). P1 had earlier ocular onset than P2 and thus had the worst BCVA (2.7 logMAR) at an earlier age (6.8 vs 11.5 yrs) (Fig. 1). At the point of similar age (11–12 yrs), P2 had far worse BCVA than P3 (2.7 logMAR vs 0.5&0.56 logMAR) (Fig. 1).
Retinal imaging
Assessment of UWF-fundus pseudocolour appearance and UWF-FAF showed a consistent bull’s eye macular appearance in all patients (Fig. 2). Additionally, P5 showed macular striae. Progress of yellow-orange macular appearance, retinal atrophy, and vessel rarefication can be observed in P1 and P2 (Fig. 3). The FAF pattern consisted of hyper-autofluorescence (hyperAF) rings surrounding a hypo-autofluorescence (hypoAF) fovea (Fig. 2). This perifoveal hyperAF ring became more apparent at first FAF FU in P1 and P2. On the second FU, the ring of hyperAF had disappeared and hypoAF had developed outside the vascular arcade corresponding to the retinal atrophy seen on fundus image (Fig. 3). Right and left eyes of the patients showed similar phenotype.
Multimodal retinal imaging for all patients. Right eye UWF-fundus pseudocolour photograph, UWF-FAF, and macular SD-OCT for P1-P5. Double black arrows indicate the margin of bull’s eye maculopathy (BEM). (P1-A) BEM and macular yellow-orange appearance found in P1. (P1-B) HyperAF ring surrounding hypoAF fovea. (P1-C) EZ loss on fovea. (P2-A) BEM was found in P2. (P2-B) HyperAF ring surrounding hypoAF fovea. (P2-C) EZ loss on fovea. (P3-A) BEM, macular yellow-orange appearance, vessel rarefication, retinal atrophy outside vascular arcade found in P3. (P3-B) HyperAF ring surrounding hypoAF fovea, hypoAF corresponding to retinal atrophy. (P3-C) EZ loss on fovea. (P4-A) BEM and vessel rarefication found in P4. Insert image of enlarged macula shows BEM. (P4-B) HyperAF ring surrounding hypoAF fovea. (P4-C) EZ loss on fovea. (P5-A) BEM, macular striae, macular yellow-orange appearance, vessel rarefication found in P5. Insert image shows clearer macular striae. (P5-B) HyperAF ring surrounding hypoAF fovea. (P5-C) Schitic changes on macula and EZ loss on fovea. (N-A,B,C) Normal control showed normal fundus pseudocolour photograph, normal UWF-FAF with foveal reduction of AF, and normal SD-OCT with normal thickness and distinct lamination. AF Autofluorescence, BEM Bull’s eye maculopathy, EZ ellipsoid zone, FAF fundus autofluorescence, SD-OCT spectral domain-optical coherence tomography, UWF ultra-wide field
Multimodal retinal imaging follow-up for selected patients. The left eye multimodal retinal imaging was selected to illustrate change over time. UWF-fundus pseudocolour photography, UWF-FAF, and SD-OCT follow-up of P1 and P2 are shown. (P1-A) Bull’s eye maculopathy (BEM) and macular yellow-orange appearance were found in P1 at 4.6 yrs. (P1-D) Macular yellow-orange appearance became more apparent at 5.3yrs. (P1-F) Macular yellow-orange appearance covering macula and retinal atrophy outside vascular arcade at 6.8 yrs. (P1-B) HyperAF ring surrounding hypoAF fovea at 4.6 yrs. (P1-E) More apparent perifoveal hyperAF ring at 5.3 yrs. (P1-G) Perifoveal hyperAF ring disappearance, hypoAF outside vascular arcade corresponding to retinal atrophy at 6.8 yrs. (P2-A) BEM was found in P2 at 5.9 yrs. (P2-D) Macular yellow-orange appearance started to bed found at 7 yrs. (P2-F) BEM, macular yellow-orange appearance, vessel rarefication, retinal atrophy on inferonasal area at 9.4 yrs. (P2-B) HyperAF ring surrounding hypoAF fovea at 5.9 yrs. (P2-E) More apparent perifoveal hyperAF ring at 7 yrs. (P2-G) Perifoveal hyperAF ring disappearance, replaced by hypoAF ring. HypoAF on inferonasal area corresponding to retinal atrophy at 9.4 yrs BL SD-OCT was taken using Heidelberg, while FU SD-OCT was taken using Cirrus device. (P1-C)(P2-C) BL SD-OCT of P1 and P2 showed disruption of foveal EZ. (P1-H)(P2-H) FU SD-OCT of P1 and P2 showed progressed disappearance of EZ. Signal hypertransmission into choroid (yellow arrow) was present in both FU SD-OCT. AF Autofluorescence, BEM Bull’s eye maculopathy, BL baseline, EZ ellipsoid zone, FAF fundus autofluorescence, FU follow-up, SD-OCT spectral domain-optical coherence tomography, UWF ultra-wide field
Spectral domain-optical coherence tomography (SD-OCT)
Foveal ellipsoid zone (EZ) disruption was found in each patient. Those with the largest central EZ disruption had the poorest eventual BCVA (P1&2) (Table 1). FU OCT was available in P1 and P2 using the Cirrus device and showed progression of EZ loss and signal hypertransmission into the choroid (Fig. 3). Macular IR appearances again showed a bull’s eye maculopathy for P1-P5 and also macular striae for P5. CSTs were ranging from 99 to 145 μm (Table 1). In the compound heterozygous CLN3 patient (P5), we identified macular inner and outer retinal microcystic changes in addition to the macular atrophy (Fig. 2). There is a concordance in SD-OCT result of each patient.
Electrophysiology
P1, P2, and P4 used skin electrode, while P3 and P5 used DTL and gold foil electrode, respectively. P4 underwent paediatric non-standard abbreviated ERG protocol with skin electrode. The electrophysiology results were as follows. The pERG recordings were noisy and almost undetectable for the 15-degree stimulus field. The 30-degree field had identifiable traces but greatly reduced. The ffERG revealed an overall electronegative ERG waveform in addition to the reduced dark adapted (DA) and light adapted (LA) responses. P2 had ERG twice with 1.1-year interval. The first one showed presence of rod function and undetectable cone response, while the second one showed worsening of rod function (Table 1) (Fig. 4).
Full-field ERGs and pattern ERG recordings. The full-field ERGs were recorded according to ISCEV protocols for paediatric ERG and in one case the non-standard abbreviated ERG protocol was used. P1 and P2 were examined using skin electrodes, P3 used DTL electrodes, P4 underwent a paediatric non-standard abbreviated ERG protocol using skin electrode, and P5 used gold foil electrodes. P4 ERG was performed using a modified Great Ormond Street Hospital (GOSH) protocol as described in the methods. All patients showed severely reduced or undetectable DA 0.01 response. All patients excluding P4 showed a reduced b:a wave ratio (electronegative) for DA 3.0 and DA 12.0. P4 had a very noisy recordings, and there is a suggestion of a reduced b wave. The LA 30 Hz and LA 3.0 were significantly reduced for P1-P2. P4’s responses were noisy but also appeared reduced. Patients P3 and P5 were at the lower of the normal range. In patients P1 to P4, the pERG 30 deg p50 amplitude was almost undetectable. Patient P5 showed an identifiable waveform, but the p50 amplitude was reduced. Despite P4 having poor compliance and cooperation during the testing which resulted in noisy recordings, the combination of the potential electronegative scotopic ERG and a significantly reduced LA 30 Hz and LA 3.0 raised the possibility of Batten disease as a potential diagnosis. DA Dark adapted, DTL Dawson, Trick, and Litzkow electrodes, ERG electroretinogram, ffERG full-field ERG, LA light adapted, pERG pattern ERG
Associated neurobehavioural issues
Neurobehavioural issues were documented in 4 patients (P1-4) at the time of presentation. The oldest patient (P5) did not have any systemic symptoms at presentation or the last ocular follow-up. Associated symptoms included autism spectrum features in P1, significant anxiety and speech delay in P2, behavioural issues in P3, and motor dyspraxia and behavioural issues (biting friends) in P4. In two patients (P2 and P4) the neurological abnormalities appeared after the visual symptoms, while P3 had onset before any eye complaint.
Discussion
CLN3-related disease commonly presents with early onset visual decline and variable neurodegeneration in childhood [12]. The visual decline in children with CLN3 disease is frequently more rapid than other early onset maculopathies such as Stargardt disease [32].
The CLN3 protein has a crucial role within neurons specifically in the synaptic space, with animal models of CLN3 disease showing this condition is primarily a disease of the inner retina, with secondary changes in the outer retina [17, 20]. CLN3 has a role in the transfer of the palmitoyl-protein thioesterases-1 (Ppt1) protein, and deficiencies in this protein have been associated with inner nuclear layer damage, particularly cone bipolar cells, and further damaging the cone photoreceptor cells over the rod [33, 34]. This pathophysiology assists in the understanding of the generation of the electronegative ERG, the one feature that was consistent across our cohort and similar to previous studies [18, 21, 22, 32], reflecting the inner retinal defects. There was significant but variable reduction in both rod and cone responses as found in other studies [9, 18, 21, 35]. The ffERG of P2 in 2 different time points showed early DA ERG preservation associated with an undetectable LA ERG, further reflecting initial cone involvement of this disease and thus resembling CRD [33, 34]. In contrast, other studies in CLN3 studies in cases without neurological phenotype showed that DA ERG is more affected that LA ERG resembling RCD [8, 9, 32, 35]. These contrasting phenotypes have electronegative ERG or at least reduced b:a wave ratio as the consistent common finding reflecting inner retina disturbance.
We found the most common pathogenic CLN3 variant of c.461-280_677 + 382del in all 5 patients [36,37,38]. In 4 patients (P1-P4), this variant was homozygous. In P5 we identified this common pathogenic variant in compound with a novel missense variant, c.680A > G p.(Tyr227Cys). This variant is likely pathogenic according to ACMG classification [39].
Batten disease is a rare paediatric degenerative disorder, and diagnosis may be delayed due to variable presenting features [18, 21, 40]. The application of electrophysiology combined with multimodal imaging in patients with reduced vision provides an opportunity of early recognition of this disease. The findings of an electronegative ERG and biomarkers of a bull’s eye maculopathy facilitate directed genetic testing. The increasing availability of genetic testing will supplant the use of peripheral blood film microscopy (vacuolated lymphocytes) and electron microscopy (storage lysosomal inclusions) as previously proposed by other authors [18].
Ophthalmic follow-up is challenging for these patients due to poor cooperation as the degenerative disorder progresses. In our study two patients had reliable measurements to enable comparison with BL. In these two patients (4 eyes) the rate of change was a loss of 0.75 (0.41) logMAR letters/year during 3.9 (2) years of FU. It is a slower rate of deterioration with longer FU compared to Wright et al. study with 2.02 (3.78) logMAR letters/year during 0.9 (0.5) years FU [18]. These results provide further evidence to the variability in disease progression in this disorder. The latest-onset patient (P5) with no documented neurological findings had the best BCVA, while the early onset patients (P1&2) had the worst BCVA at FU. P5 was the only patient with a compound heterozygous mutation. These findings were in concordance to a previous non-syndromic CLN3 study that found absence of visual loss in the late onset patients and mild visual loss in their early onset patients [9]. Later onset of the disease appears to be correlated with better BCVA. A vast majority of CLN3 disease patients (± 80%) present with vision impairment [41, 42]. A contribution to the visual decline has been postulated to arise from additional damage to the lateral geniculate nucleus and/or primary visual cortex [43].
Bull’s eye maculopathy is the most consistent and prominent macular finding in this patient cohort as also found in previous studies [18, 21, 44]. Other fundus findings reported in CLN3 disease include optic disc pallor, macular atrophy, macular striae, macular oedema, retinal pigment epithelium (RPE) atrophy, RPE granularity, bone spicule formation, epiretinal membrane, arteriolar attenuation, and even a Coats-like reaction [9, 18, 40, 45]. The fundus variability may lead to misdiagnosis of Stargardt disease or retinitis pigmentosa, demonstrating the importance of electrophysiology investigations.
UWF-FAF findings highlighted the central hypoautofluorescence (hypoAF) surrounded by a ring of hyperAF found in our patients. Through 2.2–3.5 years of UWF-FAF follow-up in P1&P2, we found that the perifoveal hyperAF ring as found in previous CLN3 study [18] became more apparent and eventually disappeared. Then hypoAF starts to emerge in the periphery corresponding to retinal atrophy [40]. As disease advances, the whole macular region shows generalised hypoAF [9, 18, 46]. Therefore, we suggest that this specific change in UWF-FAF can be used as biomarker to monitor natural disease progression. A ring of hyperAF is a common finding in rod-cone dystrophies where the ring divides healthy central retina and disturbed peripheral retina [47, 48]. Our CLN3 cases initially show the reverse pattern with an abnormal central fovea region and preserved peripheral retina.
The disrupted foveal EZ on SD-OCTs (P1-P5) is consistent with a previous review [18] and supports the CRD phenotype reflected from ERG and UWF-FAF findings in our cohort. In contrast, CLN3 cases with RCD phenotype had the predictably preserved foveal EZ while disrupted in the parafovea [8, 9]. In later stage, there is marked macular EZ disruption with difficulty identifying any remaining outer retinal structures and choroidal signal hypertransmission reflecting RPE disturbance [18, 46, 49, 50]. Inner and outer retinal microcystic changes found in P5 were also found in previous reports of CLN1 and CLN3 patients, indicating the involvement of both retinal layers [8, 9, 51]
The mechanism for retinal degeneration in CLN3 disease is yet to be understood [43]. The bull’s eye maculopathy, early DA ERG preservation, pERG disturbance, and foveal EZ disruption in our study support the notion that this disease has centrifugal (central to peripheral) progression as also found by Preising et al. in their study [52]. This condition primarily affects the inner retina with secondary defects in outer retina, as suggested in a mouse model where there were significant bipolar cell survival and preserved retinal function after gene therapy [20].
Four of our patients (P1-4) had neurological problems co-existing with their ocular symptoms, while the oldest patient (P5) did not have any systemic symptoms at presentation or the last ocular follow-up. Reflected by our P5 case, electrophysiology is the primary investigations in the event of a bull’s eye maculopathy in a child of this age. An electronegative ERG with bull’s eye maculopathy should directly lead to investigation of a genetic referral even in the case without neurological symptoms. Neurological onset is variable and may occur before, after, or concurrent with visual decline. Various neurological signs and symptoms have been reported, including: dementia, seizures, speech delay, mood fluctuations, difficult behaviour, balance, or memory changes, cognitive decline, sleep disturbances, feeding difficulties, clumsiness, and poor concentration [13, 18, 41] with seizure as the most common [13]. CLN3 has a variable phenotype as illustrated by those presenting with mild or delayed neurological defects ranging from 3- to 18-year interval between ocular and neurological onset [53,54,55,56], or no systemic features [9, 10, 15]. Ocular and neurological phenotypic variability also is frequently reported in those with the same mutations [57,58,59]. Ocular phenotype variability includes RCD and CRD [15]. CLN3 literature implies that syndromic CLN3 disease (mostly homozygous variant) is characterized by CRD with childhood onset and rapid disease progression, while the isolated retinal degeneration case (mostly compound heterozygous variant) is rather a RCD with later onset and slower progression [32]. However, genotype–phenotype correlation in CLN3 disease is not perfect and caution should be given in establishing the diagnosis [8].
Although there is no current definitive treatment for CLN3 disease, early diagnosis is important to give appropriate family counselling and establish supportive therapies at the earliest opportunity [20, 60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. In Australia there is Mackenzie’s mission a study investigating preconception for autosomal recessive disorder. CLN3 is one of the gene of 500 genes in the panel for both parents. Secondly, whole genome screening is being investigated as an expansion of the newborn screening programme to identify and enable early management of severe genetic diseases [77, 78]. There are currently 3 active CLN3 clinical trials which have ophthalmic parameter measurement as an endpoint. These include intrathecal gene therapy AT-GTX-502 (NCT03770572), oral drug PLX-200/gemfibrozil (NCT04637282), and oral drug Miglustat 100 mg (NCT05174039) which give hope that disease-modifying therapies are emerging [79]. Those studies emphasize the importance of understanding the ocular biomarkers in CLN3 disease natural history. Multimodal imaging results are similar between the two eyes in each patient, making it viable to use fellow eye as control in the event of intraocular therapeutical trials. Combination of therapies might be needed to treat this condition [73, 80, 81].
Given the retrospective nature of our study and the natural history of neurodegenerative decline in CLN3 patients, there were limitations of follow-up examinations.
Conclusions
The findings of an electronegative ERG with concurrent bull’s eye maculopathy in young age should prompt early neurological assessment for signs of neurodegeneration and referral for genomic investigation for CLN3 gene defects. Some children also experience isolated ocular presentations without neurobehavioral features. It is important that CLN3 disease is considered in electronegative ERG-bull’s eye maculopathy patients even without neurological defect. Recognition of these features will assist in establishing an early diagnosis enabling appropriate therapies, family planning, disease monitoring, and potential enrolment in clinical trials for novel therapies.
Monitoring visual function is challenging in this cohort as neurological deterioration progresses. Finding ocular biomarkers that can be consistently recorded in an outpatient setting is important for clinical trial outcome measures. Given their change throughout the natural history of the disease, EZ and FAF are the most promising structural parameters identified in our cohort.
References
Seehafer SS, Pearce DA (2009) Spectral properties and mechanisms that underlie autofluorescent accumulations in batten disease. Biochem Biophys Res Commun 382(2):247–251
Augestad LB, Flanders WD (2006) Occurrence of and mortality from childhood neuronal ceroid lipofuscinoses in Norway. J Child Neurol 21:917–922
Wisniewski KE, Zhong N, Philippart M (2001) Pheno/ genotypic correlations of neuronal ceroid lipofuscinoses. Neurology 57:576–581
The International Batten Disease Consortium (1995) Isolation of a novel gene underlying batten disease, CLN3. Cell 82:949–957
Claussen M, Heim P, Knispel J, Goebel HH, Kohlschütter A (1992) Incidence of neuronal ceroid lipofuscinoses in West Germany: variation of a method for studying autosomal recessive disorders. Am J Med Genet 42:536–538
Santorelli FM, Garavaglia B, Cardona F, Nardocci N, Bernardina BD, Sartori S et al (2013) Molecular epidemiology of childhood neuronal ceroid-lipofuscinosis in Italy. Orphanet J Rare Dis 8(19):1–7
Al-Jasmi FA, Tawfig N, Berniah A, Ali BR, Taleb M, Hertecant JL et al (2013) Prevalence and novel mutations of lysosomal storage disorders in United Arab Emirates : LSD in UAE. JIMD Rep 10:1–9
Smirnov VM, Nassisi M, Solis Hernandez C, Mejecase C, El Shamieh S, Condroyer C et al (2021) Retinal phenotype of patients with isolated retinal degeneration due to CLN3 pathogenic variants in a french retinitis pigmentosa cohort. JAMA Ophthalmol 139(3):278–291
Ku CA, Hull S, Arno G, Vincent A, Carss K, Kayton R et al (2017) Detailed clinical phenotype and molecular genetic findings in CLN3-associated isolated retinal degeneration. JAMA Ophthalmol 135(7):749–760
Chen FK, Zhang X, Eintracht J, Zhang D, Arunachalam S, Thompson JA et al (2019) Clinical and molecular characterization of non-syndromic retinal dystrophy due to c.175G>A mutation in ceroid lipofuscinosis neuronal 3 (CLN3). Doc Ophthalmol 138(1):55–70
Mole SE, Williams RE, Goebel HH (2005) Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics 6(3):107–126
Schulz A, Kohlschutter A, Mink J, Simonati A, Williams R (2013) NCL diseases - clinical perspectives. Biochim Biophys Acta 1832(11):1801–1806
Ostergaard JR (2016) Juvenile neuronal ceroid lipofuscinosis (batten disease): current insights. Degener Neurol Neuromuscul Dis 6:73–83
Mole SE, Cotman SL (2015) Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochim Biophys Acta 1852(10):2237–2241
Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V et al (2014) Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet 133(3):331–345
Sakti DH, Ali H, Korsakova M, Saakova N, Mustafic N, Fraser CL et al (2022) Electronegative electroretinogram in the modern multimodal imaging era. Clin Exp Ophthalmol 50(4):429–440
Phillips SN, Benedict JW, Weimer JM, Pearce DA (2005) CLN3, the protein associated with batten disease: structure, function and localization. J Neurosci Res 79(5):573–583
Wright GA, Georgiou M, Robson AG, Ali N, Kalhoro A, Holthaus SK et al (2020) Juvenile batten disease (CLN3): detailed ocular phenotype, novel observations, delayed diagnosis, masquerades, and prospects for therapy. Ophthalmol Retina 4(4):433–445
Bozorg S, Ramirez-Montealegre D, Chung M, Pearce DA (2009) Juvenile neuronal ceroid lipofuscinosis (JNCL) and the eye. Surv Ophthalmol 54(4):463–471
Kleine Holthaus SM, Aristorena M, Maswood R, Semenyuk O, Hoke J, Hare A et al (2020) Gene therapy targeting the inner retina rescues the retinal phenotype in a mouse model of CLN3 batten disease. Hum Gene Ther 31(13–14):709–718
Collins J, Holder GE, Herbert H, Adams GG (2006) Batten disease: features to facilitate early diagnosis. Br J Ophthalmol 90(9):1119–1124
Weleber RG (1998) The dystrophic retina in multisystem disorders the electroretinogram In neuronal ceroid Iipofuscinoses. Eye 12:580–590
Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M (2009) Resolving the clinical acuity categories “hand motion” and “counting fingers” using the freiburg visual acuity test (FrACT). Graefes Arch Clin Exp Ophthalmol 247(1):137–142
Bach M (2021) Visual acuity “cheat sheet” – high and low vision Available from: https://michaelbach.de/sci/acuity.html.
Dulz S, Atiskova Y, Wibbeler E, Wildner J, Wagenfeld L, Schwering C et al (2020) An ophthalmic rating scale to assess ocular involvement in juvenile CLN3 disease. Am J Ophthalmol 220:64–71
Sull AC, Vuong LN, Price LL, Srinivasan VJ, Gorczynska I, Fujimoto JG et al (2010) Comparison of spectral/fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina 30:235–245
Cornish EE, Vaze A, Jamieson RV, Grigg JR (2021) The electroretinogram in the genomics era: outer retinal disorders. Eye (Lond) 35(9):2406–2418
Robson AG, Frishman LJ, Grigg J, Hamilton R, Jeffrey BG, Kondo M et al (2022) ISCEV standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol 144(3):165–177
Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL et al (2013) ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol 126(1):1–7
Marmoy OR, Moinuddin M, Thompson DA (2022) An alternative electroretinography protocol for children: a study of diagnostic agreement and accuracy relative to ISCEV standard electroretinograms. Acta Ophthalmol 100(3):322–330
Kim JM, Payne JF, Yan J, Barnes CS (2012) Negative electroretinograms in the paediatric and adult population. Doc Ophthalmol 124(1):41–48
Kuper WFE, Talsma HE, van Schooneveld MJ, Pott JWR, Huijgen BCH, de Wit GC et al (2021) Recognizing differentiating clinical signs of CLN3 disease (batten disease) at presentation. Acta Ophthalmol 99(4):397–404
Appu AP, Bagh MB, Sadhukhan T, Mondal A, Casey S, Mukherjee AB (2019) Cln3-mutations underlying juvenile neuronal ceroid lipofuscinosis cause significantly reduced levels of Palmitoyl-protein thioesterases-1 (Ppt1)-protein and Ppt1-enzyme activity in the lysosome. J Inherit Metab Dis 42(5):944–954
Atiskova Y, Bartsch S, Danyukova T, Becker E, Hagel C, Storch S et al (2019) Mice deficient in the lysosomal enzyme palmitoyl-protein thioesterase 1 (PPT1) display a complex retinal phenotype. Sci Rep 9(1):14185
Eksandh L, Ponjavic V, Munroe PB, Eiberg H, Uvebrant P, Ehinger B et al (2000) Full-field ERG in patients with batten/spielmeyer-vogt disease caused by mutations in the CLN3 gene. Ophthalmic Genet 21(2):69–77
Deacon BS, Charles JM, Cheeseman EW, Cathey SS (2016) Next-generation sequencing in the diagnosis of juvenile neuronal ceroid lipofuscinosis. Pediatr Neurol 62:71–72
Filges I, Sparagana S, Sargent M, Selby K, Schlade-Bartusiak K, Lueder GT et al (2014) Brain MRI abnormalities and spectrum of neurological and clinical findings in three patients with proximal 16p11.2 microduplication. Am J Med Genet A 164A(8):2003–2012
Wibbeler E, Nickel M, Schwering C, Schulz A, Mink JW (2022) The unified batten disease rating scale (UBDRS): validation and reliability in an independent CLN3 disease sample. Eur J Paediatr Neurol 38:62–65
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 17(5):405–424
Dulz S, Wagenfeld L, Nickel M, Richard G, Schwartz R, Bartsch U et al (2016) Novel morphological macular findings in juvenile CLN3 disease. Br J Ophthalmol 100(6):824–828
Cialone J, Adams H, Augustine EF, Marshall FJ, Kwon JM, Newhouse N et al (2012) Females experience a more severe disease course in Batten disease. J Inherit Metab Dis 35(3):549–555
Marshall FJ, de Blieck EA, Mink JW, Dure L, Adams H, Messing S, Rothberg PG, Levy E, McDonough T, DeYoung J, Wang M (2005) A clinical rating scale for batten disease: reliable and relevant for clinical trials. Neurology 65(2):275–279
Weimer JM, Custer AW, Benedict JW, Alexander NA, Kingsley E, Federoff HJ et al (2006) Visual deficits in a mouse model of Batten disease are the result of optic nerve degeneration and loss of dorsal lateral geniculate thalamic neurons. Neurobiol Dis 22(2):284–293
Drack AV, Miller JN, Pearce DA (2013) A novel c. 1135_1138delCTGT mutation in CLN3 leads to juvenile neuronal ceroid lipofuscinosis. J Child Neurol 28(9):1112–1116
Hainsworth DP, Liu GT, Hamm CW, Katz ML (2009) Funduscopic and angiographic appearance in the neuronal ceroid lipofuscinoses. Retina 29:657–668
Hansen MS, Hove MN, Jensen H, Larsen M (2016) Optical coherence tomography in juvenile neuronal ceroid lipofuscinosis. Retin Cases Brief Rep 10(2):137–139
Robson AG, Tufail A, Fitzke F, Bird AC, Moore AT, Holder GE et al (2011) Serial imaging and structure-fuction correlates of high-density rings of fundus autofluorescence in retinitis pigmentosa. Retina 31:1670–1679
Robson AG, Saihan Z, Jenkins SA, Fitzke FW, Bird AC, Webster AR et al (2006) Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol 90(4):472–479
Sadda SR, Guymer R, Holz FG, Schmitz-Valckenberg S, Curcio CA, Bird AC et al (2018) Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology 125(4):537–548
Zicarelli F, Mantovani A, Preziosa C, Staurenghi G (2020) Multimodal imaging of multiple evanescent white dot syndrome: a new interpretation. Ocul Immunol Inflamm 28(5):814–820
Turriff AE, Cukras CA, Brooks BP, Huryn LA (2019) Considerations in multi-gene panel testing in paediatric ophthalmology. J AAPOS 23(3):163–165
Preising MN, Abura M, Jager M, Wassill KH, Lorenz B (2017) Ocular morphology and function in juvenile neuronal ceroid lipofuscinosis (CLN3) in the first decade of life. Ophthalmic Genet 38(3):252–259
Kuper WFE, van Alfen C, van Eck L, de Man SA, Willemsen MH, van Gassen KLI et al (2020) The c.1A > C start codon mutation in CLN3 is associated with a protracted disease course. JIMD Rep 52(1):23–27
Sarpong A, Schottmann G, Ruther K, Stoltenburg G, Kohlschutter A, Hubner C et al (2009) Protracted course of juvenile ceroid lipofuscinosis associated with a novel CLN3 mutation (p.Y199X). Clin Genet 76(1):38–45
Kuper WFE, Cv A, Lv E, Broek BTAvd, Huisman A, Genderen MMv, et al (2017) A case of unexpected adult-onset neurologic decline in CLN3-associated retinal degeneration. JAMA Ophthalmol 135(12):1451–1452
Evans LP, Gibson-Corley KN, Mullins RF, Tucker BA, Trent A, Stone EM, Jones KA (2021) An unusual presentation of CLN3-associated batten disease with classic histopathologic and ultrastructural findings. J Neuropathol Experimental Neurol 80(11):1081–1084
Gilani N, Razmara E, Ozaslan M, Abdulzahra IK, Arzhang S, Tavasoli AR et al (2021) A novel deletion variant in CLN3 with highly variable expressivity is responsible for juvenile neuronal ceroid lipofuscinoses. Acta Neurol Belg 121(3):737–748
Lebrun AH, Moll-Khosrawi P, Pohl S, Makrypidi G, Storch S, Kilian D et al (2011) Analysis of potential biomarkers and modifier genes affecting the clinical course of CLN3 disease. Mol Med 17(11–12):1253–1261
Pebrel-Richard C, Debost-Legrand A, Eymard-Pierre E, Greze V, Kemeny S, Gay-Bellile M et al (2014) An unusual clinical severity of 16p112 deletion syndrome caused by unmasked recessive mutation of CLN3. Eur J Hum Genet 22(3):369–373
Wiley LA, Burnight ER, Drack AV, Banach BB, Ochoa D, Cranston CM et al (2016) Using patient-specific induced pluripotent stem cells and wild-type mice to develop a gene augmentation-based strategy to treat CLN3-associated retinal degeneration. Hum Gene Ther 27(10):835–846
Centa JL, Jodelka FM, Hinrich AJ, Johnson TB, Ochaba J, Jackson M et al (2020) Therapeutic efficacy of antisense oligonucleotides in mouse models of CLN3 batten disease. Nat Med 26(9):1444–1451
Langin L, Johnson TB, Kovacs AD, Pearce DA, Weimer JM (2020) A tailored Cln 3(Q352X) mouse model for testing therapeutic interventions in CLN3 Batten disease. Sci Rep 10(1):10591
Tarczyluk-Wells MA, Salzlechner C, Najafi AR, Lim MJ, Smith D, Platt FM et al (2019) Combined anti-inflammatory and neuroprotective treatments have the potential to impact disease phenotypes in Cln3 (-/-) Mice. Front Neurol 10:963
Seehafer SS, Ramirez-Montealegre D, Wong AM, Chan CH, Castaneda J, Horak M et al (2011) Immunosuppression alters disease severity in juvenile batten disease mice. J Neuroimmunol 230(1–2):169–172
Ouseph MM, Kleinman ME, Wang QJ (2016) Vision loss in juvenile neuronal ceroid lipofuscinosis (CLN3 disease). Ann N Y Acad Sci 1371(1):55–67
Soldati C, Lopez-Fabuel I, Wanderlingh LG, Garcia-Macia M, Monfregola J, Esposito A et al (2021) Repurposing of tamoxifen ameliorates CLN3 and CLN7 disease phenotype. EMBO Mol Med 13(10):e13742
Sondhi D, Scott EC, Chen A, Hackett NR, Wong AM, Kubiak A et al (2014) Partial correction of the CNS lysosomal storage defect in a mouse model of juvenile neuronal ceroid lipofuscinosis by neonatal CNS administration of an adeno-associated virus serotype rh.10 vector expressing the human CLN3 gene. Hum Gene Ther 25(3):223–239
Brudvig JJ, Weimer JM (2022) On the cusp of cures: breakthroughs in Batten disease research. Curr Opin Neurobiol 72:48–54
Bosch ME, Aldrich A, Fallet R, Odvody J, Burkovetskaya M, Schuberth K et al (2016) Self-Complementary AAV9 gene delivery partially corrects pathology associated with juvenile neuronal ceroid lipofuscinosis (CLN3). J Neurosci 36(37):9669–9682
Maalouf K, Makoukji J, Saab S, Makhoul NJ, Carmona AV, Kinarivala N et al (2020) Exogenous flupirtine as potential treatment for CLN3 Disease. Cells 9(8):1872
El-Sitt S, Soueid J, Maalouf K, Makhoul N, Al Ali J, Makoukji J et al (2019) Exogenous galactosylceramide as potential treatment for CLN3 disease. Ann Neurol 86(5):729–742
Zhang X, Zhang D, Thompson JA, Chen SC, Huang Z, Jennings L et al (2021) Gene correction of the CLN3 c.175G>A variant in patient-derived induced pluripotent stem cells prevents pathological changes in retinal organoids. Mol Genet Genomic Med 9(3):e1601
Dannhausen K, Mohle C, Langmann T (2018) Immunomodulation with minocycline rescues retinal degeneration in juvenile neuronal ceroid lipofuscinosis mice highly susceptible to light damage. Dis Model Mech 11(9):033559
Warnock A, Tan L, Li C, An Haack K, Narayan SB, Bennett MJ (2013) Amlodipine prevents apoptotic cell death by correction of elevated intracellular calcium in a primary neuronal model of batten disease (CLN3 disease). Biochem Biophys Res Commun 436(4):645–649
Kovacs AD, Saje A, Wong A, Ramji S, Cooper JD, Pearce DA (2012) Age-dependent therapeutic effect of memantine in a mouse model of juvenile batten disease. Neuropharmacology 63(5):769–775
Johnson TB, Langin LM, Zhao J, Weimer JM, Pearce DA, Kovacs AD (2019) Changes in motor behavior, neuropathology, and gut microbiota of a Batten disease mouse model following administration of acidified drinking water. Sci Rep 9(1):14962
Kirk EP, Ong R, Boggs K, Hardy T, Righetti S, Kamien B et al (2021) Gene selection for the australian reproductive genetic carrier screening project (“Mackenzie’s mission”). Eur J Hum Genet 29(1):79–87
National health and medical research council. MRFF-2021 genomics health futures mission grant opportunity [Available from: https://www.nhmrc.gov.au/funding/find-funding/mrff-2021-genomics-health-futures-mission-grant-opportunity.
U. S. National library of medicine. CLN3 [Available from: https://clinicaltrials.gov/ct2/results?cond=CLN3&term=&cntry=&state=&city=&dist=.
Masten MC, Mink JW, Augustine EF (2020) Batten disease: an expert update on agents in preclinical and clinical trials. Expert Opin Investig Drugs 29(12):1317–1322
Specchio N, Ferretti A, Trivisano M, Pietrafusa N, Pepi C, Calabrese C et al (2021) Neuronal ceroid lipofuscinosis: potential for targeted therapy. Drugs 81(1):101–123
Acknowledgements
The authors thank S. Retsas, J. Nguyen, and M. Raza for their help in data collection. DHS wants to thank Indonesia Endowment Fund for Education (LPDP) scholarship for the support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was partly funded by National Health and Medical Research Council (NHMRC) Grants APP1116360, APP1099165, APP1109056, and Ophthalmic Research Institute of Australia (ORIA). The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
JRG and EEC. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by DHS, EEC, CLF, TM. S, MMJ, NAR, AMJ and JRG. BMN and RVJ collected and analysed genetic data. The first draft of the manuscript was written by DHS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DH Sakti, None; EE Cornish, None; CL Fraser, None; BM Nash, None; TM Sandercoe, None; MM Jones, None; NA Rowe; AM Johnson, None; J R Grigg; and RV Jamieson are consultant to Novartis.
Ethical approval
The study was approved by the South-Eastern Sydney Local Health District Human Research Ethics Committee and certified that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Due to retrospective nature of this study, informed consent is not required. This study does not contain images that may identify a person. Retinal images included in this study are de-identified.
Statement of human rights
The human rights of participants was recognised through the ethical conduct of the retrospective study.
Statement on the welfare of animals
This research did not involve animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakti, D.H., Cornish, E.E., Fraser, C.L. et al. Early recognition of CLN3 disease facilitated by visual electrophysiology and multimodal imaging. Doc Ophthalmol 146, 241–256 (2023). https://doi.org/10.1007/s10633-023-09930-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09930-1